Abstract
Objective
This multi-center, open-label, randomized, parallel-controlled phase II study aimed to compare the pharmacokinetics (PK), pharmacodynamics (PD) and safety profile of ripertamab (SCT400), a recombinant anti-CD20 monoclonal antibody, to rituximab (MabThera®) in patients with CD20-positive B-cell non-Hodgkin lymphoma (NHL).
Methods
Patients with CD20-positive B-cell NHL who achieved complete remission or unconfirmed complete remission after standard treatment were randomly assigned at a 1:1 ratio to receive a single dose of ripertamab (375 mg/m2) or rituximab (MabThera®, 375 mg/m2). PK was evaluated using area under the concentration-time curve (AUC) from time 0 to d 85 (AUC0−85 d), AUC from time 0 to week 1 (AUC0−1 w), AUC from time 0 to week 2 (AUC0−2 w), AUC from time 0 to week 3 (AUC0−3 w), AUC from time 0 to week 8 (AUC0−8 w), maximum serum concentration (Cmax), terminal half-life (T1/2), time to maximum serum concentration (Tmax) and clearance (CL). Bioequivalence was confirmed if the 90% confidence interval (90% CI) of the geometric mean ratio of ripertamab/rituximab was within the pre-defined bioequivalence range of 80.0%−125.0%. PD, immunogenicity, and safety were also evaluated.
Results
From December 30, 2014 to November 24, 2015, a total of 84 patients were randomized (ripertamab, n=42; rituximab, n=42) and the PK analysis was performed on 76 patients (ripertamab, n=38; rituximab, n=38). The geometric mean ratios of ripertamab/rituximab for AUC0−85 d, AUC0−inf, and Cmax were 96.1% (90% CI: 87.6%−105.5%), 95.9% (90% CI: 86.5%−106.4%) and 97.4% (90% CI: 91.6%−103.6%), respectively. All PK parameters met the pre-defined bioequivalence range of 80.0%−125.0%. For PD and safety evaluation, there was no statistical difference in peripheral CD19-positive B-cell counts and CD20-positive B-cell counts at each visit, and no difference in the incidence of anti-drug antibodies was observed between the two groups. The incidences of treatment-emergent adverse events and treatment-related adverse events were also comparable between the two groups.
Conclusions
In this study, the PK, PD, immunogenicity, and safety profile of ripertamab (SCT400) were similar to rituximab (MabThera®) in Chinese patients with CD20-positive B-cell NHL.
Keywords: Anti-CD20 monoclonal antibody, non-Hodgkin lymphoma, pharmacokinetics, ripertamab, rituximab, safety
Introduction
Non-Hodgkin lymphoma (NHL) is a common malignant hematological tumor. GLOBOCAN 2020 estimates reported 544,352 new cases and 259,793 deaths worldwide, and 92,834 new cases and 54,351 deaths in China (1). Most NHLs are of B-cell origin, and about 95% of B-cell lymphomas express CD20 (2).
Rituximab (MabThera®), a human-mouse chimeric CD20 monoclonal antibody was first approved by the U.S. Food and Drug Administration (FDA) in 1997 for patients with relapsed, low-grade, or follicular NHL (3). Since then, its efficacy has been demonstrated in patients with multiple B-cell lymphoid malignancies, including indolent and aggressive B-cell NHLs and B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) (4-9), and has been used as first-line treatment when combined with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) regimens for diffuse large B-cell lymphoma (DLBCL) (10-12).
Even though rituximab has shown favorable efficacy and safety profile in treating B-cell NHL over 20 years, the high cost has curbed its wide accessibility. In addition, its pharmacokinetic (PK) properties had not been fully characterized in Chinese patients prior to this study. Ripertamab (SCT400), a recombinant human-mouse chimeric anti-CD20 immunoglobulin G1 (IgG1) monoclonal antibody developed by Sinocelltech Ltd, Beijing, China, has the same amino acid sequence of antigen binding sites and variable region to rituximab, except one amino acid difference in position 219 of heavy chain constant region 1 (valine in ripertamab and alanine in rituximab). We have previously reported that ripertamab was well-tolerated and demonstrated encouraging efficacy at the dose level of 375 mg/m2 in patients with CD20-positive B-cell NHL (13,14). In this study, we aimed to compare the PK, pharmacodynamic (PD) and safety of ripertamab to rituximab (MabThera®) in patients with CD20-positive B-cell NHL.
