Keywords: automated analysis, β cell, calcium dynamics, cell collective, pancreas tissue slices
Abstract
The release of peptide hormones is predominantly regulated by a transient increase in cytosolic Ca2+ concentration ([Ca2+]c). To trigger exocytosis, Ca2+ ions enter the cytosol from intracellular Ca2+ stores or from the extracellular space. The molecular events of late stages of exocytosis, and their dependence on [Ca2+]c, were extensively described in isolated single cells from various endocrine glands. Notably, less work has been done on endocrine cells in situ to address the heterogeneity of [Ca2+]c events contributing to a collective functional response of a gland. For this, β cell collectives in a pancreatic islet are particularly well suited as they are the smallest, experimentally manageable functional unit, where [Ca2+]c dynamics can be simultaneously assessed on both cellular and collective level. Here, we measured [Ca2+]c transients across all relevant timescales, from a subsecond to a minute time range, using high-resolution imaging with a low-affinity Ca2+ sensor. We quantified the recordings with a novel computational framework for automatic image segmentation and [Ca2+]c event identification. Our results demonstrate that under physiological conditions the duration of [Ca2+]c events is variable, and segregated into three reproducible modes, subsecond, second, and tens of seconds time range, and are a result of a progressive temporal summation of the shortest events. Using pharmacological tools we show that activation of intracellular Ca2+ receptors is both sufficient and necessary for glucose-dependent [Ca2+]c oscillations in β cell collectives, and that a subset of [Ca2+]c events could be triggered even in the absence of Ca2+ influx across the plasma membrane. In aggregate, our experimental and analytical platform was able to readily address the involvement of intracellular Ca2+ receptors in shaping the heterogeneity of [Ca2+]c responses in collectives of endocrine cells in situ.
NEW & NOTEWORTHY Physiological glucose or ryanodine stimulation of β cell collectives generates a large number of [Ca2+]c events, which can be rapidly assessed with our newly developed automatic image segmentation and [Ca2+]c event identification pipeline. The event durations segregate into three reproducible modes produced by a progressive temporal summation. Using pharmacological tools, we show that activation of ryanodine intracellular Ca2+ receptors is both sufficient and necessary for glucose-dependent [Ca2+]c oscillations in β cell collectives.
INTRODUCTION
Beta cell exposure to high glucose stimulates insulin release. Glucose entry into the β cells through glucose transporters leads to a series of cellular events that trigger diffusion of Ca2+ ions into the cytosol. Resulting elevation in [Ca2+]c ultimately produces a complex spatio-temporal pattern of [Ca2+]c events, membrane potential changes, and exocytosis of insulin (1–3). Single-cell recording of electrophysiological parameters is accepted as the gold standard approach to follow the plasma membrane events in a β cell at a millisecond time scale (4, 5). Electrical bursts composed of subsecond spikes were measured to have a plateau frequency of a few per minute (4) at high physiological glucose, while measured [Ca2+]c oscillations were often orders of magnitude slower, with a period in a time scale of minutes (6, 7). In the past, higher frequency and long-term recordings of [Ca2+]c oscillations were limited due to the relatively weak fluorescence signal of the classical fluorophores, their high-affinity Ca2+ binding that yielded phase lags, slow sampling rates, and substantial bleaching (6–8).
There is growing evidence that intracellular Ca2+ release channels, namely the inositol (1,4,5)-trisphosphate (IP3) receptors (IP3R) and the ryanodine receptor (RYR), contribute to Ca2+-dependent insulin release in β cells (9–12). However, their exact role in physiological activation, activity, and deactivation of β cell is not clear (2). According to the current predominant view, these processes are primarily driven by plasma membrane voltage-activated Ca2+ channels (VACCs). RYRs are primarily located on endoplasmic reticulum (ER) membranes and can be activated by Ca2+ ions, ATP, and modulated by PKA (13). There are several factors that suggest that the actual role of IP3Rs and RYRs may have been underestimated. First, preincubation of β cells in 3 mM glucose empties Ca2+ from ER, putting intracellular receptors out of the game (14). Second, the maximal [Ca2+]c concentration after glucose stimulation can reach values above 10 µM that cannot be picked up with high-affinity Ca2+ indicators, nor can they be achieved by a relatively low current density of VACCs on the plasma membrane (15). The [Ca2+]c concentration achieved at the peak of the Ca2+ bursts is orders of magnitude above the resting values and slow oscillations around the baseline and is therefore physiologically of much higher significance. Third, Ca2+-induced Ca2+ release-like (CICR) phenomena in a broader sense could occur as cluster firing of fields of RYRs (or IP3Rs) (16). And fourth, the coexistence of both IP3Rs and RYRs that possess different Ca2+ activation and deactivation properties has been suggested as a basis for complex patterns of intracellular calcium regulation in other cell types (17). Furthermore, a reactive oxygen species (ROS)-dependent mechanism was described to stimulate RYRs to support glucose-dependent insulin release in male rats (18). Moreover, multiple studies suggest that disrupted activity of intracellular Ca2+ release channels may contribute to β cell dysfunction and glucose intolerance (19–21).
To assess the contribution of intracellular Ca2+ release to [Ca2+]c changes requires a much higher temporally resolved imaging. Fortunately, the advancement in the synthesis of novel fluorescent Ca2+-sensing markers (22) solved most of the aforementioned technical issues enabling the recording of [Ca2+]c with millisecond temporal and high spatial resolution simultaneously in many cells with a possibility of repeated stimulation of the same cells over prolonged periods. High data volumes generated in this manner demanded the development of an advanced data analysis, which is presented in this work. With these tools at hand, we conducted a series of experiments to revisit glucose-dependent β cell activation, activity, and deactivation, and with the help of pharmacological tools we reassessed the contribution of RYR intracellular Ca2+ release channels in these processes.
MATERIALS AND METHODS
Ethics Statement
We conducted the study in strict accordance with all national and European recommendations on care and handling experimental animals, and all efforts were made to minimize the suffering of animals. The Ministry of Education, Science and Research, Republic of Austria (No: 2020-0.488.800) and the administration of the Republic of Slovenia for Food Safety, Veterinary and Plant Protection (No: U34401-12/2015/3) approved the experimental protocol.
Tissue Slice Preparation and Dye Loading
C57BL/6J mice, 8–20 wk of age, and of either sex (Jackson Laboratories), were kept on a 12:12-h light:dark schedule in individually ventilated cages (Allentown LLC) and used to prepare pancreatic tissue slices, as described previously (8, 23). In brief, after euthanizing the mice with CO2 and cervical dislocation, we accessed the abdominal cavity via laparotomy and distally clamped the common bile duct at the major duodenal papilla. Proximally, we injected the low-melting-point 1.9% agarose (Lonza) dissolved in extracellular solution [ECS, consisting of (in mM) 125 NaCl, 26 NaHCO3, 6 glucose, 6 lactic acid, 3 myo-inositol, 2.5 KCl, 2 Na-pyruvate, 2 CaCl2, 1.25 NaH2PO4, 1 MgCl2, 0.25 ascorbic acid] at 40°C into the common bile duct. Immediately after injection, we cooled the agarose-infused pancreas with ice-cold ECS and extracted it. We prepared tissue slices with a thickness of 140 µm with a vibratome (VT 1000 S, Leica) and collected them in HEPES-buffered ECS at RT [HEPES-buffered ECS, consisting of (in mM) 125 NaCl, 10 HEPES, 10 NaHCO3, 6 glucose, 6 lactic acid, 3 myo-inositol, 2.5 KCl, 2 Na-pyruvate, 2 CaCl2, 1.25 NaH2PO4, 1 MgCl2, 0.25 ascorbic acid; titrated to pH = 7.4 using 1 M NaOH]. For staining, we incubated the slices for 50 min at RT in the dye-loading solution (6 µM Calbryte 520, AAT Bioquest), 0.03% Pluronic F-127 (wt/vol), and 0.12% dimethylsulfoxide (vol/vol) dissolved in HEPES-buffered ECS. The Ca2+ fluorescent dyes from a Calbryte series are highly fluorescent and photostable, allowing long-term recording even in deeper layers of the tissue slice. The linear part of the Ca2+-binding curve for Calbryte 520 (KD of 1.2 µM) captures the [Ca2+]c changes in β cells better than the high-affinity sensors we routinely used in our imaging experiments before. All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Stimulation Protocol and Cytosolic Calcium Imaging
We transferred individual pancreatic slices to a perifusion system containing 6 mM glucose in HEPES-buffered ECS at 34°C. We show representative traces for each experimental condition. Each of these conditions has been tested on at least three separate experimental days. Slices were exposed to a series of square-pulse-like stimulations characterized by exposure to 8 mM glucose for 25 min, followed by a washout with substimulatory 6 mM glucose concentration until all the activity switched off. Imaging was performed on standard confocal microscopes equipped with resonant scanners (Leica Microsystems TCS SP5 and SP8 or Nikon A1R) both upright and inverted with their respective sets of ×20 high numerical aperture (NA) objective lenses. Acquisition frequency was set to at least 20 Hz at 256 × 256 pixels, with pixels size close to 1 µm2 to allow for a precise quantification of [Ca2+]c oscillations. Calbryte 520 was excited by a 488 nm argon laser (Leica SP5 and Nikon A1R) or 490 nm line of a white laser (Leica SP8). The emitted fluorescence was detected by HyD hybrid detector in the range of 500–700 nm using a photon counting mode (Leica) or GaAsP PMT detectors (Nikon).
Analysis and Processing of Data
The general analysis pipeline was as follows. Experiments involving imaging of pancreatic slices typically focused on a single field of view showing up to hundreds of cells, in a recording of at least several, often dozens, gigabytes. Current tools that are widely used (e.g., ImageJ) rely on loading the recording, or its part, into memory, for viewing, analysis, and processing. It also requires laborious and long human engagement. We have developed a set of interdependent tools to automatize as much as possible the analysis pipeline (Fig. 1, Supplemental Material). Regions of interest (ROIs) were identified using a semi-automatic detection as follows. Recordings were stored as a three-dimensional (T × X × Y) numpy array (24). When the recording was stable, obtaining a mean image, or any other statistic over frame, was rather trivial. In case there was horizontal movement, it could be corrected for by aligning the frames to a template. For this we used the functionality present in CaImAn (25), except that high-frequency recordings needed to be rebinned to some moderate frequency (a few Hz), before correcting, to reduce the noise level. Once the translation offsets were obtained, we used them to correct the recording in original frequency. To define regions of interest, we blurred the representative image by a kernel of the size we expect cells to be, and at the scale double of that. The difference between these two images represents a bandpass filter of the original, where the local intensity variations were emphasized (Supplemental Fig. S7-1). We then passed through all pixels where the value of the filtered image was positive (or larger than a small positive threshold), and for each pixel we searched for a local peak in its vicinity. All the pixels that lead to the same local peak were then grouped into a single ROI. As we were mainly interested in islet cells, we chose the kernel size to approximately correspond to 10 µm, which is the characteristic length scale of the islet cells. If the pixel size is unknown, the choice of kernel is up to the person running the scripts. Representative image can be a mean over all frames or any other statistic. In addition, our code supports standard deviation, mean and standard deviation of the first derivative of the movie, and a “robust maximum” of the movie. As “robust maximum,” we defined a very high percentile of the set absolute values of a set, essentially a value close to its maximum, by default it is 10th largest. We avoid the maximum as a means to make analysis robust to outliers. This statistic is sensitive to cells that fire extremely rarely during a recording, so that the mean of those pixels is negligible. By default, we choose an average of the mean and high percentile as a representative image for bandpass filtering and ROI extraction.
