Abstract
In this review, we offer a concise overview of liver cancer epidemiology in China and worldwide from the official databases of GLOBOCAN 2020 and the National Cancer Registry in China. We also summarized the evidence for the main risk factors associated with liver cancer risk and discuss strategies implemented in China to control the liver cancer burden. Overall, liver cancer was the sixth most commonly diagnosed cancer and the third leading cause of cancer-related death worldwide in 2020. Although China contributed to nearly half of cases across the world alone, the incidence and mortality rates of liver cancer presented a declining trend owing to the persistent efforts from the governments at all levels. The current liver cancer burden in China still faces an arduous challenge due to the relatively large population base as well as the substantially low survival rate (12.1%). To better control the liver cancer burden with the lowest cost, specific measures should be conducted by reducing exposure to established risk factors such as hepatitis B infection and aflatoxin. The promotion of surveillance is also an important method to prolong the survival of liver cancer. This review will provide basic information for future direction on the control of liver cancer burden.
Keywords: Liver cancer, China, epidemiology, risk factors, prevention
Introduction
Liver cancer can be broadly subdivided into hepatocellular carcinoma (HCC) (approximately 75%−85%), intrahepatic cholangiocarcinoma, as well as other mixed types according to the pathological type (1). It ranked as the sixth most common cancer and the third preponderant cause of cancer-related deaths worldwide (2). The distribution of liver cancer presented significant geographic disparities, and China has contributed to nearly half of the global cases alone (3). Given the heavy burden of liver cancer currently, rational approaches focusing on primary prevention and surveillance have been developed. In addition, a series of policies have been enacted by the central government to enlarge the coverage of hepatitis B vaccination. These measures have shown their efficacy in preventing the development of liver cancer and decreasing overall mortality.
The epidemiology of liver cancer has been reported by domestic and international organizations, and the relevant healthcare policies and intervention measures were also conducted based on the epidemiological data in China in the last decades. However, the related sources of information are wide-ranging and lack comparisons. Thus, in this review, we aim to describe the most recent patterns of liver cancer globally and in China, and then explore the major risk factors of liver cancer and the prevention measures conducted in China.
Global epidemiology of liver cancer
Incidence rates
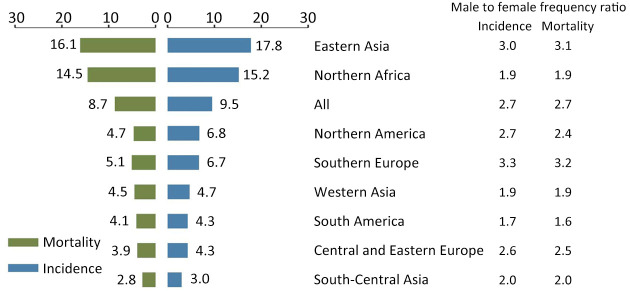
An estimated 905,677 people were diagnosed with liver cancer worldwide in 2020 with an age-standardized incidence rate of 9.5 per 100,000 (2). The age-standardized incidence rate of liver cancer was 14.1 per 100,000 for males and 5.2 per 100,000 for females. The liver cancer burden has dramatic geographic variations, which was most pronounced in transitioning regions (Figure 1). The highest incidence of liver cancer was observed in Eastern Asia with an age-standardized rate of 17.8 per 100,000, followed by Northern Africa (15.2 per 100,000). In contrast, the lowest incidence rates in the world occurred in South-Central Asia (3.0 per 100,000), Central and Eastern Europe (4.3 per 100,000), and South America (4.3 per 100,000). Northern America had an intermediate level of incidence rate (6.8 per 100,000) (Figure 1). However, the incidence of liver cancer in several regions of Europe and the United States gradually presented increasing trends (4).
Figure 1.
Incidence and mortality rates of liver cancer by regions (per 100,000).
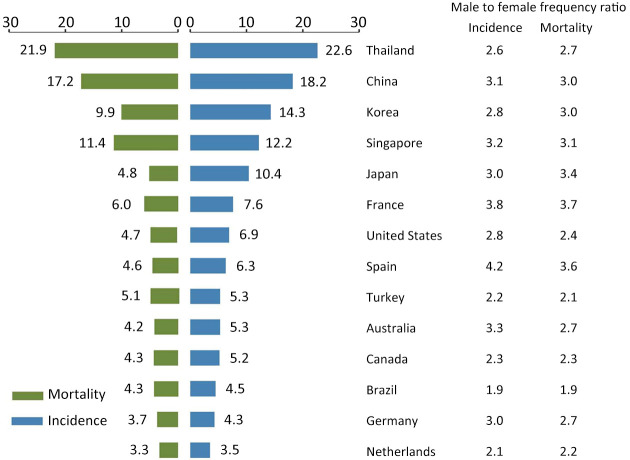
Individually, owing to the large population base, China is responsible for approximately half of the global burden of liver cancer. But the maximum age-standardized incidence rate was found in Mongolia (85.6 per 100,000). The age-standardized incidence rates among the developed countries were estimated at less than 10.0 per 100,000 (Figure 2), such as United States (6.9 per 100,000), France (7.6 per 100,000) and Australia (5.3 per 100,000).
Figure 2.
Incidence and mortality rates of liver cancer by countries (per 100,000).
Mortality rates
In 2020, there were about 830,180 deaths caused by liver cancer internationally which accounted for almost 8.3% of total cancer deaths. Nearly 69.6% of liver cancer deaths were males, with an age-standardized mortality rate of 12.9 per 100,000. Females had a relatively lower age-standardized mortality rate (4.8 per 100,000), compared to their counterparts. The estimated age-standardized mortality rate for liver cancer in Eastern Asia (particularly Mongolia) was more 5 folds than that of South-Central Asia (16.1 per 100,000 vs. 2.8 per 100,000). The lowest age-standardized mortality rates were observed in South-Central Asia (2.8 per 100,000), followed by Central and Eastern Europe (3.9 per 100,000) (Figure 1).