Materials and methods
Study design and patients
All patients signed the informed consent form before any study operation, the protocol was approved by the ethics committees of each study hospital and implemented in compliance with Good Clinical Practice guideline and Declaration of Helsinki. This study was registrated with ClinicalTrials.gov (NCT02456207).
Patients were eligible if they satisfied the following criteria: 1) aged between 18 and 75 years; 2) histologically confirmed CD20-positive B-cell NHL; 3) achieved a complete remission (CR) or unconfirmed complete remission (CRu) after the standard therapy according to International Working Group 1999 Criteria (15); and 4) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0−1 with estimated life expectancy greater than 6 months. Patients were excluded if they had any of the following: 1) previously treated with rituximab or other anti-CD20 monoclonal antibody within one year before enrollment; 2) received anti-cancer therapy including corticosteroid within 4 weeks prior to enrollment or failed to recover from toxicities of prior therapy; 3) serious hematologic, hepatic, or renal dysfunctions; 4) co-existing other malignant tumors or serious benign tumor diseases; 5) history of allergic reaction to protein product including murine proteins; or 6) use of live vaccines within four weeks before enrollment. Patients were randomized 1:1 to receive a single dose of ripertamab (375 mg/m2) or rituximab (MabThera®, Shanghai Roche Pharmaceuticals Ltd, 375 mg/m2) intravenously (IV) and were followed-up for 12 weeks (85 d). Block randomization with a block size of 4 was performed by an independent statistician using the PROC PLAN process (SAS software; Version 9.2; SAS Institute Inc., Cary, NC, USA). An independent statistician generated the allocation sequences via central randomization. The randomized blocks were allocated to each study hospital, and allocation was proceeded by randomly picking one of the orderings of a block and assigning the next block of participating patients to study groups based on the specified sequence. The allocation sequences were concealed from the patients and the investigation groups until the moment of the assignment. The study was open-label and the data were analyzed un-blindly.
Assessments
The primary PK endpoint was area under the concentration-time curve (AUC) of from time 0 to d 85 (AUC0−85 d). The AUC 0−85 d was the primary endpoint based on our previous PK study of ripertamab, which showed that d 85 covered more than 5 half-lives of ripertamab (13). The key secondary endpoints included AUC from time 0 to infinity (AUC0−inf), maximum serum concentration (Cmax), PD parameters of the absolute counts and percentages of peripheral CD19-positive B-cells and CD20-positive B-cells relative to baseline at each time point, positive rate of anti-drug antibodies (ADAs) and safety in the two groups. In addition, AUC from time 0 to week 1 (AUC0−1 w), AUC from time 0 to week 2 (AUC0−2 w), AUC from time 0 to week 3 (AUC0−3 w), AUC from time 0 to week 8 (AUC0−8 w), time to maximum serum concentration (Tmax) and clearance (CL) were also analyzed.
PK evaluation
Serum concentrations of ripertamab and rituximab were detected by validated enzyme-linked immunosorbent assay. The PK samples were collected in PK analysis set at pre-infusion, 3 h after the beginning of infusion, end of infusion, 2 h and 6 h post-infusion, as well as on d 2, d 3, d 5, d 8, d 15, d 22, d 29, d 57 and d 85. PK analysis set included all patients who received study drugs infusion with available PK data, and had no protocol deviations with impact on the PK data.
PD evaluation
The absolute counts and percentages of peripheral blood CD19-postive B-cells and CD20-postive B-cells were detected using flow cytometry. Blood samples were taken in PD analysis set prior to infusion of study drugs, and on d 2, d 3, d 8, d 29, d 57 and d 85 after infusion of study drugs. PD analysis set included all patients who received study drugs infusion with available PD data.