Figure 1.
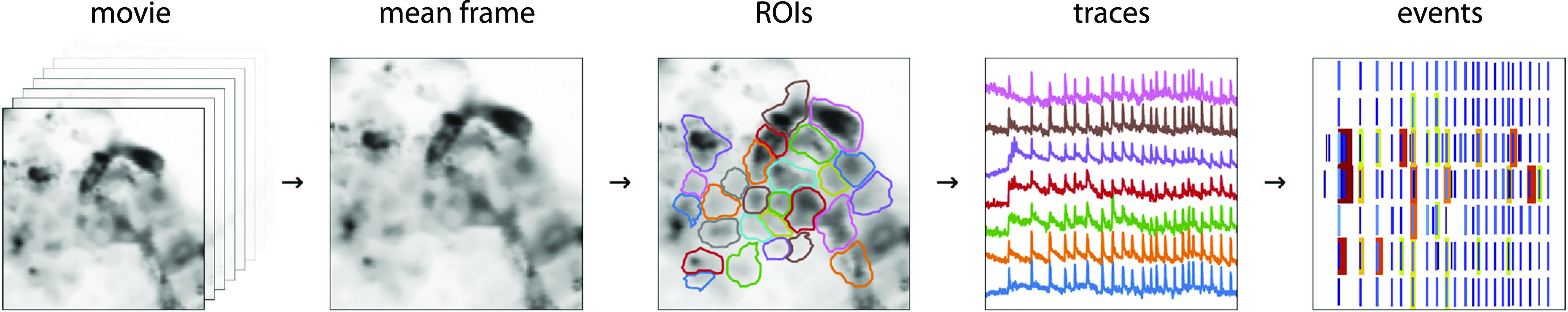
Processing pipeline to automatically detect regions of interest (ROIs) and [Ca2+]c events at all time scales within an experiment. From a full movie, we calculated the mean (or other statistic) across all frames. We passed the mean image through a bandpass filter and define ROIs by detecting local peaks of light intensity. We then saved ROIs with all the important information (time traces, ROI coordinates, movie statistics, recording frequency, pixel size, etc.). Traces contained features at very different timescales—with different timescales presumably being important for different cell types. We collected them into separable events for analysis.
Trace processing was conducted as follows. In an ideal detector, recording a time trace of a single pixel in the absence of any signal would consist of independent values of the number of photons detected during the dwell time. The values (x) are then distributed according to the Poisson distribution, with standard deviation (σ1) being equal to the square root of the mean µ1, . Transformation to standard score or z-score (Supplemental Fig. S7-2) is then performed that recasts the initial quantity x in the units of standard deviation from the expected mean.
A noisy trace in z spends 95% of the time between −2 and 2, and 99.7% between −3 and 3. Probability of z > 3 is very small P < 0.0013, which is why it is often considered a threshold value for pointing out the outliers. In general, the mean slow component needed to be inferred, typically by low-pass filtering. Throughout this project, we used cascaded second-order section (sos) filtering, implemented in scipy.signal module (25). The cut-off frequency fcut of a filter determines the timescale of the events that can be detected in z-score.
Fast Fourier transform naturally distorts signals, but the inferred z-score can be used to correct for it. We constructed an iterative filtering algorithm, where at each iteration, we neglect the outlier values of the original trace, substitute them with the values of the slow component, and reapply the filter. At each iteration, the distortion is less prominent, increasing the z-score. In Supplemental Fig. S7-2, we show the result after 10 iterations, though we found three iterations as a more conservative choice, and a reasonable compromise between results and computing time.
All aforementioned refers also to a sum of pixel traces, but, crucially, not to their mean. A sum of two pixels a and b (x = xa + xb), with means µa and µb, would have a standard deviation as expected . But, if we were to consider the average , standard deviation would be times smaller . Therefore, when calculating z-score for a ROI trace, we always considered the sum, rather than the average trace of the underlying pixels. When we visualized traces, we show them averaged, only to keep the scales comparable. The same reasoning holds for rebinning a single trace, where the resulting trace, rebinned by a factor of n, had a standard deviation times smaller than the original.
Experiments discussed in this manuscript were recorded on standard Leica SP5 or SP8, as well as NIKON A1R confocal microscopes. In cases where we used a Hybrid detector in the photon counting mode (Leica), we saw no significant departure from our assumption of Poisson distribution. Even with nonunit gain, the linear dependence between variance and mean remains, though the slope was different from 1 (Supplemental Fig. S7-3). Other types of detectors introduce additional sources of noise other than Poisson (e.g., thermal), but mostly they were still dominated by Poisson in our experience, at least as long as the values between neighboring pixels and frames were independent.
Traces contain features spanning orders of magnitude in time: from tens of milliseconds, to tens of minutes. We aimed to investigate how these events at different timescales and a connection between them and the islets’ environment interact. For this, we devised a two-step algorithm to identify intrinsic timescale of events and to minimize false positives (Supplemental Fig. S7-4). In the first step, we performed a sequential filtering of the traces at timescales starting from 0.5 s, and increasing by a factor of , until the timescale of the longest event of interest was achieved. At each timescale, we transformed the trace to z-score, and identify regions where z > 4 as candidate events.
Events were characterized by the start time (t0), its maximal height, and the width at the half of the height (half-width, δt), which is our measurement of its duration. For simplicity, we defined end time as tend = t0 + δt, although events arguably last much longer after the intensity drops at half of the peak.
For an event to be considered real, it needed to be detected at multiple timescales, and will have started around the same time and will have approximately the same halfwidth. We specify a tolerance of 20% of the halfwidth as to whether two candidate events should be considered equal; if their start and end times were within 20% of the halfwidth, they were considered cognates, having arisen due to the same real event. For a set of cognates, we estimated the start and end time of the real underlying event as a median over the set. If the resulting estimate for the halfwidth is larger than 2 s, we required that a set consists of at least four candidate events (corresponding to event being detectable when filtered at timescales that differ at least twofold). For shorter events, we required only that an event is not unique to a single timescale.
We also neglected events that last less than three time-frames, as well as those too close to the beginning or end of the recording (within δt/2), which we ascribed to artifacts from zero-padding for the filtering. We termed this procedure event distilling (Fig. 1, Supplemental Fig. S7-4).
RESULTS
Imaging of β Cell [Ca2+]c in Pancreas Slices at Physiological Glucose Concentration
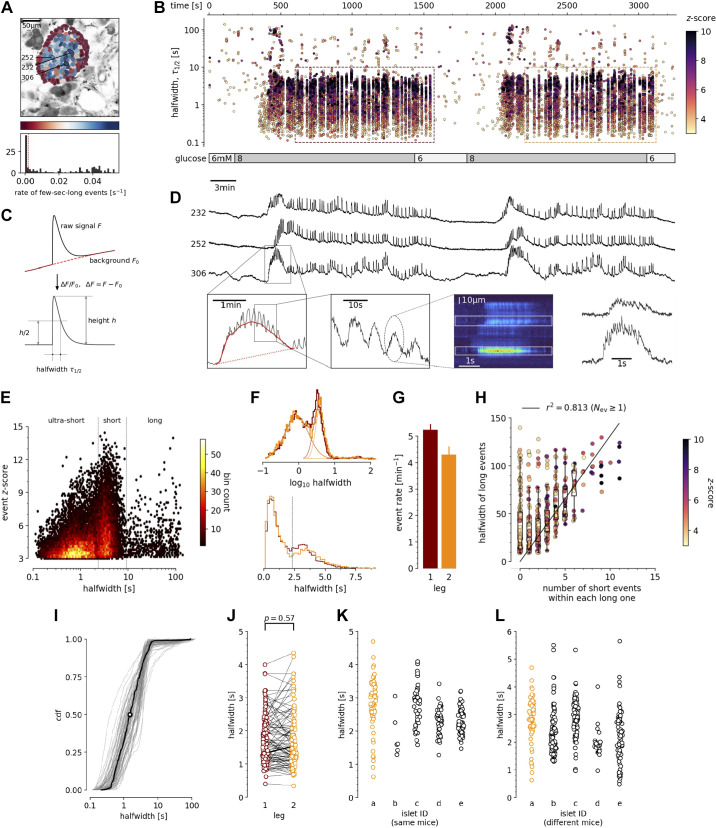
Pancreas tissue slices offer the opportunity to study β cells in situ (Fig. 2; Supplemental Video S1) and closer to physiological conditions compared with isolated islets and dispersed islet cells (26). At substimulatory glucose concentrations (6 mM), β cells from adult mice displayed a stable resting [Ca2+]c, which can be seen during the prestimulatory and subsequent washout phases (Fig. 2, B and D). From a baseline of 6 mM, a physiological stimulatory glucose concentration (8 mM) after a typical delay of solution exchange and glucose metabolism, triggered a biphasic [Ca2+]c response (Fig. 2, B and D). This biphasic response is composed of so-called initial increase in [Ca2+]c, previously termed the transient or asynchronous phase, followed by a prolonged plateau phase (Fig. 2, B and D) (8, 27). In both phases, we detected [Ca2+]c events at different time scales, spanning from millisecond to a hundred of seconds range (Fig. 2, B, D–F, and I–L). These [Ca2+]c events show several levels of temporal summation following a self-similarity pattern, as long events were a temporal summation of series of short events, a feature that could be indicative of CICR-like behavior (Fig. 2, D and H). In many aspects, the arrangement of short and long events reliably follows the electrical activity recorded in classical studies (4).
Figure 2.
Quantification and analysis of [Ca2+]c dynamics in physiological stimulatory glucose. A: regions of interest (ROIs) obtained by our segmentation algorithm. The color indicates the number of events identified in the ROI trace, upon a high-pass filtering at 0.2 Hz. We discarded ROIs with number of events below the threshold (red dashed line in the histogram in the bottom). Indicated are the ROI numbers whose filtered traces correlate best with the average trace for the whole islet; the correlation coefficients being 0.835, 0.818, and 0.807 (from top down in D). B: events’ halfwidth duration through time. Note the ranges of halfwidth duration occurring, the events’ synchronicity, and the phenotype’s reproducibility in sequential glucose stimulation. Color indicates the statistical significance in terms of z-score. Only events with a z-score higher than 3 were included. The stimulation protocol is indicated in the bar at the bottom of the pane. The plateau phases in both stimulations are indicated by a color coded rectangles. There is a prominent superposition of the short events on the plateau phases between the ROIs. C: an schematic of a transient event to describe the features of [Ca2+]c events from background subtraction of the raw data, to height and halfwidth duration. Peak-point is the time of highest amplitude of an event. D, top: time courses from ROIs indicated in A, exposed to a double stimulation protocol, and rebinned to 2 Hz (recorded 20 Hz). The abscissa is shared with B and is indicated there. Bottom: illustration of the compounding nature of transients. The left-most panel is a closeup of the trace above to highlight the long transient in red. In the second closeup, we emphasize the structure within the long transient. The third panel shows data from a different experiment, in same conditions (8 mM glucose), recorded as a line scan. In the final panel, we plot the time course of the indicated spatial regions, to illustrate the structure of subsecond events. E: in a z-score vs. halfwidth density plot, the events are clearly separated into three groups, which we named ultra-short, short, and long, with a typical separation between the groups at 2 s and 8.5 s. The dominant time scale of the events had a halfwidth duration of 2–5 s. F: normalized Gaussian fit through the logarithmic distribution of halfwidth duration for events for both first and second stimulation as indicated in B. G: the rate of events for the dominant short [Ca2+]c time scale for both stimulations indicated. H: evidence that the long events resulted from a progressive temporal summation of the (ultra-)short events. Some of the long events are less likely to contain substructure of short events, have lower z-scores, and contribute only little to the least square fit. I: cumulative distribution frequency (CDF) of the halfwidth duration of dominant short events during the plateau phase of the first stimulation for a randomly selected ROI. Thick black line indicates the median distribution. J: comparison between the halfwidth durations of events in individual ROIs during first and second glucose stimulation. K: comparison of halfwidth durations of events from all ROIs from different islets of the same mouse. L: comparison of halfwidth durations of events of all ROIs from islets of different mice.