5-year survival rates
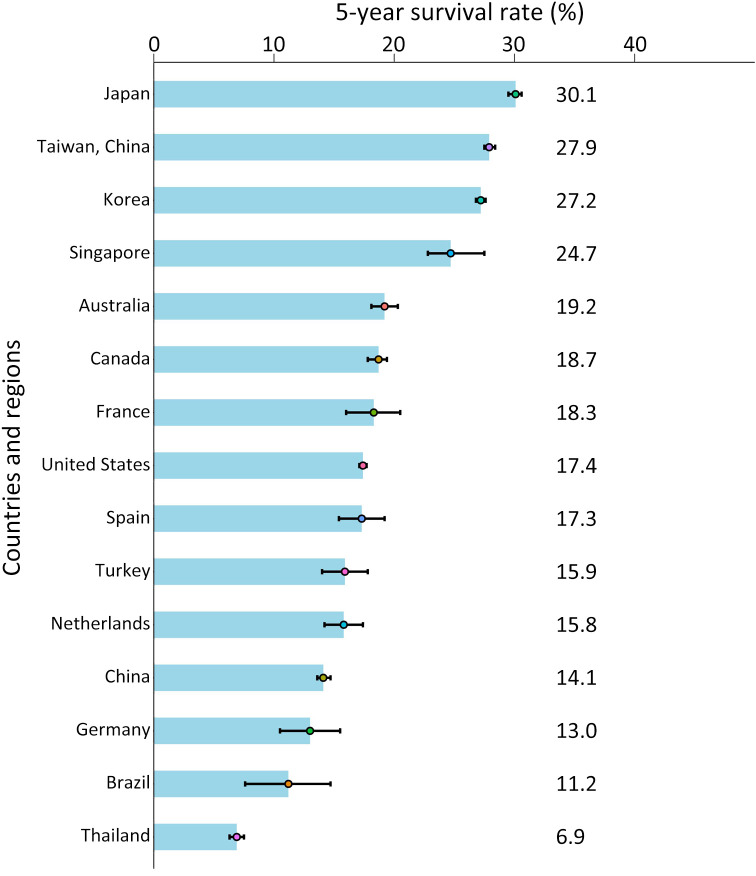
From 2000 to 2014, the age-standardized 5-year net survival rates ranged from 5.0% to 30.0% in most countries according to the estimation of CONCORD-3. From 2010 to 2014, a relatively high survival rate of liver cancer was shown in Japan at 30.1% and in the range of 20.0%−29.0% in Taiwan, China (27.9%), Korea (27.2%), and Singapore (24.7%) (Figure 3). In most countries, the 5-year survival rates of liver cancer were generally increasing, as survival increased by more than 10% in Korea, Singapore, and Norway from 2000 to 2014 (5).
Figure 3.
5-year survival rates of liver cancer by countries and regions.
Liver cancer epidemiology in China
Incidence rates
In 2016, liver cancer was the fourth most common cancer in China after lung cancer, colorectal cancer, and gastric cancer. An estimated 388,800 cases were diagnosed with liver cancer according to the most updated data from the National Cancer Center with a crude incidence rate of 28.1 per 100,000 and an age-standardized incidence rate (using Segi’s world standard population in 2000) of 17.7 per 100,000 (Table 1). About 74.3% (or about 288,800) of the reported cases were males, which was the third most prevalent malignancy in males. In females, liver cancer was the seventh most diagnosed cancer. The age-standardized incidence rates also showed significant sex disparities, and males had more three folds than the rate of females (26.7 per 100,000 vs. 8.7 per 100,000).
Table 1. Estimated liver cancer cases, deaths, and corresponding rates by sex and regions in China, 2016 (6,7).
| Regions | Sex | Incidence | Mortality | ||||
| Crude rate (1/100,000) | ASR (1/100,000) | Crude rate (1/100,000) | ASR (1/100,000) | ||||
| ASR, age-standardized rate. The incidence and mortality rates were standardized by Segi’s standard population structure. | |||||||
| All areas | Both | 28.1 | 17.7 | 24.3 | 15.1 | ||
| Male | 40.8 | 26.7 | 35.3 | 22.9 | |||
| Female | 14.8 | 8.7 | 12.9 | 7.3 | |||
| Urban areas | Both | 27.0 | 16.3 | 23.5 | 13.9 | ||
| Male | 39.7 | 24.9 | 34.5 | 21.3 | |||
| Female | 14.1 | 7.8 | 12.4 | 6.6 | |||
| Rural areas | Both | 29.7 | 19.3 | 25.9 | 16.6 | ||
| Male | 42.8 | 28.8 | 37.4 | 25.0 | |||
| Female | 16.1 | 9.7 | 13.9 | 8.2 | |||
| Eastern areas | Both | 27.4 | 15.8 | 24.0 | 13.5 | ||
| Male | 40.1 | 24.1 | 35.1 | 20.8 | |||
| Female | 14.5 | 7.6 | 12.7 | 6.4 | |||
| Central areas | Both | 27.6 | 18.8 | 23.7 | 16.0 | ||
| Male | 38.8 | 27.5 | 33.6 | 23.7 | |||
| Female | 15.8 | 10.2 | 13.4 | 8.4 | |||
| Western areas | Both | 30.9 | 20.9 | 26.9 | 18.1 | ||
| Male | 45.8 | 31.7 | 39.7 | 27.4 | |||
| Female | 15.4 | 9.9 | 13.6 | 8.6 | |||
Age- and region-specific incidence
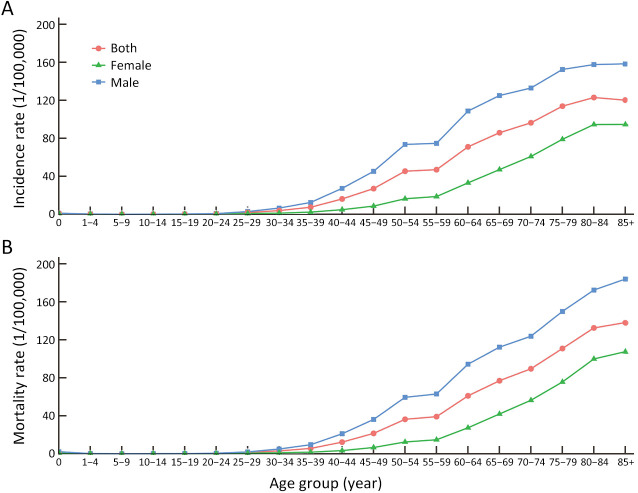
In terms of age-specific characteristics, liver cancer was most commonly diagnosed at age 60−79 years (129.0 thousand, 44.7%), followed by age 45−59 years (107.8 thousand, 37.3%), and age 15−44 years (29.1 thousand, 10.1%) in males. In contrast, liver cancer was not the main cancer type in females aged less than 60, and the diagnosed cases were 53.4 thousand and 17.8 thousand for females at age 60−79 years and over 80 years, respectively. We also abstracted the age-specific incidence rate of liver cancer from the National Cancer Registry Annual Report (6), which suggested that the incidence rate of liver cancer increased with age smoothly (Figure 4A). The incidence of liver cancer in China varied geographically. For both sexes combined, the estimated age-standardized rates of liver cancer in urban areas were lower than that in rural areas (16.3 per 100,000 vs. 19.3 per 100, 000) (Table 1). South China showed the highest age-standardized incidence rate (26.1 per 100,000), followed by the Northeast regions (18.6 per 100,000). To be more specific, the recent estimated data showed the southern rural areas had the highest liver cancer incidence (30.0 per 100,000), and the lowest age-standardized incidence rate was observed in Northern urban areas (10.5 per 100,000).