Immunogenicity
The presence of ADAs was assessed by enzyme-linked immunosorbent assay (ELISA). Peripheral blood samples for immunogenicity assessments were collected before infusion of study drugs, on d 15, d 29, d 57 and d 85 after study drug infusion.
Safety evaluation
All adverse events (AEs), treatment emergent adverse events (TEAEs), treatment-related adverse events (TRAEs), serious adverse events (SAEs), and abnormal findings from physical examinations, vital signs and laboratory tests were evaluated in safety set (SS). AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Laboratory assessments included peripheral blood routine examination, peripheral blood biochemical examination, immunoglobulin and T cell examination, urine routine test, as well as 12-lead electrocardiography. SS included all patients who received study drugs infusion at least once with available safety assessments.
Statistical analysis
Statistical analyses were performed using the SAS software (Version 9.2; SAS Institute Inc., Cary, NC, USA). The PK parameters were calculated using the non-compartmental model (Phoenix WinNonlin 6.3 software). The ripertamab/rituximab ratio and 90% confidence interval (90% CI) of the geometric mean of PK parameters were calculated. 90% CI of the geometric mean ratio of AUC0−85 d was estimated by MIXED procedure. The PK bioequivalence was confirmed if the primary endpoint (AUC0−85 d) fell within the pre-defined bioequivalence range of 80.0%−125.0%. A team of clinical investigators and statisticians determined the sample size before the study started. The sample size was calculated with nQuery Advisor (Version 7.0; Statistical Solutions, Cork, Ireland). Assuming the geometric mean ratio of ripertamab/rituximab for AUC was between 0.95−1.05 and the coefficient of variation (CV) was 0.3, a sample size of 80 patients (40 in each group) was sufficient to estimate 90% power to conclude bioequivalence with two-sided error rate of 0.05. For PD analysis, the changes of the percentage/absolute count of peripheral blood CD19-positive B-cells and CD20-positive B-cells relative to baseline were calculated at each time point. The differences between two groups were analyzed using t-test or Wilcoxon rank sum test, and P<0.05 was considered statistically significant. Sensitivity analysis was performed in the following population sets: all randomized patients; patients with negative ADAs; and patients whose serum study drug concentration before infusion of study drugs was less than 5% of Cmax. The analysis was performed based on the observed data only without any imputation to the missing data.
Results
Enrollment and patient characteristics
This multi-center, open-label, randomized, parallel-controlled phase II study was conducted across 14 hospitals in China. From December 30, 2014 to November 24, 2015, a total of 95 patients with CD20-positive B-cell NHL were screened, and among them, 84 patients were randomly assigned to ripertamab group or rituximab group with 42 patients in each group. Forty patients in the ripertamab group and 41 patients in the rituximab group completed the study. Thirty-eight patients in each group were included in the PK analysis set. All 84 patients were included in the PD analysis set and SS. The patient distribution flow chart is shown in Figure 1.
Figure 1.
Patient distribution flow chart. HBsAg, hepatitis B surface antigen; TEAE, treatment emergent adverse event; PK, pharmacokinetics; ADA, anti-drug antibody; SAE, serious adverse event; CR, complete remission; CRu, unconfirmed complete remission; PD, pharmacodynamics; SS, safety set. *, Rituximab refers to MabThera®.
The baseline patient characteristics are shown in Table 1. Overall, these parameters were well balanced with no statistical difference between the two groups.
Table 1. Patient baseline characteristics.