The initial transient phase is characterized by sizeable delays between the activation of individual β cell groups within an islet (28). In the initial phase, we recorded one or more large transient [Ca2+]c events with a mean duration of tens of seconds (Fig. 2D), and with or without discernible superimposed shorter [Ca2+]c events. The long transient events were also occasionally recorded outside the initial phase. In less than 30% of recorded islets or in a small fraction of the cells in a typical islet, long events were more prominent and were apparent in both initial as well as in plateau phase.
Meanwhile, during the plateau phase we recorded mostly [Ca2+]c events with a mean halfwidth duration of a few seconds (Fig. 2, D–G), occasionally exhibiting temporal summation into slowly rising and decaying long events (Fig. 2, D and H). These short events were a dominant time scale in the control conditions at 8 mM glucose in all experiments. The short [Ca2+]c events within the plateau phase were commonly well synchronized among the β cells in an islet (Fig. 2, B and D). The short [Ca2+]c events on the plateau phase were regenerative and could be stably recorded for hours at physiological glucose concentrations (29). Due to the regenerative nature of the short [Ca2+]c events, we could design a multiple stimulation protocol on the same slice. The protocols were designed in a way that in the initial section we tested the responsiveness to the stimulation with 8 mM glucose, in the second section we tested the effect of a specific pharmacological treatment on stimulation with 8 mM glucose, and in the last section we applied another 8 mM glucose control stimulation. A detailed inspection of the short [Ca2+]c events in the plateau phase showed that they were compound events composed of even shorter [Ca2+]c events with a mean halfwidth duration below one second (Fig. 2E, Fig. 3C, and Fig. 6C). Similar ultra-short events were until now observed only as spikes on top of the bursts of the electrical activity, and were attributed to Ca2+ action potentials (30–32), mediated by the dominant contribution of L-type VACCs (33). Our approach offered insight into [Ca2+]c events on times scales spanning several orders of magnitude (Fig. 2E), with temporal resolution comparable with that achieved with electrophysiological approach, and with an upgrade of simultaneously visualizing the activity of all islet cells in an optical plane permitting the interrogation of a collective behavior in situ (Fig. 2A).
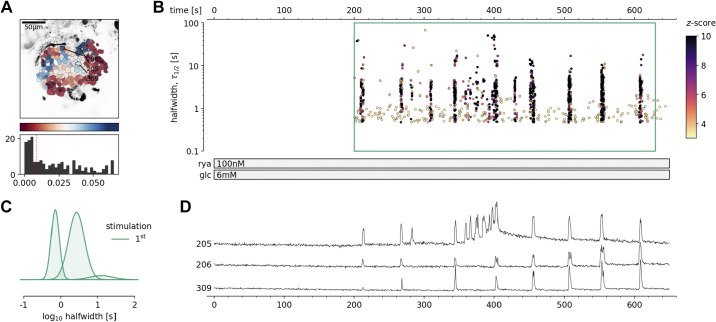
Figure 3.
Pharmacological activation of intracellular ryanodine receptor (RYR) Ca2+ receptors in mouse β cells at subthreshold glucose concentration. A: regions of interest (ROIs) obtained by our segmentation algorithm. The color indicates the number of events identified in the ROI trace, upon a high-pass filtering at 0.2 Hz. We discarded ROIs with number of events below the threshold (red dashed line in the histogram in the bottom). Indicated are the ROI numbers whose filtered traces correlate best with the average trace for the whole islet. B: events’ halfwidth duration through time. Note the ranges of halfwidth duration occurring, the events’ synchronicity, and the phenotype’s reproducibility in ryanodine stimulation. Color indicates the statistical significance in terms of z-score. The stimulation protocol is indicated in the bar at the bottom of the pane. There is a prominent superposition of the short events on the plateau phases between the ROIs. C: normalized Gaussian fit through the logarithmic distribution of halfwidth duration during ryanodine stimulation, indicated temporal summation producing three discrete modes. D: time courses from ROIs indicated in A, exposed to a double stimulation protocol, and rebinned to 2 Hz (recorded at 20 Hz). The abscissa is shared with B and is indicated there.
Figure 6.
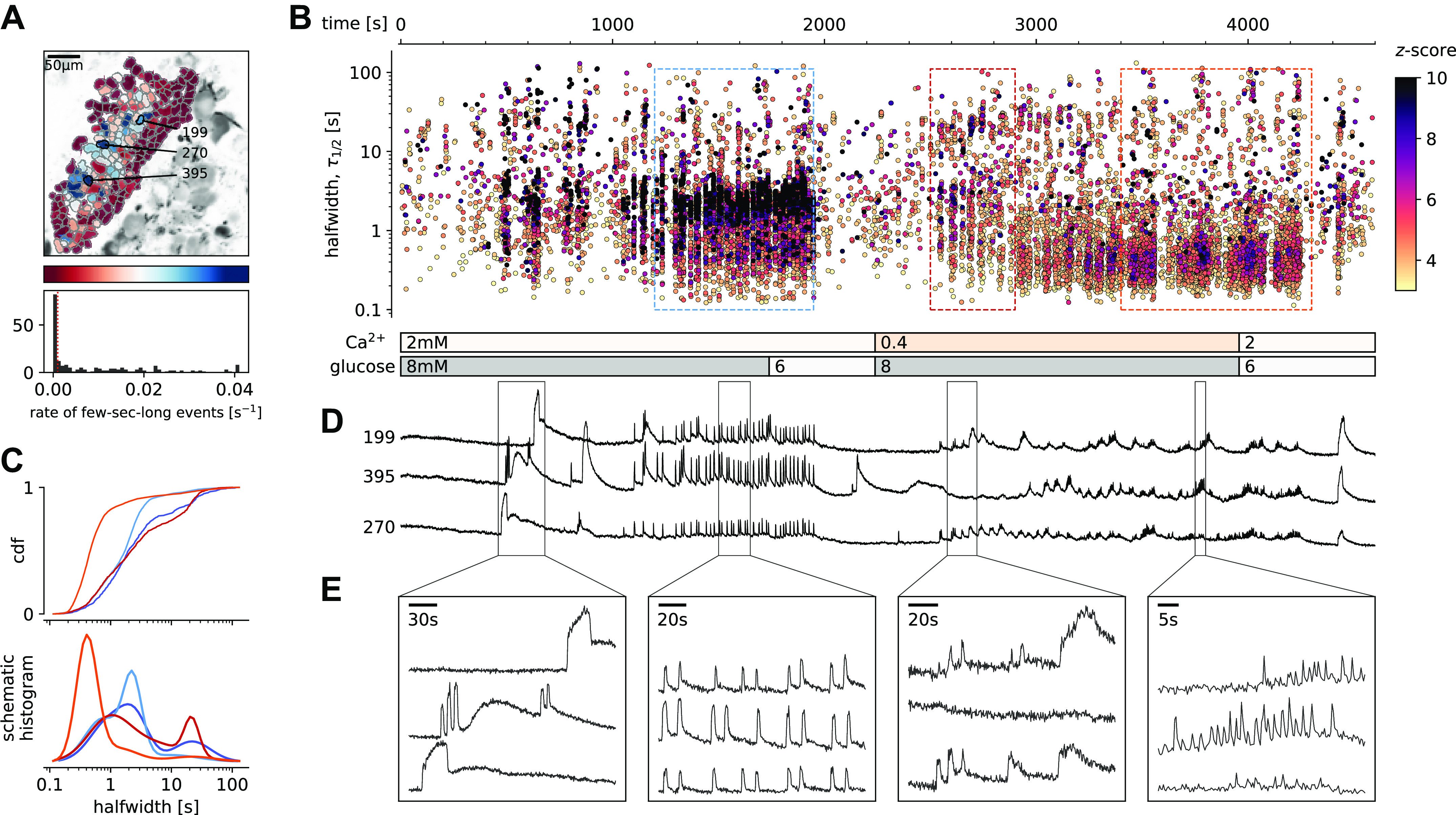
Glucose-dependent activation of β cells at subphysiological extracellular Ca2+ concentration. A: regions of interest (ROIs) obtained by our segmentation algorithm. The color indicates the number of events identified in the ROI trace, upon a high-pass filtering at 0.2 Hz. We discarded ROIs with number of events below the threshold (red dashed line in the histogram in the bottom). Indicated are the ROI numbers whose filtered traces correlate best with the average trace for the whole islet. B: events’ halfwidth duration through time for an islet exposed to a double 8 mM glucose stimulation protocol. Subphysiological extracellular Ca2+ level (400 µM) was applied in the second section of the protocol. The stimulation protocol is indicated in the bar at the bottom of the pane. There is a prominent superposition of the short events on the plateau phases between the ROIs. C, top: cumulative distribution frequency (CDF) of a mean halfwidth duration of events during the plateau phase of the both stimulations. Events from the initial transient phase are in dark colors, events from the plateau phase are in light color. Bottom: normalized Gaussian fit through the logarithmic distribution of halfwidth duration during control conditions and inhibition of voltage-activated Ca2+ channels (VACCs). Note a shift toward shorter events during the plateau phase in the conditions of subphysiological extracellular Ca2+ concentration. D: time courses from ROIs indicated in A, exposed to a double stimulation protocol, and rebinned to 2 Hz (recorded at 20 Hz). The abscissa is shared with B and is indicated there. E: expanded time traces from a representative ROI indicating (as indicated in C) the a long event from the initial transient phase, followed by a plateau phase in control, long event during initiation of the second stimulation, and short events from early plateau phase in low extracellular Ca2+ concentration.
The dynamic continuity of the time scales reflected in observed functional self-similarity resulted in a tri-modal distribution of halfwidth durations of the individual events that could be individually fitted with Gaussian function (Fig. 2F). The reproducibility of the individual modes between different experiments was high, suggesting relatively stable lengths of unitary and compound [Ca2+]c events (Fig. 2E), and comparable degree of reproducibility and variability between the individual events on the plateau phase in the same ROI (Fig. 2I), within the same ROIs during sequential stimulation with glucose (Fig. 2J), within ROIs of different islet of the same mice (Fig. 2K), as well as among islets from different mice (Fig. 2L). However, the frequencies of the events of all time scales were typically not compared between different islets, since this parameter showed larger degree of variability, confirming previous reports (28). In addition, reporting shorter modes of [Ca2+]c dynamics compared with classical approaches by using low-affinity indicators, we point out that these dominant short events reach at least an order of magnitude higher [Ca2+]c and make a major contribution to Ca2+-dependent insulin release (34).