Figure 4.
Age-specific incidence and mortality rates per 100,000 of liver cancer. (A) Incidence; (B) Mortality.
Incidence trends
Liver cancer incidence has shown a favorable decline trend in recent years, although China contributed nearly half of the global burden (8). The age-standardized incidence rates of liver cancer in males were decreasing significantly by 2.2% per year between 2000-2016 and during the same period, the age-standardized incidence in females decreased by 2.7% per year (7).
Mortality rates
Liver cancer was the second most common malignancy of death in 2016 (7). In China, a total of 336,400 deaths (249,600 cases in males and 86,800 cases in females) of liver cancer were reported. The crude mortality rate was 24.3 per 100,000 (35.3 per 100,000 in males and 12.9 per 100,000 in females) and the age-standardized mortality rate was 15.1 per 100,000 (22.9 per 100,000 in males and 7.3 per 100,000 in females).
Age- and region-specific mortality
For the age-specific deaths of liver cancer among males, the population aged 60−79 years had the largest number of deaths (115.0 thousand, 46.1%), followed by age 45−59 years (88.0 thousand, 35.3%). The lowest number of deaths was observed in the population at the age of 0−14 years (0.3 thousand). Liver cancer was the second cancer related-deaths among the population aged 60−69 years in females, second only to lung cancer. The recorded frequencies of deaths were 0.2 thousand, 4.1 thousand, 17.4 thousand, and 19.0 thousand among the population at age 0−14 years, 15−44 years, 45−59 years, and over 80 years, respectively. According to data from the National Cancer Registry Annual Report (6), the highest mortality rate of liver cancer was observed in the age group of 85+ for both sexes combined (Figure 4B).
The geographical disparities were demonstrated in terms of liver cancer mortality. Rural areas had slightly higher mortality than urban areas (16.6 per 100,000 vs. 13.9 per 100,000) (Table 1). Similar to the incidence, a higher mortality rate was observed in the southern area (22.3 per 100,000), especially in the rural (26.6 per 100,000). Specifically, in a survey covering nine areas with high mortality rates from liver cancer (Qidong, Haimen, Dafeng, Taixing, Yangzhong of Jiangsu province, Jiashan of Zhejiang province, Changle, Tongan of Fujian province, and Fusui of Guangxi Zhuang autonomous region), it was found that Qidong and Taixing showed the highest mortality rate (77.42 and 57.70 per 100,000), which was almost 3-fold higher than the national average level (9). The lowest mortality rate was observed in North China (10.5 per 100,000) (7).
Mortality trends
A recognizable decreased trend in liver cancer mortality rate was observed in both sexes with average annual percentage changes (AAPCs) of 2.7% on average in men and 3.3% in women, respectively (7). The temporal trends of liver cancer mortality were described after adjusting the effects of age, period, and cohort stratified by sex and region, which suggested that a significant decline was observed in liver cancer mortality among urban residents (AAPC = −1.1% for men, and AAPC = −1.4% for women), whereas liver cancer mortality remained stable among rural residents (AAPC = −0.1% for men, and AAPC = −0.9% for women) (10).
Prevalence rates
Liver cancer was the sixth most prevalent cancer after colorectal cancer, breast cancer, lung cancer, thyroid cancer, and gastric cancer (5th in males and 11th in females) in 2020. The recorded prevalent cases of liver cancer were 422,633 cases with a 5-year prevalence proportion of 29.2 per 100,000 (11). For men, the number of prevalent cases increased from 268.2 thousand in 2011 to 310.6 thousand in 2020 and so had the age-standardized prevalence proportion from 38.8 per 100,000 in 2011 to 41.9 per 100,000 in 2020. While the liver cancer prevalence proportion was lower for women compared to men, with the age-standardized prevalence proportion from 14.6 per 100,000 to 15.9 per 100,000 between 2011 and 2020 (12).
Staging and 5-year survival rates
Among all cancers in China, liver cancer has the second-lowest survival rate because of the late diagnosis. A hospital-based retrospective analysis including 6,241 patients with primary HCC reported that the proportions of patients in stage 0/A, stage B, stage C, and stage D based on the Barcelona Clinic Liver Cancer staging system were 28.9%, 16.2%, 53.6 and 1.3%, respectively (13).