| Characteristics | n (%) | P | |
| Ripertamab (N=42) | Rituximab* (N=42) | ||
| BSA, body surface area; ECOG, Eastern Cooperative Oncology Group; PS, performance status; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MZL, marginal zone lymphoma; *, rituximab refers to MabThera®. | |||
Age (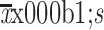 ) (year) ) (year) |
56.1±11.7 | 55.2±13.0 | 0.929 |
| Gender | 0.662 | ||
| Male | 24 (57.1) | 21 (50.0) | |
| Female | 18 (42.9) | 21 (50.0) | |
BSA (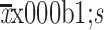 ) (m2) ) (m2) |
1.7±0.2 | 1.7±0.2 | 0.915 |
| ECOG PS | 0.488 | ||
| 0 | 30 (71.4) | 26 (61.9) | |
| 1 | 12 (28.6) | 16 (38.1) | |
| Ann Arbor stage | 1.000 | ||
| I | 8 (19.0) | 7 (16.7) | |
| II | 10 (23.8) | 11 (26.2) | |
| III | 12 (28.6) | 12 (28.6) | |
| IV | 12 (28.6) | 12 (28.6) | |
| Histological classification | 0.374 | ||
| DLBCL | 22 (52.4) | 26 (61.9) | |
| FL | 12 (28.6) | 10 (23.8) | |
| MZL | 3 (7.1) | 0 (0) | |
| Others | 5 (11.9) | 6 (14.3) | |
PK
Ripertamab and rituximab showed similar drug concentration-time curve (Figure 2, Table 2) and reached maximum concentration at median time of 5.00 h and 5.24 h after infusion of study drugs, respectively. The bioequivalence analysis showed that the geometric mean ratio of ripertamab/rituximab for AUC0−85 d were 96.1% (90% CI: 87.6%−105.5%), geometric mean ratio for AUC0−inf and Cmax were 95.9% (90% CI: 86.5%−106.4%) and 97.4% (90% CI: 91.6%−103.6%), respectively, which all fell within the pre-defined bioequivalence range of 80.0%−125.0%. Other PK parameters such as AUC0−1 w, AUC0−2 w, AUC0−3 w, AUC0−8 w, Tmax and CL were also similar between the two groups (Table 2,3).
Figure 2.
Serum concentration over time for ripertamab and rituximab after single dose 375 mg/m2 infusion. Rituximab refers to MabThera®.
Table 2. Geometric means of PK parameters of ripertamab and rituximab.
| Parameters | Geometric mean [CV (%)] | |
| Ripertamab (n=38) | Rituximab* (n=38) | |
| PK, pharmacokinetics; AUC, area under the concentration-time curve; AUC0−85 d, AUC from time 0 to d 85; AUC0−1 w, AUC from time 0 to week 1; AUC0−2 w, AUC from time 0 to week 2; AUC0−3 w, AUC from time 0 to week 3; AUC0−8 w, AUC from time 0 to week 8; AUC0−inf, AUC from time 0 to infinity; Cmax, maximum serum concentration; t1/2, terminal elimination half-life; Tmax, time to maximum serum concentration; CL, clearance; CV, coefficient of variation; *, rituximab refers to MabThera®. | ||
| AUC0−85 d (hr·μg/mL) | 63,448.164 (22.63) | 66,020.898 (27.38) |
| AUC0−1 w (hr·μg/mL) | 19,221.729 (15.00) | 19,859.049 (17.36) |
| AUC0−2 w (hr·μg/mL) | 30,630.996 (15.17) | 31,545.376 (17.22) |
| AUC0−3 w (hr·μg/mL) | 38,360.029 (15.74) | 39,756.863 (18.04) |
| AUC0−8 w (hr·μg/mL) | 58,355.858 (19.50) | 60,652.183 (23.88) |
| AUC0−inf (hr·μg/mL) | 67,304.617 (25.56) | 70,190.499 (32.04) |
| Cmax (μg/mL) | 217.891 (11.07) | 223.701 (19.06) |
| t1/2 (h) | 466.910 (27.85) | 475.822 (26.46) |
| Tmax (h) [median (range)] | 5.00 (4.32−11.03) | 5.24 (4.13−10.42) |
| CL (mL/h/m2) | 5.572 (25.53) | 5.343 (26.22) |
Table 3. Geometric mean and ratio of PK parameters of ripertamab and rituximab.