Intracellular Ca2+ Channels Are Sufficient to Generate [Ca2+]c Oscillations at Substimulatory Glucose
Next, we directly assessed the contribution of RYR intracellular Ca2+ channels, which in mouse β cells are predominantly RYR2 (21), in glucose-dependent [Ca2+]c dynamics. We first tested the pharmacological RYR activation (Fig. 3, Supplemental Video 2). We showed that at subthreshold glucose, direct stimulation with stimulatory concentrations of ryanodine (100 nM) occasionally elicited regenerative Ca2+ events (Fig. 3). Predominantly short [Ca2+]c events were triggered at stimulatory ryanodine concentration. Both short and long [Ca2+]c events induced by ryanodine stimulation were a progressive temporal summation of the events in a subsecond range, and of the same duration as events observed under 8 mM glucose stimulation (Fig. 2). The onset of the activity varied between individual β cells in an islet (Fig. 3, B and D).
These results, together with the previously published data (35), suggest that pharmacological activation of both RYR and IP3 intracellular release channel could produce [Ca2+]c events in β cells that would be regularly observed during the 8 mM glucose stimulation. Both stimulations generated compound [Ca2+]c events through intermolecular CICR and presented two distinct kinetic components for intracellular release of Ca2+ and distinct regimes of intercellular coordination. Together, these data further confirm that a selective stimulation of Ca2+ release from the intracellular Ca2+ stores can contribute to [Ca2+]c oscillations in β cells that are both coordinated (Fig. 3) and noncoordinated (35).
Intracellular Ca2+ Channels Are Necessary for Glucose-Induced [Ca2+]c Oscillations
After demonstrating that [Ca2+]c events can be evoked at glucose concentrations just below the glucose activation threshold with RYR activation, we used specific pharmacological tools to selectively block the activity of these intracellular channels and consequently determine the contribution of these receptors to the glucose-dependent stimulation of [Ca2+]c events. We blocked RYR intracellular Ca2+ channels with an inhibitory ryanodine concentration (100 µM) (Fig. 4, Supplemental Video 3). High ryanodine selectively and completely inhibited the dominant time scale of events and its superimposed ultra-short events during the plateau phase, leaving initial long transient events intact (Fig. 4, B and D). Immediately after the washout of the high ryanodine, we observed prominent long [Ca2+]c oscillations, which is consistent with stimulatory effects of the low ryanodine concentration (Fig. 4, B–D). The exposure to high ryanodine concentration was fully reversible and subsequent exposure to control 8 mM glucose stimulation resulted in a response comparable with the initial control stimulation (Fig. 4B).
Figure 4.
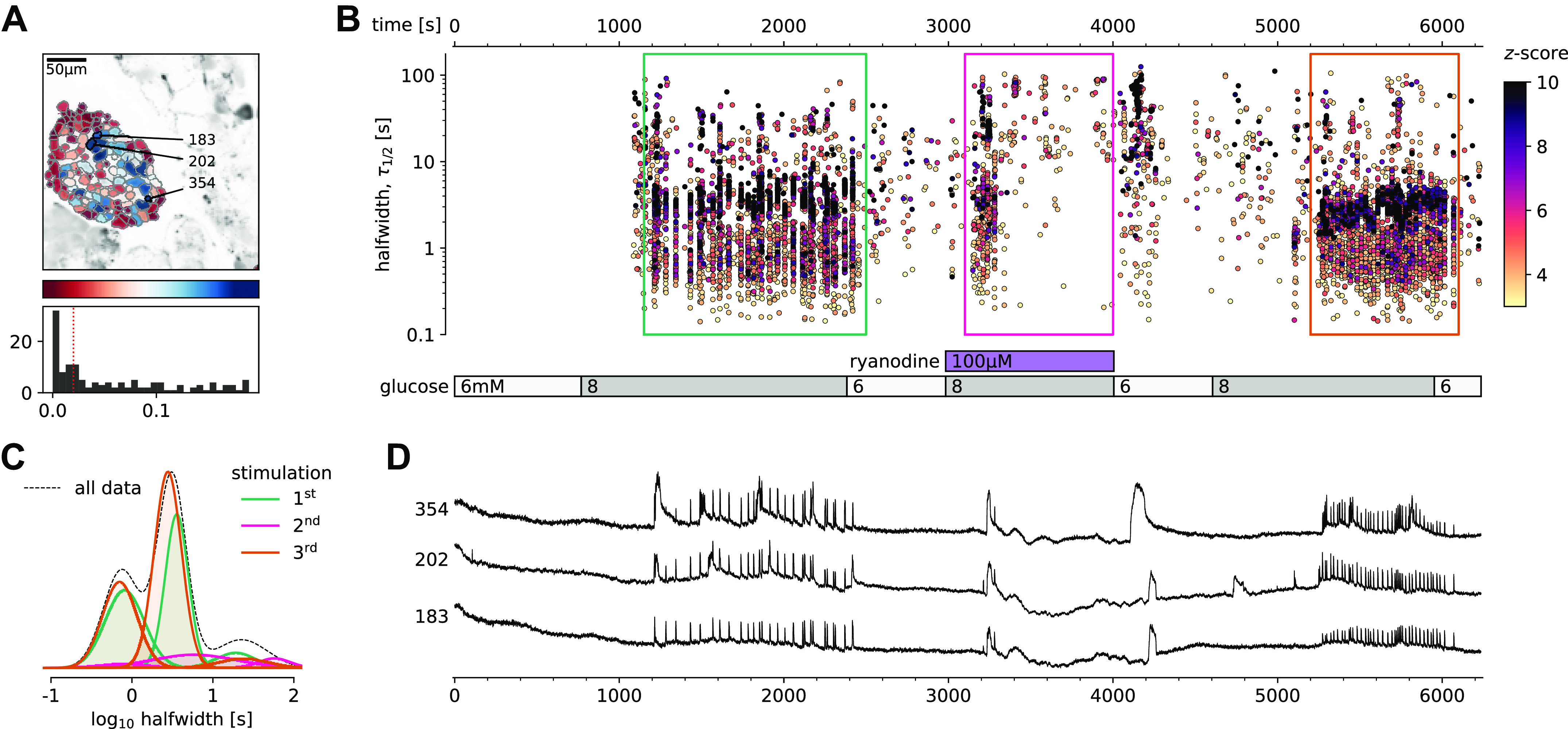
Pharmacological inhibition of intracellular ryanodine receptor (RYR) Ca2+ channels in mouse β cells selectively inhibits the plateau [Ca2+]c oscillations. A: regions of interest (ROIs) obtained by our segmentation algorithm. The color indicates the number of events identified in the ROI trace, upon a high-pass filtering at 0.2 Hz. We discarded ROIs with number of events below the threshold (red dashed line in the histogram in the bottom). Indicated are the ROI numbers whose filtered traces correlate best with the average trace for the whole islet. B: events’ halfwidth duration through time for an islet exposed to a triple 8 mM glucose stimulation protocol. Inhibitory ryanodine (100 µM) was applied in the middle section of the protocol. The stimulation protocol is indicated in the bar at the bottom of the pane. There is a prominent superposition of the short events on the plateau phases between the ROIs. C: normalized Gaussian fit through the logarithmic distribution of halfwidth duration during control conditions and RYR inhibition, indicated temporal summation producing three discrete peaks. Note a complete absence of short events during the plateau phase of the second stimulation in the presence of high ryanodine. D: time courses from ROIs indicated in A, exposed to a triple stimulation protocol, and rebinned to 2 Hz (recorded at 20 Hz). The abscissa is shared with B and is indicated there. Note the reduced [Ca2+]c level during the exposure to high ryanodine.
[Ca2+]c Events Can Be Initiated and Maintained during L-Type VACC Block
Our next step toward elucidating the molecular mechanisms of the aforementioned cytosolic Ca2+ events was to determine to what extent these multiple time scales of [Ca2+]c events depended on the activity of L-type VACCs. Previous experiments performed on β cell-selective Cav1.2 Ca2+ channel null mice showed that, in the absence of the Ca2+ channel, [Ca2+]c events were only moderately affected during the first minutes of the glucose stimulation, with a characteristic change in bursting pattern observed during acute stimulation (33). In the present study, we used the double stimulation protocol to test the effect of a saturating concentration of isradipine (5 µM), a specific L-type VACC blocker (Fig. 5, Supplemental Video 4). Consistent with the knockout mouse data, we observed a transient phase and initial plateau activity with a halfwidth duration with a median value of 3.1 s (Q1 1.8 s, Q3 4.6 s) during the first section and 3.4 s (Q1 2.1 s, Q3 4.3 s, P = 0.249) in the second section of the double protocol, where we co-applied isradipine. However, in the continuation of the plateau phase, the pattern of [Ca2+]c events was characterized by shorter and smaller events with the median value of halfwidth duration of 2.4 s (Q1 1.9 s, Q3 3.1.s, P < 10−15) [Fig. 5C (red traces)]. Instead of CICR-like compound events of the dominant time scale, a reduced number of events with a mean halfwidth duration below 2 s remained that could reflect progressively lower probability of CICR after blockage of VACCs and a switch toward isolated ultra-short events (Fig. 5E). Within minutes after the change in the pattern of [Ca2+]c events, the events disappeared completely.
Figure 5.