The age-standardized 5-year relative survival rate of liver cancer from 2012 to 2015 was 12.1% [95% confidence interval (95% CI): 11.7−12.6] in both sexes (14), which increased significantly from 10.1% between 2003 and 2005 year with an average annual change of about 0.7% (95% CI: −1.2−2.6) during the twelve years. Males had a lower 5-year survival rate, compared to those females (12.2% vs. 13.1%). Disparities were also observed in the survival rates between urban and rural areas. The age-standardized 5-year relative survival rate from 2012 to 2015 was 14.0% (95% CI: 13.3−14.7) in urban areas, with an average change of −0.7% (95% CI: −3.1−1.8) between 2003 and 2015. And the age-standardized 5-year relative survival rate from 2012 to 2015 was 11.2% (95% CI: 10.6−11.8) in rural areas, with an average change of 1.6% (95% CI: −0.4−3.6) between 2003 and 2015 (14). For the age-specific survival, we found individuals aged 0−64 years had a similar 5-year survival rate, however, the 5-year survival rate decreased dramatically for people aged over 65 years (14).
Economic burden
Inpatient expenses for liver cancer accounted for a demonstrating fraction of total cancer (15), from 4.5 billion CNY in 2008 to 20.3 billion CNY in 2017. Notably, the increasing rates of inpatient expenses for liver cancer significantly became to slow down after 2013. Substantial differences across types of the hospital were identified, both in levels and trends (Table 2). Among the three types of hospitals, inpatient expenses for liver cancer were highest for grade 3 general hospitals and lowest for grade 2 general hospitals (15). Additionally, prevalence-based research from a societal perspective reports the overall economic burden of liver cancer in China is 76.7 billion CNY in 2019, including 21.6 billion CNY for direct expenditure and 55.1 for indirect expenditure, respectively (16).
Table 2. Inpatient expenses of liver cancer overall and by types of hospital in China during 2008−2017 (15).
| Year | Inpatient expenses (×100 million) (CNY) | |||
| Total | General, grade 2 | General, grade 3 | Cancer specialized | |
| APC, annual percentage change; 95% CI, 95% confidence interval; CNY, Chinese Yuan. Data were extracted from Contemporary trends on expenditure of hospital care on total cancer and its subtypes in China during 2008−2017. | ||||
| 2008 | 44.9 | 6.7 | 21.9 | 4.3 |
| 2009 | 53.8 | 8.3 | 25.9 | 5.3 |
| 2010 | 64.2 | 10.4 | 34.7 | 6.3 |
| 2011 | 80.5 | 11.8 | 43.2 | 9.8 |
| 2012 | 92.8 | 11.4 | 50.1 | 10.1 |
| 2013 | 144.6 | 13.8 | 71.4 | 12.9 |
| 2014 | 176.6 | 15.0 | 71.0 | 14.1 |
| 2015 | 164.7 | 15.7 | 81.4 | 16.6 |
| 2016 | 178.3 | 14.8 | 96.3 | 19.4 |
| 2017 | 202.9 | 15.6 | 109.6 | 20.6 |
| Overall Change | 351.9 | 132.8 | 400.5 | 379.1 |
| APC (95% CI) (%) | ||||
| 2008−2013 | 22.0 (16.3, 27.7) | 13.4 (7.7, 19.1) | 23.2 (19.8, 26.6) | 22.5 (16.8, 28.2) |
| 2013−2017 | 6.9 (0, 13.8) | 2.3 (−1.9, 6.6) | 11.6 (5.9, 17.4) | 12.6 (9.3, 15.8) |
Main risk factors of liver cancer
Unlike other malignant tumors, liver cancer has relatively definite risk factors. Notably, chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection are the two strongest causes of liver cancer, which contributed to approximately 80% of all liver cancer cases worldwide (17,18). In China, it has been estimated that HBV infection was responsible for the 56.0% (95% CI: 52.0−60.0) fraction of liver cancer burden (19), followed by HCV, and alcohol consumption. In addition, a growing number of epidemiological studies showed that cirrhosis, aflatoxin exposure, diabetes, and metabolic risk factors were also reported to have significant associations with the occurrence of liver cancer (7,16,19-21).
HBV infection
Chronic HBV infection is one of the well-recognized risk factors for liver cancer worldwide. According to the data estimated by the International Agency for Research on Cancer (IARC), more than 300 million people are infected with HBV at a global scale currently and most of them would develop liver cancer. Approximately two out of three cases of liver cancer attributable to HBV infection occurred in less developing countries, particularly in China, which presented a high degree of geographical aggregation (22). A recent large-scale prospective population-based study recruiting 496,732 participants from China Kadoorie Biobank cohort showed that individuals who were HBsAg seropositive had a higher risk of HCC [hazard ratio (HR)=15.77, 95% CI, 14.15−17.57], compared to HBsAg seronegative participants. Such association could increase when combined with other risk factors (23,24). Individuals with seropositive HBsAg and a family history of liver cancer had a 30-fold increase in developing liver cancer risk, in comparison to HBsAg-seronegative individuals without a family history (23).
HCV infection
HCV infection was considered the second important risk factor of liver cancer. Approximately 30% of HCC cases could be attributable to HCV infection worldwide (25). The rate of HCC among persons with chronic infection of HCV ranged from 1% to 3% over 30 years (26). And a much number of cross-section and case-control studies showed that HCV infection is associated with a 15-to-20-fold increase in the risk of HCC when compared with HCV-negative subjects (26).
Cirrhosis
Cirrhosis is a significant co-factor in the development of liver cancer. The annual incidence of liver cancer was estimated at 2%−4% among individuals diagnosed with cirrhosis (27). Cirrhosis could be caused by chronic viral hepatitis, alcohol consumption, and inherited metabolic diseases (25). In HBV-related cirrhosis, the 5-year cumulative HCC risk is 15% in high endemic areas (28). And the 5-year cumulative HCC risk doubled among individuals with the infection of HCV and cirrhosis (28). A cohort study conducted in Taiwan, China revealed that the odds ratio (OR) for progression to HCC in patients with alcoholic cirrhosis alone, HBV-related, or HCV-related cirrhosis was 4.5, 12.6, and 15.3 respectively (23).