| Parameters | Geometric mean and ratio | 90% CI | ||
| Ripertamab (n=38) | Rituximab* (n=38) | ratio† (%) | ||
| PK, pharmacokinetics; AUC, area under the concentration-time curve; AUC0−85 d, AUC from time 0 to d 85; AUC0−inf, AUC from time 0 to infinity; Cmax, maximum serum concentration; 90% CI, 90% confidence interval. †, Ratio refers to geometric means of PK parameters of ripertamab/rituximab; *, rituximab refers to MabThera®. | ||||
| AUC0−85 d | 63,448.164 | 66,020.898 | 96.1 | 87.6−105.5 |
| AUC0−inf | 67,304.617 | 70,190.499 | 95.9 | 86.5−106.4 |
| Cmax | 217.891 | 223.701 | 97.4 | 91.6−103.6 |
Sensitivity analysis of the AUC0−t, AUC0−inf, and Cmax was performed in the following populations: all randomized patients; patients with negative ADAs; and patients whose serum drug concentration before infusion of study drugs was less than 5% of Cmax. The 90% CIs of the geometric mean ratios for AUC0−85 d, AUC0−inf and Cmax in these subgroups were within the pre-defined bioequivalence range of 80.0%−125.0% (Table 4).
Table 4. Sensitive analysis between ripertamab and rituximab*.
| Variables | n | AUC0−85 d (hr·μg/mL) | AUC0−inf (hr·μg/mL) | Cmax (μg/mL) |
| 90% CI, 90% confidence interval; ADA, anti-drug antibody; Cmax, maximum serum concentration; AUC, area under the concentration-time curve; AUC0−85 d, AUC from time 0 to d 85; AUC0−inf, AUC from time 0 to infinity. †, Ratio refers to geometric means of PK parameters of ripertamab/rituximab; *, rituximab refers to MabThera®. | ||||
| All patients | ||||
| Ripertamab | 42 | 59,484.139 | 62,945.650 | 216.326 |
| Rituximab | 42 | 60,514.288 | 66,646.350 | 220.969 |
| ratio† (%) | − | 98.3 | 94.4 | 97.9 |
| 90% CI | − | 84.9−113.8 | 82.8−107.8 | 92.2−103.9 |
| Patients with negative ADAs | ||||
| Ripertamab | 34 | 65,099.881 | 69,345.257 | 218.381 |
| Rituximab | 36 | 66,573.500 | 70,865.924 | 224.079 |
| ratio† (%) | − | 97.8 | 97.9 | 97.5 |
| 90% CI | − | 88.7−107.8 | 87.8−109.1 | 91.2−104.2 |
| Serum concentration <5% Cmax before infusion of study drugs | ||||
| Ripertamab | 38 | 63,448.164 | 67,304.617 | 217.891 |
| Rituximab | 39 | 65,774.654 | 69,839.756 | 222.884 |
| ratio† (%) | − | 96.5 | 96.4 | 97.8 |
| 90% CI | − | 88.0−105.7 | 87.0−106.8 | 91.9−103.9 |
PD
Both the absolute counts of peripheral blood CD19-positive B-cells and CD20-positive B-cells in two groups decreased rapidly after ripertamab and rituximab infusion, and reached a nadir on d 7. The CD19-positive B-cell counts (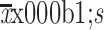 ) were 154.67±137.16 pieces/µL (pcs/µL) and 132.51±125.77 pcs/µL (P=0.448) at baseline, and 4.12±9.05 pcs/µL and 4.78±11.67 pcs/µL (P=0.450) at d 85 for the ripertamab group and the rituximab group, respectively. The CD20-positive B-cell counts (