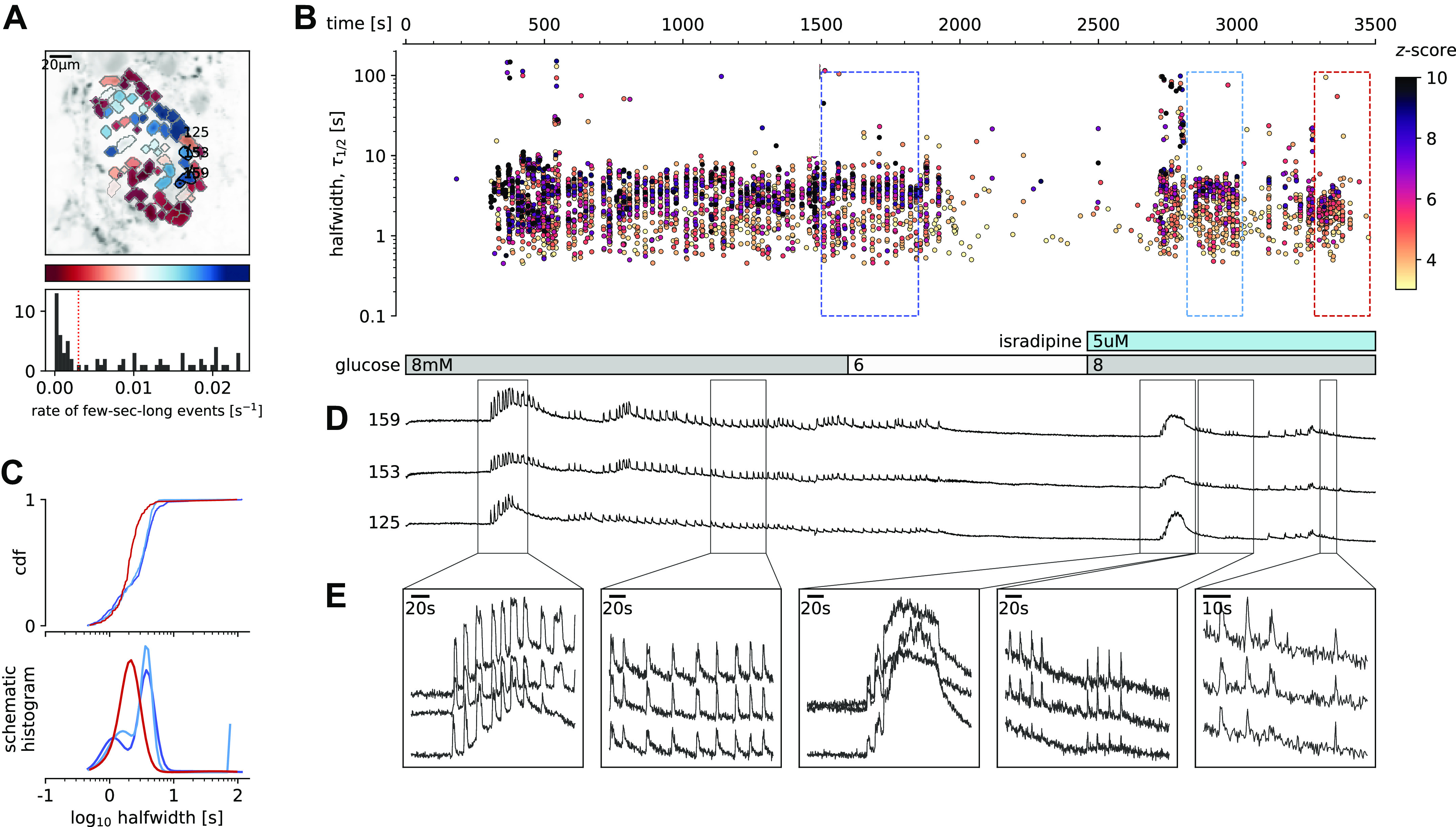
Glucose-dependent activation of β cells in the presence of inhibitory isradipine concentration to block voltage-activated Ca2+ channels (VACCs). A: regions of interest (ROIs) obtained by our segmentation algorithm. The color indicates the number of events identified in the ROI trace, upon a high-pass filtering at 0.2 Hz. We discarded ROIs with number of events below the threshold (red dashed line in the histogram in the bottom). Indicated are the ROI numbers whose filtered traces correlate best with the average trace for the whole islet. B: events’ halfwidth duration through time for an islet exposed to a double 8 mM glucose stimulation protocol. Saturating concentration of VACC blocker isradipine (5 µM) was applied in the second section of the protocol. The stimulation protocol is indicated in the bar at the bottom of the pane. There is a prominent superposition of the short events on the plateau phases between the ROIs. C, top: cumulative distribution frequency (CDF) of a mean halfwidth duration of events during the plateau phase of the both stimulations. Bottom: normalized Gaussian fit through the logarithmic distribution of halfwidth duration during control conditions and inhibition of VACCs. Note a shift toward shorter events during the late plateau phase in the presence of isradipine. D: time courses from ROIs indicated in A, exposed to a double stimulation protocol, and rebinned to 2 Hz (original frequency is 20 Hz). The abscissa is shared with B and is indicated there. E: expanded time traces from a representative ROI indicating (as indicated in C) the a long event from the initial transient phase, followed by a plateau phase in control, long event during initiation of the second stimulation, short events from early plateau phase, and further shortened events from a late plateau phase.
Blocking L-type VACCs with isradipine during the plateau phase produced a similar change in the phenotype of [Ca2+]c events, with progressively shorter and smaller events, before events subsided completely (Supplemental Fig. S4-1). A complete inhibition of the glucose-dependent [Ca2+]c events was obtained when slices were exposed to 100 µM diazoxide before the onset of glucose dependence (Supplemental Fig. S4-2). At this concentration, diazoxide clamps the membrane potential close to the resting membrane potential, which is around the diffusion equilibrium potential for K+ (EK; −90 mV) (7, 36) and outside the range where VACCs could be activated (37). However, when we applied the same high diazoxide concentration on the plateau phase, as is shown in the Supplemental Fig. S4-3, similarly to isradipine, 100 µM diazoxide first changes the quality of the oscillations, before the activity stops completely in most of the cells or islets. One out of five islets did not switch off completely during 100 µM diazoxide. In line with the standard model, the washout of diazoxide in high glucose happened only very slowly, with a delay of 15 min after the start of diazoxide washout, as has been shown also by others (38). When β cells eventually activated, their oscillations were initially ultra-short and only with time developed into a dominant short phenotype.
Our experiments confirm a role of L-type VACCs for the regenerative glucose-dependent activity of β cells during the plateau phase (39). However, we also confirm previous observations that these channels play only part of the role during the first minutes of β cells activation, and that the plasma membrane depolarization plays an independent role in stimulation of intracellular Ca2+ release (40).
[Ca2+]c Oscillations in Low Extracellular Ca2+ Conditions
To further test the potential role of intracellular Ca2+ channels on ER in the spatio-temporal regulation of [Ca2+]c events, we used a classical approach with the reduction of extracellular Ca2+ concentration (40). Incubation of β cells with 0.4 mM extracellular Ca2+ uncovered two major phenomena (Fig. 6, Supplemental Video 5). First, β cells within the islets tended to functionally dissociate, losing coordinated and global intercellular Ca2+ events with a phenotype resembling the Cx36 ablated or pharmacologically blocked cell-cell electrical coupling (41, 42). Second, low extracellular Ca2+ also changed the pattern of [Ca2+]c events (Fig. 6). The most prominent effect of low extracellular Ca2+ concentration was the complete absence of the short dominant time scale events (Fig. 6), where the median halfwidth duration of 2.2 s (Q1 1.6 s, Q3 3.0 s) from the control section, decomposed to ultra-short events with a median halfwidth duration of 0.6 s (Q1 0.5 s, Q3 1.7 s, P < 10−200) during the treatment section. In addition, the median amplitude of the events was significantly smaller in the section with low extracellular Ca2+ concentration. The decomposition of the dominant time scale short [Ca2+]c events resulted in a series of smaller, but higher frequency events (Fig. 6, B–E), confirming previous electrophysiological measurements(7). The coherence of responses between the individual β cells within the islet was more localized in low extracellular Ca2+ concentration. Also long events were significantly longer in the low extracellular Ca2+ concentration with median halfwidth duration of 24 s (Q1 14 s, Q3 33 s) in comparison to 184 20 s (Q1 11 s, Q3 32 s, P < 0.0001) during the control section. Our results suggest that in the presence of sufficient extracellular Ca2+ concentration, the CICR-like [Ca2+]c oscillations are likely to occur in glucose-stimulated β cells in situ and that these events decompose at subphysiologically low extracellular [Ca2+]. The spectrum of recorded patterns of β cell activities confirms previously described role of CICR through intracellular Ca2+ channels in glucose-dependent activity of these cells (43, 44).
The low extracellular Ca2+ concentration recordings provide further evidence regarding the existence of two distinct phases for the activation and activity of β cells. The initial phase during the activation of β cells in the fresh pancreas slice consisted of one or few long IP3-dependent [Ca2+]c transients (some tens of seconds long) as reported earlier (35), followed by a plateau phase comprising of a series of short RYR-mediated events.
DISCUSSION
Decades of electrophysiological experiments on pancreatic β cells have established a critical role for plasma membrane ion channels in controlling excitability, dynamics of cytosolic Ca2+ events, and insulin exocytosis (2, 30). In standard electrophysiology experiments, β cells are kept at 3 mM glucose where they are electrically silent, after which they are activated by an instantaneous increase to glucose levels well in excess of 10 mM (28, 42).
In the current study, we combined fast confocal microscopy, a photostable and bright low affinity Ca2+ sensor, and tools of data sciences to obtain new insights into physiological activation of β cells within intact islets in fresh pancreas tissue slices. Specific pharmacological modulation of RYR in intact β cell collectives stimulated with 8 mM glucose and recorded with a unique spatio-temporal resolution demands an updated model of β cell activation and bursting activity. According to our model, multiple time scales of events are generated with a progressive temporal superposition of ultra-short events, which represent CICR of either IP3 (35) or RYR Ca2+ release channels (Fig. 7). Both intracellular channels can be directly stimulated by glucose. The ultra-short events represent unitary activity of intracellular Ca2+ release channels, which can engage in intermolecular CICR, and as described for other cell types, depend on sufficient Ca2+ ER load (16, 45).
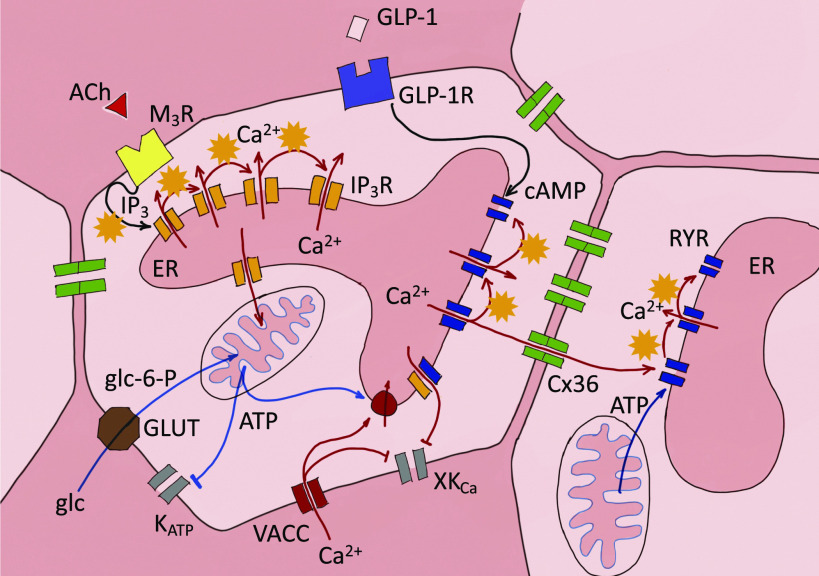
Figure 7.
Proposed model of the role of intracellular ryanodine receptor (RYR) and inositol (1,4,5)-trisphosphate (IP3) Ca2+ release channels in activation and activity of the mouse β cell. The basic principle in different patterns of [Ca2+]c oscillation is intermolecular Ca2+-induced Ca2+ release-like (CICR, orange stars). Physiological glucose stimulation activates both IP3R and RYR activity, which is followed by repetitive bursts of RYRs activity. The subcellular arrangement of RYRs enables intercellular communication through the Cx36 proteins and synchronized propagation of the Ca2+ events. ATP-dependent closure of KATP channels contributes to the synchronization. VACCs are critical for refilling of the internal stores. [Ca2+]c changes are registered by different plasma membrane K+ channels (XKCa). GLP-1R stimulation of cAMP production and ATP from the mitochondrial metabolism can modulate the RYR activity. Ca2+ concentration in the extracellular space and in different compartments of the cell is color coded, with the lowest [Ca2+]c in the cytosol.