Aflatoxin exposure
The IARC listed aflatoxin B1 as a class I carcinogen in 1987 (29). Long-term, low-level dietary exposure to aflatoxin is a risk factor for the development of liver cancer, especially in regions of sub-Saharan Africa, Southeast Asia, and East Asia (4). The meta-analysis reported that aflatoxin exposure could increase the risk of liver cancer individually and interact with the chronic infection of HBV. The OR of liver cancer risk for detected aflatoxin in urine among individuals who were negative for HBsAg was 1.9, but the conjunction risk of liver cancer was 12.5 fold for those who were HBsAg positive, compared with individuals who were not exposed to aflatoxin and HBV infection (30). Liu and Wu estimated that 4.6%−28.2% of global liver cancer cases were related to aflatoxin exposure and most cases occurred in Southeast Asia and China (31). It is estimated that the average aflatoxin exposure in China was 17−30 ng/kg body weight/day (31). But the current study shows that the level of aflatoxin exposure has been a dramatic decline from the 1980s to the present (32).
Other potential etiologies
Smoking has been widely considered an established risk factor contributing to the development of multiple neoplasms. Three well-designed meta-analyses provided confirmed evidence that smoking is associated with an increased incidence of liver cancer, incorporating 191 epidemiological studies in total (33). Alcohol consumption is an important part of Chinese culture, especially in rural areas of China. According to the Chinese Health and Nutrition Survey, the alcohol consumption rate was around 34%.
A cross-sectional study conducted in 30 provinces in China among patients with liver diseases suggested that 60.44% of the participant were alcohol drinkers, showing a high dependence on alcohol consumption in this population (34). In addition, several studies have shown that alcohol consumption could cause cirrhosis and further increase the risk of liver cancer (35).
Mounting evidence suggested that nonalcoholic fatty liver disease (NAFLD) and other metabolic factors including obesity and diabetes are strongly associated with the development of liver cancer (36-38). In patients with NAFLD, the reported annual HCC incidence ranged from 0.04% to 0.6%, and a higher HCC incidence was observed when nonalcoholic steatohepatitis, diabetes, and advanced fibrosis were further identified (39). Currently, NAFLD has grown to become a public health problem in China and across the whole world with the dramatic changes in lifestyles (40,41). If obesity and type 2 diabetes mellitus level off in the future, China will face the highest growth of NAFLD prevalence between 2016 and 2030 (42).
Prevention measures implementation in China
Prevention should always be put into the priority to reduce the occurrence of liver cancer and its related mortality. During the past decades, China has made a great effort to reduce the burden of liver cancer. Among the established measures conducted in China, primary and secondary prevention are the most crucial and cost-effective.
Primary prevention
Vaccination
The initiation of the hepatitis B vaccination service played an important role in preventing HBV transmission. Hepatitis B vaccination received in early life has been shown dramatically to reduce persistent infection, providing 72% of vaccine efficacy against liver cancer occurrence (43,44). In 1992, hepatitis B vaccination was incorporated into the routine immunization schedule for infants with the self-reimbursed service by the central government. All neonates were encouraged to receive three doses of vaccination as per the defined schedule of 0, 1, and 6 months. In 2002, the neonatal hepatitis B vaccine was included in the Expanded Program of Immunization to offer free universal immunization for newborns, and further administration costs were then reimbursed by the government starting in 2005 (45). From 2009 to 2011, as a major public health project of the National Medical and health system reform, the Chinese government carried out a catch-up strategy of hepatitis B vaccine for people under the age of 15, and more than 68 million people benefited from this program (7).
Due to the persistent efforts and vigorous promotion, the coverage of three doses of hepatitis B vaccination showed a dramatic upward trend. According to a national survey in 2006, the weighted three doses coverage of hepatitis B vaccination reached 93.4% (46). In 2010, the immunization coverage of hepatitis B vaccination increased to 94% at least, and the immunization coverage of timely birth dose increased to 88% (47). As a consequence, the prevalence of hepatitis B surface antigen decreased largely. It is estimated that the weighted prevalence rates of HBsAg for the population aged 1−59 years were 7.2% and only 1.0% for children aged <5 years old (45). However, due to the time-lag action of the vaccination and the deprived opportunities to receive the hepatitis B vaccination among the population born before 1992, the count of HBV carriers is still considerable in China (about 70 million HBsAg carriers) (48,49). In 2016, the World Health Organization set the goal of eliminating the hepatitis threat by 2030 (50). To achieve this aim, there is still a long way to go in China.
Blood inspection
Blood transmission is an important channel to spread HBV and HCV. To ensure a safe blood supply environment, the Chinese government passed the Blood Donation Law in 1998 (51). Subsequently, the blood banking system was established (52). In 2011, the revised Blood donors’ health inspection requirements is a complement to the development of our unpaid blood donation work (53). The improved quality of donated blood promoted the decline of the HCV infection rate among children aged 15 years. However, the number of new cases of HCV infection increased from 70,681 to 201,622 between 2006 and 2012 (54). Thus, the control of HCV infection should be given enough attention, particularly focusing on antiviral therapy.
Food preservation and improved the source of water
Maize, peanut, and their derived products are the main maples in China, which were susceptible to aflatoxin exposure. To reduce the exposure level of aflatoxin, the primary prevention model of liver cancer in rural China was created. Two major factors were emphasized. The ditch and pond water should be replaced by piped tap water to make sure the quality of the source water. Changing the preservation method of staple foods is also encouraged, such as drying the crop in the sun, on a mat, discarding visibly moldy kernels or nuts before storage, and using natural fiber sacks for storage (55,56).