) were 154.67±137.16 pieces/µL (pcs/µL) and 132.51±125.77 pcs/µL (P=0.448) at baseline, and 4.12±9.05 pcs/µL and 4.78±11.67 pcs/µL (P=0.450) at d 85 for the ripertamab group and the rituximab group, respectively. The CD20-positive B-cell counts ( ) were 157.78±138.42 pcs/µL and 134.70±127.01 pcs/µL (P=0.434) at baseline, and 3.37±9.88 pcs/µL and 3.08±7.87 pcs/µL (P=0.374) at d 85 for the ripertamab group and the rituximab group, respectively. There were no significant differences in counts of CD19-positive B-cell or CD20-positive B-cell between the ripertamab group and the rituximab group at all measured time points (Table 5). Compared to baseline, the percentage reduction of CD19-positive B-cells on d 7 were 98.5% in the ripertamab group and 98.9% in the rituximab group; the percentage reduction of CD20-positive B-cells on d 7 were 99.5% in the ripertamab group and 99.6% in the rituximab group, respectively. A slight recovery was observed on d 85: Compared to baseline, the percentage reduction of CD19-positive B-cells at d 85 were 97.3% in the ripertamab group and 96.4% in the rituximab group; the percentage reduction of CD20-positive B-cells were 97.9% in the ripertamab group and 97.7% in the rituximab group, respectively. At 12-week post drug infusion, the absolute counts of CD19-positive B-cells of 2 patients in the ripertamab group (4.8%) and 3 patients in the rituximab group (7.1%) returned to their baseline level. The absolute counts of CD20-positive B-cells of 1 patient in the ripertamab group (2.4%) and 2 patients in the rituximab group (4.8%) recovered to the baseline level. The changes in the CD19-positive B-cells and CD20-positive B-cell counts relative to the baseline in the two groups were similar, with no significant difference in the changes of CD19-positive B-cell absolute counts (Figure 3) and CD20-positive B-cell absolute counts (Figure 4) between the two groups at all visit time points.
) were 157.78±138.42 pcs/µL and 134.70±127.01 pcs/µL (P=0.434) at baseline, and 3.37±9.88 pcs/µL and 3.08±7.87 pcs/µL (P=0.374) at d 85 for the ripertamab group and the rituximab group, respectively. There were no significant differences in counts of CD19-positive B-cell or CD20-positive B-cell between the ripertamab group and the rituximab group at all measured time points (Table 5). Compared to baseline, the percentage reduction of CD19-positive B-cells on d 7 were 98.5% in the ripertamab group and 98.9% in the rituximab group; the percentage reduction of CD20-positive B-cells on d 7 were 99.5% in the ripertamab group and 99.6% in the rituximab group, respectively. A slight recovery was observed on d 85: Compared to baseline, the percentage reduction of CD19-positive B-cells at d 85 were 97.3% in the ripertamab group and 96.4% in the rituximab group; the percentage reduction of CD20-positive B-cells were 97.9% in the ripertamab group and 97.7% in the rituximab group, respectively. At 12-week post drug infusion, the absolute counts of CD19-positive B-cells of 2 patients in the ripertamab group (4.8%) and 3 patients in the rituximab group (7.1%) returned to their baseline level. The absolute counts of CD20-positive B-cells of 1 patient in the ripertamab group (2.4%) and 2 patients in the rituximab group (4.8%) recovered to the baseline level. The changes in the CD19-positive B-cells and CD20-positive B-cell counts relative to the baseline in the two groups were similar, with no significant difference in the changes of CD19-positive B-cell absolute counts (Figure 3) and CD20-positive B-cell absolute counts (Figure 4) between the two groups at all visit time points.
Table 5. CD19 positive B-cell and CD20 positive B-cell counts at measured time points.