Evidence for CICR in β cells involving both IP3 and RYR channels has been previously reported (46–48). When, however, the ER Ca2+ load decreases, CICR bursts switch to subsecond events produced by individual or more localized clusters of intracellular Ca2+ channels before events eventually stop. Decomposed CICR bursts, therefore, appear to oscillate at higher frequency, a phenomenon reported after ER emptying using thapsigargin (49), genetic ablation of L-type VACCs (33), or lowered extracellular Ca2+ (7, 49). Our experiments show that physiological glucose concentrations (e.g., 8 mM) support sufficient ER Ca2+ load (50) to enable CICR bursts triggered by direct pharmacological stimulation of RYR and IP3 Ca2+ channels. Addition of suprathreshold glucose concentration first activates a prominent IP3-dependent transient Ca2+ release lasting for several tens of seconds (35). IP3 activation is localized to segregated β cell clusters and not coordinated within the whole islet (51). Next, Ca2+-dependent mitochondrial metabolism of glucose boosts ATP production (20), that directly decreases the opening probability of KATP channels (52), and directly activates RYR Ca2+ channels (13, 53). High input resistance due to KATP channel closure enhances the coordination between β cells during the regenerative RYR-CICR activity. We also observed coherent intercellular activity after ryanodine stimulation at substimulatory glucose and this activity was specifically blocked by inhibitory concentrations of ryanodine, suggesting that RYR independently promotes intercellular coordination due to strategic cellular localization of these receptors. The long-term activity of intracellular Ca2+ receptors during the plateau phase of β cells must be supported with mobilization of Ca2+ from the extracellular space, where VACCs could activate through a large ER Ca2+ depletion as has been suggested earlier (49). Our results do not discount a role of plasma membrane KATP channels and voltage-gated Ca2+ entry in β cell function. We speculate that KATP channels play a critical depolarizing role to support activation of L-type VACCs, reloading the ER Ca2+ stores, promoting cell-cell coordination and the long-term regenerative activity, consistent with the human genetics of β cell responsiveness and their clinical utility as targets of sulfonylurea therapy (54). KATP channels may also play an outsized role in the response to an instantaneous glucose increase from hypoglycemic levels (2–3 mM) to supraphysiological levels (15–25 mM). This is consistent with previous observations suggesting that closure of KATP channels is not the sole mechanism to depolarize β cells (55). Indeed, other plasma membrane ionic currents have been invoked to explain the complex oscillatory behavior of β cells (2, 30). As an example, multiple Ca2+-dependent K+ and other K+ channel activities have been bundled to explain the elusive, so-called Kslow conductance, which could critically shape the typical electrical bursting pattern of β cells after the initial β cell activation (56–59). The details about the physiological glucose responses are even less clear in human β cells and still need to be performed (60).
Glucose plays an essential role in the filling, and therefore also emptying, intracellular Ca2+ stores (50). Forced depletion of intracellular Ca2+ stores using thapsigargin or extracellular EGTA, increases cytosolic Ca2+ concentration, and produces a sustained depolarization, with increased frequency of membrane potential oscillations (7, 49). Slow [Ca2+]c oscillations have been shown to persist with only minor modifications when SERCA is blocked with thapsigargin (61, 62) or when SERCA3 has been ablated (63–65). Similarly, reduced activity of the SERCA2 pump to maintain ER Ca2+ loading has been shown to disrupt glucose-stimulated calcium signaling (66). Increased frequency of [Ca2+]c events was also recorded in our experiments using low extracellular Ca2+ concentration. We demonstrate that glucose around the threshold (6–7 mM glucose) supports ER Ca2+ release through IP3 (35) and RYR release channels. In addition to supporting sufficient Ca2+ load in the ER, glucose-dependent effects in β cells provide all key substrates, such as ATP and cAMP, to directly trigger and modulate the activation of intracellular Ca2+ release channels, even in the initial absence of plasma membrane depolarization that would increase the opening probability of VACCs. Included in these stimuli is the parasympathetic release of ACh binding to muscarinic ACh receptors (mAChRs) and inducing insulin release via the production of IP3 and Ca2+ release from the intracellular stores (35, 67–69). Previous studies, including our own, systematically underestimated the role of RYR and IP3 receptors due to pre-emptying of intracellular Ca2+ stores in too low (0–3 mM) extracellular glucose.
The prominent role for intracellular Ca2+ release had strong early support from 45Ca2+ flux studies (39), but subsequent electrophysiological work challenged this paradigm (49, 53) and evidence accumulated in favor of the dominance of plasma membrane K+ channels and VACCs in patterning the dynamics of [Ca2+]c events. Even the presence of RYR Ca2+ channels in β cells was debated (41, 70). There has been less controversy regarding the expression and roles of IP3Rs (71, 72). However, we and others have documented RYR activity and confirmed RYR2 as the most abundant isoform expressed in rat, mouse, and human β cells (18, 19, 21, 73, 74). Indeed, the unique localization and Ca2+-release kinetics of each intracellular Ca2+ release channel enables the coding of Ca2+ signals to control specific cellular (73, 75–77) or intercellular functions. The duality of contributions of both IP3 and ryanodine receptors has been previously described to underly complex patterns of intracellular Ca2+ regulation of neuronal activity (17). As proposed in our recent theoretical paper, β cell and islet collectives could utilize dual Ca2+ sources to adequately respond to a variety of metabolic challenges from those requiring transient hormonal adjustments to those requiring major release of insulin (78). Such a functional arrangement would support both a form of a circuit memory, and risk of a stochastic editing contributing to the pathogenesis of diabetes mellitus (79).
It was proposed that a β cell’s ER is extraordinarily leaky to Ca2+ (14). ER Ca2+ leak is accelerated by ER stress, leading to β cell apoptosis (19, 21). In addition, excitotoxicity and ER Ca2+ overload have been implicated in β cell apoptosis (21) and may also contribute to diabetes pathogenesis. There is strong evidence that ER dysfunction is involved in the pathogenesis of both type 1 and type 2 diabetes (80). In type 1 diabetes, ER dysfunction is a prominent and early feature (81). In type 2 diabetes, Genome-wide association studies have identified multiple RYR2 SNPs with suggestive evidence in dozens of glycemic traits (www.type2diabetesgenetics.org, accessed Feb 2021) (82). Both ER Ca2+ load as well as intracellular Ca2+ channels can serve as targets for therapy of diabetes mellitus (83).
Pharmacological inhibition of VACCs activity with verapamil was recently reported to have a positive effect on β cell function and survival in adults with recently diagnosed T1D (84), consistent with previous preclinical studies (85). Preclinical studies also demonstrate that inhibition of voltage-gated Na+ channels can protect β cells from cytokine-induced death, and reduces diabetes incidence in NOD mice (86, 87). Also, intracellular Ca2+ release channels are druggable and were implicated in successful diabetes therapies. For example, RYR2 receptors can be activated by both ATP and PKA (13) and, can therefore be directly stimulated by glucose and modulated by incretins, catecholamines, and peptide hormones. RYRs are a proposed therapeutic target in Wolfram syndrome (88). Abundant physiological data also indicate that CICR from intracellular stores can serve as an “amplifier” of glucose-induced insulin granule exocytosis and plays a central role in incretin-induced insulin secretion (43, 89).
The major strength and limitations of our approach are essentially the same as they have been described for a fresh pancreatic slice preparation, which we introduced in 2001 (26). This is one of the first studies where we could use high spatial and temporal resolution imaging to specifically address intracellular Ca2+ receptors. Some of the observed long events may result from phenomena not necessarily connected to insulin secretion, such as movement, cilia protrusion, metabolism, drift of the objective of the microscope. At the moment we do not have a full understanding of the direction and the magnitude of all potential biases beyond the presented experimental evidence. In summary, using powerful new rapid imaging and data analysis methods, we show here that RYR play an important role in glucose stimulated Ca2+ signaling in β cells in situ.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Material: https://doi.org/10.6084/m9.figshare.20279532.v2.
GRANTS
M.S.R. received grants by the Austrian Science Fund/Fonds zur Förderung der Wissenschaftlichen Forschung (Bilateral Grants I3562-B27 and I4319-B30). M.S.R., C.E.-M., and J.D.J. received financial support from National Institutes of Health (NIH) R01DK127236. M.S.R., A.S., and D.K. further received financial support from the Slovenian Research Agency (Research Core Funding Program P3-0396 and Projects N3-0048, N3-0133 and J3-9289). J.D.J. received Grants from CIHR and Diabetes Canada.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S. and M.S.R. conceived and designed research; S.P., J.P., V.P., L.K.B., N.S., M.S.K., and J.D. performed experiments; S.P., S.S., J.P., N.S., D.K., and M.S.R. analyzed data; S.P., S.S., J.P., L.K.B., D.K., A.S., C.E.-M., J.D.J., and M.S.R. interpreted results of experiments; S.P., S.S., J.P., and M.S.R., prepared figures; S.P., S.S., and M.S.R. drafted manuscript; S.P., S.S., J.P., V.P., L.K.B., N.S., M.S.K., J.D., D.K., A.S., C.E.-M., J.D.J., and M.S.R edited and revised manuscript; S.P., S.S., J.P., V.P., L.K.B., N.S., M.S.K., J.D., D.K., A.S., C.E.-M., J.D.J., and M.S.R. approved final version of manuscript.