Secondary prevention
The prognosis of liver cancer is largely dependent on the stage at diagnosis. It is estimated that more than 60% of patients are not eligible for curative therapies when the disease was detected (57). Screening for liver cancer is considered an important potential channel to improve patients’ survival. At present, the existing population-based liver cancer screening programs in China include the Early Detection and Early Treatment of Cancer in Rural Cancer initiated in 2005, the Early Detection and Early Treatment of Cancer in Huaihe River Basin in 2007, and the Cancer Screening Program in Urban China in 2012 (58). How to conduct an effective surveillance program should concentrate on the target population, suitable surveillance modalities, and optimal intervals.
High-risk population
Surveillance among the population at high risk for developing liver cancer was recommended by several domestic and international liver societies (25,59). Population with the viral infection were considered at high risk for liver cancer, who were subjected to screening by some official guidelines. In addition, the American Association for the Study of Liver Diseases recommends offering surveillance when the expected risk of liver cancer is at least 1.5% per year in patients with hepatitis C and the incidence is greater than 0.2% per year among individuals with the infection of HBV (60). With regard to the potential population for liver cancer screening, plenty of models were constructed to identify and classify individuals at different levels of risk (61-63). Specifically, the aMAP score involving age, male, albumin-bilirubin, and platelets performed excellently in assessing HCC risk (C-index: 0.82−0.87), regardless of etiologies and ethnicities (64). Recently, the prevalence of NAFLD-related liver cancer presented an increasing trend. Whether these populations should be regarded the high risk still needs further discussion.
The coverage of screening plays an important role in achieving the effects of screening. A large-scale rural population-based study reported that the screening rate among the high-risk population was just around 45.4% (65). Another research conducted in urban areas reported the screening rate was 37.5% between 2013 and 2017 (66). Nevertheless, the coverage of liver cancer screening is still relatively low which was estimated to be about only 0.09% in 2019 across the country among the population aged 35−74 years (67).
Screening modalities
Alpha-fetoprotein (AFP) combined with ultrasound for liver cancer screening has reached a basic consensus. A recent meta-analysis showed that the ultrasound can identify HCC at any stage with a sensitivity of 76%−92%, but the efficacy is lower for early liver cancer with 33%−61% of sensitivity (68). The combination of AFP could increase the diagnostic performance, showing a sensitivity of 91%−99% given that ultrasound examination may be affected by the experience of the clinical doctor, patients’ and lesions’ characteristics (68). Relatively, CT and MRI are not suitable offered to large-scale screening programs due to the high cost and radiation exposure.
With the development of technology and science, a number of novel biomarkers, such as des-gamma carboxy prothrombin (DCP) and lectin-bound AFP (AFP-L3) are identified. The sensitivity and specificity for DCP were 87% and 85%, for AFP 69% and 87%, and for AFP-L3 56% and 90% 56.1%, respectively (69). ALP-L3 and DCP have been incorporated into guidelines in Japan (70). A non-invasive liquid biopsy method based on circulating tumor DNA could differentiate HCC and normal tissue accurately and less time-consuming. But no convincing evidence showed that such a method has definite cost-effectiveness (71).
Screening interval
The optimal interval for the surveillance program plays a critical role in finding the curable stage of liver cancer. However, the best surveillance schedule for liver cancer remains unknown, ranging from 3 months to 12 months. A multicenter randomized trial including patients with alcoholic cirrhosis comparing 3-month periodicity and 6-month period indicated that every 3 months period for ultrasonographic surveillance did not significantly increase the likelihood to detect small (≤3 cm) HCC (79% vs. 70%), and extended 5-year survival (85% vs. 86%) (72). Another community-based randomized study conducted in the population of positive hepatitis B surface antigen (HBsAg) or antibody to HCV or a platelet count <150 (109/L) compared the surveillance effectiveness at 4 months or 12 months, but no different survival benefits were observed (73). Up to now, a 6-month surveillance interval is the most recommended for the high-risk population according to the growth rate of liver cancer. And the suitable interval could be adjusted according to the local resources and population characteristics.
Conclusions
The burden of liver cancer in China contributes to nearly half of cases across the world but presents a decreasing trend over the past decade. Achievements have been made by progress in the prevention and control associated with a reduction in the established risk factors exposure, aimed at hepatitis B vaccination as well as aflatoxin, and enlarging the coverage of screening in the high-risk population. However, it deserves attention on the increasing prevalence of risk factors like NAFLD, alcohol, and tobacco use. Considering the large population base of liver cancer cases in China, specific intervention through primary and secondary prevention modalities combined with policies should continue to be emphasized and promoted.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81974492), the National Natural Science Foundation of China (No. 82273721), and the Sanming project of Medicine in Shenzhen (No. SZSM201911015).
References
- 1.He J, Chen WQ, Shen HB, et al China guideline for liver cancer screening (2022, Beijing) Lin Chuang Gan Dan Bing Za Zhi (in Chinese) 2022;38:1739–58. doi: 10.3969/j.issn.1001-5256.2022.08.007. [DOI] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Rudolph KL Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 4.McGlynn KA, Petrick JL, London WT Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin liver Dis. 2015;19:223–38. doi: 10.1016/j.cld.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allemani C, Matsuda T, Di Carlo V, et al Global surveillance of trends in cancer survival: analysis of individual records for 37, 513, 025 patients diagnosed with one of 18 cancers during 2000-2014 from 322 population-based registries in 71 countries (CONCORD-3) Lancet. 2018;391:1023–75. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He J, Chen WQ. Beijing: China Cancer Registry Annual People’s Medical Publishing House, 2020.