| Variables | Counts (pcs/µL) (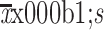 ) [reduction (%)]* ) [reduction (%)]* |
P** | |
| Ripertamab (N=42) | Rituximab# (N=42) | ||
| pcs, pieces. *, reduction (%) was calculated based on the mean values of the counts at baseline and the counts at the time points post infusion of study drugs; **, t-test or Wilcoxon rank-sum test was used for the inter-group comparison; #, rituximab refers to MabThera®. | |||
| CD19-positive B-cells | |||
| Baseline | 154.67±137.16 | 132.51±125.77 | 0.448 |
| D 1 post infusion | 4.19±8.69 (97.3) | 3.98±5.58 (97.0) | 0.488 |
| D 2 post infusion | 3.91±6.15 (97.5) | 2.78±3.25 (97.9) | 0.454 |
| D 7 post infusion | 2.38±4.21 (98.5) | 1.42±2.18 (98.9) | 0.430 |
| D 29 post infusion | 2.01±3.58 (98.7) | 2.28±3.55 (98.3) | 0.344 |
| D 57 post infusion | 1.84±4.39 (98.8) | 2.42±5.08 (98.2) | 0.337 |
| D 85 post infusion | 4.12±9.05 (97.3) | 4.78±11.67 (96.4) | 0.450 |
| CD20-positive B-cells | |||
| Baseline | 157.78±138.42 | 134.70±127.01 | 0.434 |
| D 1 post infusion | 0.77±1.49 (99.5) | 1.21±4.00 (99.1) | 0.419 |
| D 2 post infusion | 0.72±1.44 (99.5) | 0.80±2.13 (99.4) | 0.395 |
| D 7 post infusion | 0.77±1.94 (99.5) | 0.54±1.36 (99.6) | 0.348 |
| D 29 post infusion | 0.71±1.57 (99.6) | 0.71±1.43 (99.5) | 0.310 |
| D 57 post infusion | 1.70±4.98 (98.9) | 1.47±5.59 (98.9) | 0.324 |
| D 85 post infusion | 3.37±9.88 (97.9) | 3.08±7.87 (97.7) | 0.374 |
Figure 3.
Changes of peripheral blood CD19-positive B-cell absolute counts. Rituximab refers to MabThera®. pcs, pieces.
Figure 4.
Changes of peripheral blood CD20-positive B-cell absolute counts. Rituximab refers to MabThera®. pcs, pieces.
Immunogenicity
The immunogenicity characteristics of ripertamab and rituximab were similar. Eight patients were tested positive for ADA throughout the study period, including 5 (12.2%) patients in the ripertamab group and 3 (7.1%) patients in the rituximab group. Among them, 2 patients were tested positive for ADA at baseline (1 patient in each group). There was no statistically significant difference in ADA positive rates between the two groups at baseline, d 15, d 29, d 57 and d 85.
Safety
Similar safety profile was observed between the ripertamab group and the rituximab group. The percentages of patients who experienced at least one TEAE were 78.6% and 81.0% in the ripertamab group and the rituximab group, respectively. The most common TEAEs in the ripertamab group and the rituximab group were pruritus (9.5% vs. 4.8%), upper respiratory tract infection (7.1% vs. 14.3%), and alanine aminotransferase increased (7.1% vs. 7.1%). The incidences of TRAEs were 50.0% and 61.9% in the ripertamab group and the rituximab group, respectively. Four patients in the ripertamab group experienced grade ≥3 TEAEs: herpes zoster (1 patient), soft tissue infection (1 patient) and infusion-related reaction (IRR) (2 patients manifested as chills, laryngeal oedema, dyspnoea, and tremor). One patient in the rituximab group developed neutrophil count decrease. IRR occurred in 23.8% of patients in both groups with the most common IRRs being skin reactions and hypersensitivity reaction. The median time of IRR occurrence was 68.0 (range: 60−120) min and 91.5 (range: 40−240) min after ripertamab and rituximab infusion, respectively. A total of 3 patients experienced one SAE, including 1 herpes zoster and 1 cerebral infarction in the ripertamab group, and 1 arteriosclerosis coronary artery in the rituximab group. No death occurred in both groups. The summary of AEs is shown in Table 6.
Table 6. Summary of AEs.