REFERENCES
- 1. Drews G, Krippeit-Drews P, Düfer M. Electrophysiology of islet cells. Adv Exp Med Biol 654: 115–163, 2010. doi: 10.1007/978-90-481-3271-3_7. [DOI] [PubMed] [Google Scholar]
- 2. Rorsman P, Ashcroft FM. Pancreatic β-cell electrical activity and insulin secretion: of mice and men. Physiol Rev 98: 117–214, 2018. doi: 10.1152/physrev.00008.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skelin Klemen M, Dolenšek J, Slak Rupnik M, Stožer A. The triggering pathway to insulin secretion: functional similarities and differences between the human and the mouse β cells and their translational relevance. Islets 29, 109–139, 2017. doi: 10.1080/19382014.2017.1342022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cook DL. Isolated islets of Langerhans have slow oscillations of electrical activity. Metabolism 32: 681–685, 1983. doi: 10.1016/0026-0495(83)90124-5. [DOI] [PubMed] [Google Scholar]
- 5. Sanchez-Andres JV, Pomares R, Malaisse WJ. Adaptive short-term associative conditioning in the pancreatic β-cell. Physiol Rep 8: e14403, 2020. doi: 10.14814/phy2.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bertram R, Satin LS, Sherman AS. Closing in on the mechanisms of pulsatile insulin secretion. Diabetes 67: 351–359, 2018. doi: 10.2337/dbi17-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilon P, Henquin JC. Influence of membrane potential changes on cytoplasmic Ca2+ concentration in an electrically excitable cell, the insulin-secreting pancreatic B-cell. J Biol Chem 267: 20713–20720, 1992. doi: 10.1016/S0021-9258(19)36744-4. [DOI] [PubMed] [Google Scholar]
- 8. Stožer A, Dolenšek J, Rupnik MS. Glucose-stimulated calcium dynamics in islets of langerhans in acute mouse pancreas tissue slices. PLoS One 8: e54638, 2013. doi: 10.1371/journal.pone.0054638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilon P, Chae HY, Rutter GA, Ravier MA. Calcium signaling in pancreatic β-cells in health and in Type 2 diabetes. Cell Calcium 56: 340–361, 2014. doi: 10.1016/j.ceca.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 10. Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium 32: 269–278, 2002. doi: 10.1016/s0143416002001884. [DOI] [PubMed] [Google Scholar]
- 11. Montero M, Brini M, Marsault R, Alvarez J, Sitia R, Pozzan T, Rizzuto R. Monitoring dynamic changes in free Ca2+ concentration in the endoplasmic reticulum of intact cells. EMBO J 14: 5467–5475, 1995. doi: 10.1002/j.1460-2075.1995.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santulli G, Nakashima R, Yuan Q, Marks AR. Intracellular calcium release channels: an update. J Physiol 595: 3041–3051, 2017. doi: 10.1113/JP272781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Petegem F. Ryanodine receptors: structure and function. J Biol Chem 287: 31624–31632, 2012. doi: 10.1074/jbc.R112.349068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klec C, Madreiter-Sokolowski CT, Stryeck S, Sachdev V, Duta-Mare M, Gottschalk B, Depaoli MR, Rost R, Hay J, Waldeck-Weiermair M, Kratky D, Madl T, Malli R, Graier WF. Glycogen synthase kinase 3 β controls presenilin-1-mediated endoplasmic reticulum Ca2+ leak directed to mitochondria in pancreatic islets and β-cells. Cell Physiol Biochem 52: 57–75, 2019. doi: 10.33594/000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rose T, Efendic S, Rupnik M. Ca2+-secretion coupling is impaired in diabetic Goto Kakizaki rats. J Gen Physiol 129: 493–508, 2007. doi: 10.1085/jgp.200609604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo T, Gillespie D, Fill M. Ryanodine receptor current amplitude controls Ca2+ sparks in cardiac muscle. Circ Res 111: 28–36, 2012. doi: 10.1161/CIRCRESAHA.112.265652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bezprozvanny L, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of lns(l,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351: 751–754, 1991. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 18. Llanos P, Contreras-Ferrat A, Barrientos G, Valencia M, Mears D, Hidalgo C. Glucose-dependent insulin secretion in pancreatic β-cell islets from male rats requires Ca2+ release via ROS-stimulated ryanodine receptors. PLoS One 10: e0129238, 2015. doi: 10.1371/journal.pone.0129238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luciani DS, Gwiazda KS, Yang TL, Kalynyak TB, Bychkivska Y, Frey MH, Jeffrey KD, Sampaio AV, Underhill TM, Johnson JD. Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and β-cell death. Diabetes 58: 422–432, 2009. doi: 10.2337/db07-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Santulli G, Pagano G, Sardu C, Xie W, Reiken S, D'Ascia SL, Cannone M, Marziliano N, Trimarco B, Guise TA, Lacampagne A, Marks AR. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J Clin Invest 125: 1968–1978, 2015. doi: 10.1172/JCI79273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamamoto WR, Bone RN, Sohn P, Syed F, Reissaus CA, Mosley AL, Wijeratne AB, True JD, Tong X, Kono T, Evans-Molina C. Endoplasmic reticulum stress alters ryanodine receptor function in the murine pancreatic β cell. J Biol Chem 294: 168–181, 2019. doi: 10.1074/jbc.RA118.005683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liao J, Patel D, Zhao Q, Peng R, Guo H, Diwu Z. A novel Ca2+ indicator for long-term tracking of intracellular calcium flux. Biotechniques 70: 271–277, 2021. doi: 10.2144/btn-2020-0161. [DOI] [PubMed] [Google Scholar]
- 23. Speier S, Rupnik M. A novel approach to in situ characterization of pancreatic β-cells. Pflugers Arch 446: 553–558, 2003. doi: 10.1007/s00424-003-1097-9. [DOI] [PubMed] [Google Scholar]
- 24. Harris CR, Millman KJ, Van Der Walt SJ, Gommers R, Virtanen P, Cournapeau D, Wieser E, Taylor J, Berg S, Smith NJ, Kern R, Picus M, Hoyer S, Van Kerkwijk MH, Brett M, Haldane A, Del Río JF, Wiebe M, Peterson P, Gérard-Marchant P, Sheppard K, Reddy T, Weckesser W, Abbasi H, Gohlke C, Oliphant TE. Array programming with NumPy. Nature 585: 357–362, 2020. doi: 10.1038/s41586-020-2649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giovannucci A, Friedrich J, Gunn P, Kalfon J, Brown BL, Koay SA, Taxidis J, Najafi F, Gauthier JL, Zhou P, Khakh BS, Tank DW, Chklovskii DB, Pnevmatikakis EA. CaImAn an open source tool for scalable calcium imaging data analysis. eLife 8: e38173, 2019. doi: 10.7554/eLife.38173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marciniak A, Cohrs CM, Tsata V, Chouinard JA, Selck C, Stertmann J, Reichelt S, Rose T, Ehehalt F, Weitz J, Solimena M, Slak Rupnik M, Speier S. Using pancreas tissue slices for in situ studies of islet of Langerhans and acinar cell biology. Nat Protoc 9: 2809–2822, 2014. doi: 10.1038/nprot.2014.195. [DOI] [PubMed] [Google Scholar]
- 27. Stožer A, Markovič R, Dolenšek J, Perc M, Marhl M, Slak Rupnik M, Gosak M. Heterogeneity and delayed activation as hallmarks of self-organization and criticality in excitable tissue. Front Physiol 10: 869, 2019. doi: 10.3389/fphys.2019.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stozer A, Skelin Klemen M, Gosak M, Križančić Bombek L, Pohorec V, Rupnik SM, Dolensek J. . Glucose-dependent activation, activity, and deactivation of β cell networks in acute mouse pancreas tissue slices. Am J Physiol Endocrinol Metab 321: E305–E323, 2021. doi: 10.1152/ajpendo.00043.2021. [DOI] [PubMed] [Google Scholar]
- 29. Gosak M, Stožer A, Markovič R, Dolenšek J, Perc M, Rupnik MS, Marhl M. Critical and supercritical spatiotemporal calcium dynamics in β cells. Front Physiol 8: 1106, 2017. doi: 10.3389/fphys.2017.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic β-cell. Prog Biophys Mol Biol 54: 87–143, 1989. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 31. Göpel S, Kanno T, Barg S, Galvanovskis J, Rorsman P. Voltage-gated and resting membrane currents recorded from B-cells in intact mouse pancreatic islets. J Physiol 521: 717–728, 1999. doi: 10.1111/j.1469-7793.1999.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Henquin JC, Meissner HP. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia 40: 1043–1052, 1984. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- 33. Schulla V, Renström E, Feil R, Feil S, Franklin I, Gjinovci A, Jing XJ, Laux D, Lundquist I, Magnuson MA, Obermüller S, Olofsson CS, Salehi A, Wendt A, Klugbauer N, Wollheim CB, Rorsman P, Hofmann F. Impaired insulin secretion and glucose tolerance in β cell-selective Cav1.2 Ca2+ channel null mice. EMBO J 22: 3844–3854, 2003. doi: 10.1093/emboj/cdg389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skelin M, Rupnik M. CAMP increases the sensitivity of exocytosis to Ca2+ primarily through protein kinase A in mouse pancreatic β cells. Cell Calcium 49: 89–99, 2011. doi: 10.1016/j.ceca.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 35. Sluga N, Postic S, Sarikas S, Huang YC, Stozer A, Rupnik MS. Dual mode of action of acetylcholine on cytosolic calcium oscillations in pancreatic β and acinar cells in situ . Cells 10: 1580, 2021. doi: 10.3390/cells10071580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zünkler BJ, Lenzen S, Männer K, Panten U, Trube G. Concentration-dependent effects of tolbutamide, meglitinide, glipizide, glibenclamide and diazoxide on ATP-regulated K+ currents in pancreatic B-cells. Naunyn Schmiedebergs Arch Pharmacol 337: 225–230, 1988. doi: 10.1007/BF00169252. [DOI] [PubMed] [Google Scholar]
- 37. Larsson-Nyrén G, Sehlin J, Rorsman P, Renström E. Perchlorate stimulates insulin secretion by shifting the gating of L-type Ca2+ currents in mouse pancreatic B-cells towards negative potentials. Pflugers Arch 441: 587–595, 2001. doi: 10.1007/s004240000426. [DOI] [PubMed] [Google Scholar]
- 38. Balboa D, Barsby T, Lithovius V, Saarimäki-Vire J, Omar-Hmeadi M, Dyachok O, Montaser H, Lund P-E, Yang M, Ibrahim H, Näätänen A, Chandra V, Vihinen H, Jokitalo E, Kvist J, Ustinov J, Nieminen AI, Kuuluvainen E, Hietakangas V, Katajisto P, Lau J, Carlsson P-O, Barg S, Tengholm A, Otonkoski T. Functional, metabolic and transcriptional maturation of human pancreatic islets derived from stem cells. Nat Biotechnol 40: 1042–1055, 2022. doi: 10.1038/s41587-022-01219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wollheim CB, Sharp GW. Regulation of insulin release by calcium. Physiol Rev 61: 914–973, 1981. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- 40. Roe MW, Lancaster ME, Mertz RJ, Worley JF 3rd, Dukes ID. Voltage-dependent intracellular calcium release from mouse islets stimulated by glucose. J Biol Chem 268: 9953–9956, 1993. [PubMed] [Google Scholar]
- 41. Ravier MA, Güldenagel M, Charollais A, Gjinovci A, Caille D, Söhl G, Wollheim CB, Willecke K, Henquin J-C, Meda P. Loss of Connexin36 channels alters β-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes 54: 1798–1807, 2005. doi: 10.2337/diabetes.54.6.1798. [DOI] [PubMed] [Google Scholar]
- 42. Speier S, Gjinovci A, Charollais A, Meda P, Rupnik M. Cx36-mediated coupling reduces β-cell heterogeneity, confines the stimulating glucose concentration range, and affects insulin release kinetics. Diabetes 56: 1078–1086, 2007. doi: 10.2337/db06-0232. [DOI] [PubMed] [Google Scholar]