- 7.Zheng R, Zhang S, Zeng H, et al Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2:1–9. doi: 10.1016/j.jncc.2022.02.002>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi JF, Cao M, Wang Y, et al Is it possible to halve the incidence of liver cancer in China by 2050. Int J Cancer. 2021;148:1051–65. doi: 10.1002/ijc.33313. [DOI] [PubMed] [Google Scholar]
- 9.Chen JG, Zhang SW Liver cancer epidemic in China: past, present and future. Semin Cancer Biol. 2011;21:59–69. doi: 10.1016/j.semcancer.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Wang Y, Li M, et al Long-term trends of liver cancer mortality by gender in urban and rural areas in China: an age-period-cohort analysis. BMJ Open. 2018;8:e020490. doi: 10.1136/bmjopen-2017-02049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Agency for Research on Cancer. Global cancer observatory. Cancer today 2020. Available online: https://gco.iarc.fr/today/online-analysis-table
- 12.Zheng R, Zeng H, Zhang S, et al National estimates of cancer prevalence in China, 2011. Cancer Lett. 2016;370:33–8. doi: 10.1016/j.canlet.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Xiang X, Zhong JH, Wang YY, et al Distribution of tumor stage and initial treatment modality in patients with primary hepatocellular carcinoma. Clin Transl Oncol. 2017;19:891–7. doi: 10.1007/s12094-017-1621-6. [DOI] [PubMed] [Google Scholar]
- 14.Zeng H, Chen W, Zheng R, et al Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555–67. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 15.Cai Y, Chen W, Wang X, et al Contemporary trends on expenditure of hospital care on total cancer and its subtypes in China during 2008-2017. Chin J Cancer Res. 2021;33:627–36. doi: 10.21147/j.issn.1000-9604.2021.05.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao MD, Liu CC, Wang H, et al The population-level economic burden of liver cancer in China, 2019-2030: prevalence-based estimations from a societal perspective. Cost Eff Resour Alloc. 2022;20:36. doi: 10.1186/s12962-022-00370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Martel C, Maucort-Boulch D, Plummer M, et al World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190–200. doi: 10.1002/hep.27969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omata M, Cheng AL, Kokudo N, et al Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–70. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao M, Ding C, Xia C, et al Attributable deaths of liver cancer in China. Chin J Cancer Res. 2021;33:480–9. doi: 10.21147/j.issn.1000-9604.2021.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YG, Yang CW, Chung CH, et al The association between metabolic risk factors, nonalcoholic fatty liver disease, and the incidence of liver cancer: a nationwide population-based cohort study. Hepatol Int. 2022;16:807–16. doi: 10.1007/s12072-021-10281-9. [DOI] [PubMed] [Google Scholar]
- 21.Im PK, Millwood IY, Kartsonaki C, et al Alcohol drinking and risks of liver cancer and non-neoplastic chronic liver diseases in China: a 10-year prospective study of 0. 5 million adults. BMC Med. 2021;19:216. doi: 10.1186/s12916-021-02079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maucort-Boulch D, de Martel C, Franceschi S, et al Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142:2471–7. doi: 10.1002/ijc.31280. [DOI] [PubMed] [Google Scholar]
- 23.Loomba R, Liu J, Yang HI, et al Synergistic effects of family history of hepatocellular carcinoma and hepatitis B virus infection on risk for incident hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2013;11:1636–45.e1-3. doi: 10.1016/j.cgh.2013.04.043>PMID:23669307>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu MW, Lin CL, Liu CJ, et al Influence of metabolic risk factors on risk of hepatocellular carcinoma and liver-related death in men with chronic hepatitis B: A large cohort study. Gastroenterology. 2017;153:1006–17.e5. doi: 10.1053/j.gastro.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 25.European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 26.El-Serag HB Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villanueva A Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–62. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 28.Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127(5 Suppl 1): S35-50.
- 29.International Agency for Research on Cancer (IARC) Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. IARC Monogr Eval Carcinog Risks Hum Suppl. 1987;7:1–440. [PubMed] [Google Scholar]
- 30.Ross RK, Yuan JM, Yu MC, et al Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992;339:943–6. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Wu F Global burden of aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ Health Perspect. 2010;118:818–24. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen JG, Egner PA, Ng D, et al Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. Cancer Prev Res (Phila) 2013;6:1038–45. doi: 10.1158/1940-6207.CAPR-13-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marti-Aguado D, Clemente-Sanchez A, Bataller R Cigarette smoking and liver diseases. J Hepatol. 2022;77:191–205. doi: 10.1016/j.jhep.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Xie YD, Zhao CQ, Wang JP, et al Alcohol consumption analysis among patients with liver disease in China. Chin Med J (Engl) 2019;132:420–30. doi: 10.1097/CM9.0000000000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhadoria AS, Mohapatra A Alcohol-related cirrhosis and liver cancer: a call for inclusion in non-communicable disease action plans. Lancet Gastroenterol Hepatol. 2021;6:982. doi: 10.1016/S2468-1253(21)00373-3. [DOI] [PubMed] [Google Scholar]
- 36.Streba LA, Vere CC, Rogoveanu I, et al Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: an open question. World J Gastroenterol. 2015;21:4103–10. doi: 10.3748/wjg.v21.i14.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sohn W, Lee HW, Lee S, et al Obesity and the risk of primary liver cancer: A systematic review and meta-analysis. Clin Mol Hepatol. 2021;27:157–74. doi: 10.3350/cmh.2020.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jun BG, Kim M, Shin HS, et al Impact of overweight and obesity on the risk of hepatocellular carcinoma: a prospective cohort study in 14. 3 million Koreans. Br J Cancer. 2022;127:109–15. doi: 10.1038/s41416-022-01771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang DQ, El-Serag HB, Loomba R Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–38. doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou J, Zhou F, Wang W, et al Epidemiological features of NAFLD From 1999 to 2018 in China. Hepatology. 2020;71:1851–64. doi: 10.1002/hep.31150. [DOI] [PubMed] [Google Scholar]