- 43. Holz GG, Leech CA, Heller RS, Castonguay M, Habener JF. cAMP-dependent mobilization of intracellular Ca2+ stores by activation of ryanodine receptors in pancreatic β-cells. A Ca2+ signaling system stimulated by the insulinotropic hormone glucagon-like peptide-1-(7-37). J Biol Chem 274: 14147–14156, 1999. doi: 10.1074/jbc.274.20.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li W-h, Harbeck M, Roe MW, Holz GG. A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic β cells. J Physiol 566: 173–188, 2005. doi: 10.1113/jphysiol.2005.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen W, Wang R, Chen B, Zhong X, Kong H, Bai Y, Zhou Q, Xie C, Zhang J, Guo A, Tian X, Jones PP, O'Mara ML, Liu Y, Mi T, Zhang L, Bolstad J, Semeniuk L, Cheng H, Zhang J, Chen J, Tieleman DP, Gillis AM, Duff HJ, Fill M, Song L-S, Chen SR. The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias. Nat Med 20: 184–192, 2014. doi: 10.1038/nm.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dyachok O, Gylfe E. Ca2+-induced Ca2+ release via inositol 1,4,5-trisphosphate receptors is amplified by protein kinase A and triggers exocytosis in pancreatic β-cells. J Biol Chem 279: 45455–45461, 2004. doi: 10.1074/jbc.M407673200. [DOI] [PubMed] [Google Scholar]
- 47. Dyachok O, Tufveson G, Gylfe E. Ca2+-induced Ca2+ release by activation of inositol 1,4,5-trisphosphate receptors in primary pancreatic β-cells. Cell Calcium 36: 1–9, 2004. doi: 10.1016/j.ceca.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 48. Islam MS, Rorsman P, Berggren P-O. Ca2+-induced Ca2+ release in insulin-secreting cells. FEBS Lett 296: 287–291, 1992. doi: 10.1016/0014-5793(92)80306-2. [DOI] [PubMed] [Google Scholar]
- 49. Worley JF 3rd, McIntyre MS, Spencer B, Mertz RJ, Roe MW, Dukes ID. Endoplasmic reticulum calcium store regulates membrane potential in mouse islet β-cells. J Biol Chem 269: 14359–14362, 1994. [PubMed] [Google Scholar]
- 50. Tengholm A, Hellman B, Gylfe E. The endoplasmic reticulum is a glucose-modulated high-affinity sink for Ca2+ in mouse pancreatic β-cells. J Physiol 530: 533–540, 2001. doi: 10.1111/j.1469-7793.2001.0533k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Markovič R, Stožer A, Gosak M, Dolenšek J, Marhl M, Rupnik MS. Progressive glucose stimulation of islet β cells reveals a transition from segregated to integrated modular functional connectivity patterns. Sci Rep 5: 7845–7845, 2015. doi: 10.1038/srep07845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Speier S, Yang SB, Sroka K, Rose T, Rupnik M. KATP-channels in β-cells in tissue slices are directly modulated by millimolar ATP. Mol Cell Endocrinol 230: 51–58, 2005. doi: 10.1016/j.mce.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 53. Islam MS. The ryanodine receptor calcium channel of β-cells: molecular regulation and physiological significance. Diabetes 51: 1299–1309, 2002. doi: 10.2337/diabetes.51.5.1299. [DOI] [PubMed] [Google Scholar]
- 54. Pearson ER, Flechtner I, Njølstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, Shield J, Robert J-J, Holst JJ, Clark PM, Ellard S, Søvik O, Polak M, Hattersley AT, Neonatal Diabetes International Collaborative Group. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med 355: 467–477, 2006. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 55. Arkhammar P, Nilsson T, Rorsman P, Berggren O. Inhibition of ATP-regulated K+ channels precedes depolarization-induced increase in cytoplasmic free Ca2+ concentration in pancreatic β cells. J Biol Chem 262: 5448–5454, 1987. [PubMed] [Google Scholar]
- 56. Ammälä C, Bokvist K, Larsson O, Berggren PO, Rorsman P. Demonstration of a novel apamin-insensitive calcium-activated K+ channel in mouse pancreatic B cells. Pflugers Arch 422: 443–448, 1993. doi: 10.1007/BF00375069. [DOI] [PubMed] [Google Scholar]
- 57. Ammälä C, Larsson O, Berggren PO, Bokvist K, Juntti-Berggren L, Kindmark H, Rorsman P. Inositol trisphosphate-dependent periodic activation of a Ca2+-activated K+ conductance in glucose-stimulated pancreatic β-cells. Nature 353: 849–852, 1991. doi: 10.1038/353849a0. [DOI] [PubMed] [Google Scholar]
- 58. Gopel SO, Kanno T, Barg S, Eliasson L, Galvanovskis J, Renström E, Rorsman P. Activation of Ca2+-dependent K+ channels contributes to rhythmic firing of action potentials in mouse pancreatic β cells. J Gen Physiol 114: 759–770, 1999. doi: 10.1085/jgp.114.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tamarina NA, Wang Y, Mariotto L, Kuznetsov A, Bond C, Adelman J, Philipson LH. Small-conductance calcium-activated K+ channels are expressed in pancreatic islets and regulate glucose responses. Diabetes 52: 2000–2006, 2003. doi: 10.2337/diabetes.52.8.2000. [DOI] [PubMed] [Google Scholar]
- 60. Henquin JC. Glucose-induced insulin secretion in isolated human islets: does it truly reflect β-cell function in vivo? Mol Metab 48: 101212, 2021. doi: 10.1016/j.molmet.2021.101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu Y-J, Tengholm A, Grapengiesser E, Hellman B, Gylfe E. Origin of slow and fast oscillations of Ca2+in mouse pancreatic islets. J Physiol 508: 471–481, 1998. doi: 10.1111/j.1469-7793.1998.471bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gilon P, Arredouani A, Gailly P, Gromada J, Henquin J-C. Uptake and release of Ca2+by the endoplasmic reticulum contribute to the oscillations of the cytosolic Ca2+ concentration triggered by Ca2+ influx in the electrically excitable pancreatic B-cell. J Biol Chem 274: 20197–20205, 1999. doi: 10.1074/jbc.274.29.20197. [DOI] [PubMed] [Google Scholar]
- 63. Zhang IX, Ren J, Vadrevu S, Raghavan M, Satin LS. ER stress increases store-operated Ca2+entry (SOCE) and augments basal insulin secretion in pancreatic β cells. J Biol Chem 295: 5685–5700, 2020. doi: 10.1074/jbc.RA120.012721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Arredouani A, Guiot Y, Jonas J-C, Liu LH, Nenquin M, Pertusa JA, Rahier J, Rolland J-F, Shull GE, Stevens M, Wuytack F, Henquin J-C, Gilon P. SERCA3 ablation does not impair insulin secretion but suggests distinct roles of different sarcoendoplasmic reticulum Ca2+ pumps for Ca2+ homeostasis in pancreatic β-cells. Diabetes 51: 3245–3253, 2002. doi: 10.2337/diabetes.51.11.3245. [DOI] [PubMed] [Google Scholar]
- 65. Ravier MA, Daro D, Roma LP, Jonas JC, Cheng-Xue R, Schuit FC, Gilon P. Mechanisms of control of the free Ca2+ concentration in the endoplasmic reticulum of mouse pancreatic β-cells: interplay with cell metabolism and [Ca2+]c and role of SERCA2b and SERCA3. Diabetes 60: 2533–2545, 2011. doi: 10.2337/db10-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tong X, Kono T, Anderson-Baucum EK, Yamamoto W, Gilon P, Lebeche D, Day RN, Shull GE, Evans-Molina C. SERCA2 deficiency impairs pancreatic β-cell function in response to diet-induced obesity. Diabetes 65: 3039–3052, 2016. doi: 10.2337/db16-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Caicedo A. Paracrine and autocrine interactions in the human islet: more than meets the eye. Semin Cell Dev Biol 24: 11–21, 2013. doi: 10.1016/j.semcdb.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dolenšek J, Rupnik MS, Stožer A. Structural similarities and differences between the human and the mouse pancreas. Islets 7: e1024405, 2015. doi: 10.1080/19382014.2015.1024405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic β-cell function. Endocr Rev 22: 565–604, 2001. doi: 10.1210/edrv.22.5.0440. [DOI] [PubMed] [Google Scholar]
- 70. Beauvois MC, Arredouani A, Jonas JC, Rolland JF, Schuit F, Henquin JC, Gilon P. Atypical Ca2+-induced Ca2+ release from a sarco-endoplasmic reticulum Ca2+-ATPase 3-dependent Ca2+ pool in mouse pancreatic β-cells. J Physiol 559: 141–156, 2004. doi: 10.1113/jphysiol.2004.067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chen Z, Li Z, Peng G, Chen X, Yin W, Kotlikoff MI, Yuan Z-Q, Ji G. Extracellular ATP-induced nuclear Ca2+ transient is mediated by inositol 1,4,5-trisphosphate receptors in mouse pancreatic β-cells. Biochem Biophys Res Commun 382: 381–384, 2009. doi: 10.1016/j.bbrc.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 72. Hagar RE, Ehrlich BE. Regulation of the type III InsP3 receptor and its role in β cell function. Cell Mol Life Sci 57: 1938–1949, 2000. doi: 10.1007/pl00000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Johnson JD, Kuang S, Misler S, Polonsky KS. Ryanodine receptors in human pancreatic β cells: localization and effects on insulin secretion. FASEB J 18: 878–880, 2004. doi: 10.1096/fj.03-1280fje. [DOI] [PubMed] [Google Scholar]
- 74. Takasawa S, Kuroki M, Nata K, Noguchi N, Ikeda T, Yamauchi A, Ota H, Itaya-Hironaka A, Sakuramoto-Tsuchida S, Takahashi I, Yoshikawa T, Shimosegawa T, Okamoto H. A novel ryanodine receptor expressed in pancreatic islets by alternative splicing from type 2 ryanodine receptor gene. Biochem Biophys Res Commun 397: 140–145, 2010. doi: 10.1016/j.bbrc.2010.05.051. [DOI] [PubMed] [Google Scholar]
- 75. Berridge MJ. The AM and FM of calcium signalling. Nature 386: 759–760, 1997. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- 76. Berridge MJ. Unlocking the secrets of cell signaling. Annu Rev Physiol 67: 1–21, 2004. doi: 10.1146/annurev.physiol.67.040103.152647. [DOI] [PubMed] [Google Scholar]
- 77. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2000. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 78. Korošak D, Stožer A, Rupnik MS. Collective biological computation of metabolic economy (Preprint). arXiv 220607787, 2022. doi: 10.48550/arXiv.2206.07787. [DOI]
- 79. Durant F, Morokuma J, Fields C, Williams K, Adams DS, Levin M. Long-term, stochastic editing of regenerative anatomy via targeting endogenous bioelectric gradients. Biophys J 112: 2231–2243, 2017. doi: 10.1016/j.bpj.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol Metab 22: 266–274, 2011. doi: 10.1016/j.tem.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lombardi A, Tomer Y. Interferon alpha impairs insulin production in human β cells via endoplasmic reticulum stress. J Autoimmun 80: 48–55, 2017. doi: 10.1016/j.jaut.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445: 881–885, 2007. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 83. Henderson MJ, Trychta KA, Yang S-M, Bäck S, Yasgar A, Wires ES, Danchik C, Yan X, Yano H, Shi L, Wu K-J, Wang AQ, Tao D, Zahoránszky-Kőhalmi G, Hu X, Xu X, Maloney D, Zakharov AV, Rai G, Urano F, Airavaara M, Gavrilova O, Jadhav A, Wang Y, Simeonov A, Harvey BA. target-agnostic screen identifies approved drugs to stabilize the endoplasmic reticulum-resident proteome. Cell Rep 35: 109040, 2021. doi: 10.1016/j.celrep.2021.109040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ovalle F, Grimes T, Xu G, Patel AJ, Grayson TB, Thielen LA, Li P, Shalev A. Verapamil and β cell function in adults with recent-onset type 1 diabetes. Nat Med 24: 1108–1112, 2018. doi: 10.1038/s41591-018-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chen J, Cha-Molstad H, Szabo A, Shalev A. Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am J Physiol Endocrinol Metab 296: E1133–E1139, 2009. doi: 10.1152/ajpendo.90944.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lee JTC, Shanina I, Chu YN, Horwitz MS, Johnson JD. Carbamazepine, a β-cell protecting drug, reduces type 1 diabetes incidence in NOD mice. Sci Rep 8: 4588, 2018. doi: 10.1038/s41598-018-23026-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yang YHC, Vilin YY, Roberge M, Kurata HT, Johnson JD. Multiparameter screening reveals a role for Na+ channels in cytokine-induced β-cell death. Mol Endocrinol 28: 406–417, 2014. doi: 10.1210/me.2013-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fischer TT, Ehrlich BE. Wolfram syndrome: a monogenic model for diabetes mellitus and neurodegeneration. Curr Opin Physiol 17: 115–123, 2020. doi: 10.1016/j.cophys.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bruton JD, Lemmens R, Shi CL, Persson-Sjögren S, Westerblad H, Ahmed M, Pyne NJ, Frame M, Furman BL, Islam MS. Ryanodine receptors of pancreatic β-cells mediate a distinct context-dependent signal for insulin secretion. FASEB J 17: 301–303, 2003. doi: 10.1096/fj.02-0481fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material: https://doi.org/10.6084/m9.figshare.20279532.v2.
Data Availability Statement
Data will be made available upon reasonable request.