- 41.Estes C, Razavi H, Loomba R, et al Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–33. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estes C, Anstee QM, Arias-Loste MT, et al Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 43.Cao M, Fan J, Lu L, et al Long term outcome of prevention of liver cancer by hepatitis B vaccine: Results from an RCT with 37 years. Cancer Lett. 2022;536:215652. doi: 10.1016/j.canlet.2022.215652. [DOI] [PubMed] [Google Scholar]
- 44.Qu C, Chen T, Fan C, et al Efficacy of neonatal HBV vaccination on liver cancer and other liver diseases over 30-year follow-up of the Qidong hepatitis B intervention study: a cluster randomized controlled trial. PLoS Med. 2014;11:e1001774. doi: 10.1371/journal.pmed.1001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang X, Bi S, Yang W, et al Epidemiological serosurvey of hepatitis B in China-declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550–7. doi: 10.1016/j.vaccine.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 46.Luo Z, Li L, Ruan B Impact of the implementation of a vaccination strategy on hepatitis B virus infections in China over a 20-year period. Int J Infect Dis. 2012;16:e82–8. doi: 10.1016/j.ijid.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 47.National Administration of Disease Prevention and Control. Remarkable achievements in controlling hepatitis B in China 2013. Available online: http://www.nhc.gov.cn/jkj/s3582/201307/518216575e544109b2caca07fca3b430.shtml
- 48.Polaris Observatory Collaborators Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, Liang W, Jing W, et al Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ. 2019;97:230–8. doi: 10.2471/BLT.18.219469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030. 2022 Available online: https://www.who.int/publications/i/item/9789240053779
- 51.The central people’s governments of the People’s Republic of China. The Blood Donation Law of People’s Republic of China. 1998. Available online:http://www.gov.cn/banshi/2005-08/01/content_18963.htm
- 52.Shan H, Wang JX, Ren FR, et al Blood banking in China. Lancet. 2002;360:1770–5. doi: 10.1016/S0140-6736(02)11669-2. [DOI] [PubMed] [Google Scholar]
- 53.The central people’s governments of the People’s Republic of China. Requirements for health examination of blood donors (GB 18467-2001). 2012. Available online: http://www.gov.cn/govweb/gzdt/2012-07/09/content_2179382.htm
- 54.Zhang Q, Qi W, Wang X, et al Epidemiology of hepatitis B and hepatitis C infections and benefits of programs for hepatitis prevention in northeastern China: A cross-sectional study. Clin Infect Dis. 2016;62:305–12. doi: 10.1093/cid/civ859. [DOI] [PubMed] [Google Scholar]
- 55.Hall AJ, Wild CP Liver cancer in low and middle income countries. BMJ. 2003;326:994–5. doi: 10.1136/bmj.326.7397.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun Z, Chen T, Thorgeirsson SS, et al Dramatic reduction of liver cancer incidence in young adults: 28 year follow-up of etiological interventions in an endemic area of China. Carcinogenesis. 2013;34:1800–52. doi: 10.1093/carcin/bgt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Altekruse SF, McGlynn KA, Reichman ME Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao M, Li H, Sun D, et al Cancer screening in China: The current status, challenges, and suggestions. Cancer Lett. 2021;506:120–7. doi: 10.1016/j.canlet.2021.02.017. [DOI] [PubMed] [Google Scholar]
- 59.Korean Liver Cancer Association (KLCA); National Cancer Center (NCC), Goyang, Korea 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Korean J Radiol. 2019;20:1042–113. doi: 10.3348/kjr.2019.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Wang M, Li H, et al A male-ABCD algorithm for hepatocellular carcinoma risk prediction in HBsAg carriers. Chin J Cancer Res. 2021;33:352–63. doi: 10.21147/j.issn.1000-9604.2021.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee MH, Yang HI, Liu J, et al Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles. Hepatology. 2013;58:546–54. doi: 10.1002/hep.26385. [DOI] [PubMed] [Google Scholar]
- 63.Kim JH, Kim YD, Lee M, et al Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J Hepatol. 2018;69:1066–73. doi: 10.1016/j.jhep.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 64.Fan R, Papatheodoridis G, Sun J, et al aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol. 2020;73:1368–78. doi: 10.1016/j.jhep.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 65.Li J, Li H, Zeng H, et al Trends in high-risk rates and screening rates for the population-based cancer screening program on esophageal, stomach and liver cancer in China, 2010-2016. J Natl Cancer Cent. 2021;1:101–7. doi: 10.1016/j.jncc.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen WQ, Li N, Cao MM, et al Preliminary Analysis of Cancer Screening Program in Urban China from 2013 to 2017. Zhongguo Zhong Liu. 2020;29:1–6. [Google Scholar]
- 67.Shi JF, Cao MD, Yan XX, et al Accessibility of liver cancer screening in our population: an exploratory analysis. Zhonghua Liu Xing Bing Xue Za Zhi. 2022;43:906–14. doi: 10.3760/cma.j.cn112338-20211112-00879. [DOI] [PubMed] [Google Scholar]
- 68.Tzartzeva K, Obi J, Rich NE, et al Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: A meta-analysis. Gastroenterology. 2018;154:1706–18.e1. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Durazo FA, Blatt LM, Corey WG, et al Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1541–8. doi: 10.1111/j.1440-1746.2008.05395.x. [DOI] [PubMed] [Google Scholar]
- 70.Song P, Gao J, Inagaki Y, et al Biomarkers: evaluation of screening for and early diagnosis of hepatocellular carcinoma in Japan and China. Liver Cancer. 2013;2:31–9. doi: 10.1159/000346220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu RH, Wei W, Krawczyk M, et al Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater. 2017;16:1155–61. doi: 10.1038/nmat4997. [DOI] [PubMed] [Google Scholar]
- 72.Trinchet JC, Chaffaut C, Bourcier V, et al Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology. 2011;54:1987–97. doi: 10.1002/hep.24545. [DOI] [PubMed] [Google Scholar]
- 73.Wang JH, Chang KC, Kee KM, et al Hepatocellular carcinoma surveillance at 4- vs. 12-month intervals for patients with chronic viral hepatitis:a randomized study in community. Am J Gastroenterol. 2013;108:416–24. doi: 10.1038/ajg.2012.445. [DOI] [PubMed] [Google Scholar]