| AEs | n (%) | |
| Ripertamab (N=42) | Rituximab* (N=42) | |
| AE, adverse event; TEAE, treatment emergent adverse event; TRAE, treatment-related adverse event; SAE, serious adverse event; IRR, infusion related reaction. *, Rituximab refers to MabThera®. | ||
| TEAEs | 33 (78.6) | 34 (81.0) |
| TRAEs | 21 (50.0) | 26 (61.9) |
| SAEs | 2 (4.8) | 1 (2.4) |
| Treatment discontinuation due to TEAEs | 1 (2.4) | 0 (0) |
| IRRs | 10 (23.8) | 10 (23.8) |
| TEAEs with incidence ≥5% in either group | ||
| Hematological toxicity | ||
| White blood cell count decreased |
2 (4.8) | 10 (23.8) |
| Neutrophil count decreased | 2 (4.8) | 8 (19.0) |
| Lymphocyte count decreased |
0 (0) | 3 (7.1) |
| Non-hematological toxicity | ||
| Pruritus | 4 (9.5) | 2 (4.8) |
| Upper respiratory tract infection |
3 (7.1) | 6 (14.3) |
| Alanine aminotransferase increased |
3 (7.1) | 3 (7.1) |
| Blood lactate dehydrogenase increased |
3 (7.1) | 1 (2.4) |
| Blood glucose increased | 3 (7.1) | 0 (0) |
| Urinary tract infection | 3 (7.1) | 0 (0) |
| Rash | 3 (7.1) | 0 (0) |
| Constipation | 3 (7.1) | 0 (0) |
| Insomnia | 3 (7.1) | 0 (0) |
| Hypersensitivity | 1 (2.4) | 6 (14.3) |
| Aspartate aminotransferase increased |
1 (2.4) | 4 (9.5) |
Discussion
Ripertamab (SCT400) has identical amino acid sequence in the antigen binding sites and variable region to rituximab, and one amino acid difference in position 219 of heavy chain constant region 1 (i.e. valine in ripertamab and alanine in rituximab). The alanine in Fc portion of rituximab is uniquely different from that of other marketed IgG1 antibodies. Roche Pharmaceuticals Ltd. corrected this mutation and chose valine in their subsequent versions of CD20 antibodies. In vitro test revealed that ripertamab and rituximab both exhibited highly similar immunological characteristics and biological function (data unpublished). Results of the phase III study also demonstrated the similar efficacy and safety between ripertamab and rituximab (Mabthera®) in combination with CHOP in patients with previously untreated CD20-positive DLBCL (14). The V219A difference had no influence on Fab and Fc function, antibody-dependent cell-mediated cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC) activities compared to rituximab.
In this multi-center, open-label, randomized, parallel-controlled phase II study, ripertamab demonstrated PK bioequivalence to rituximab in patients with CD20-positive B-cell NHL who had achieved a CR or CRu after the standard treatment. In addition, the PD, immunogenicity, and safety profile of ripertamab were also consistent with those of rituximab.
The PK results showed that the 90% CIs of the geometric mean ratios for PK parameters, AUC0−85 d, AUC0−inf and Cmax were all within the pre-defined bioequivalence range of 80.0%−125.0%. Ripertamab and rituximab showed a nearly identical drug concentration-time curve, reaching maximum concentration at the median time of 5.000 h and 5.240 h, respectively. Other PK parameters such as AUC0−1 w, AUC0−2 w, AUC0−3 w, AUC0−8 w, Tmax and CL were also similar between the two groups. This study also provided PK characteristics of rituximab in Chinese CD20-positive B-cell NHL patients. The calculated mean Cmax and t1/2 of a single dose of rituximab (375 mg/m2) in Chinese patients with CD20-positive B-cell NHL were 223.701 µg/mL and 475.822 h respectively, which are similar to the reported Cmax (243 µg/mL) and t1/2 (528 h) in patients from U.S (16).
The equivalent PD data of ripertamab and rituximab were as expected as the two drugs share identical Fab sequences. The peripheral blood CD19-positive B-cells and CD20-positve B-cells in the ripertamab group and the rituximab group decreased rapidly after study drugs infusion, and reached a nadir on d 7. Compared to baseline, the percentage reduction of CD19-positive B-cells were 98.5% in the ripertamab group and 98.9% in the rituximab group, the percentage reduction of CD20-positive B-cells on d 7 were 99.5% in the ripertamab group and 99.6% in the rituximab group, respectively, indicating a rapid depletion of peripheral B-cells. There was no significant difference in the clearance effect targeted to CD19-positive B-cells and CD20-positive B-cells between the two groups, which was consistent with the results of phase I clinical study of ripertamab and reports in the literature of rituximab (13,17-21).
The phase I study results of ripertamab showed that the serum concentration of ripertamab correlated negatively with the initial tumor size or baseline level of B-cells in the blood circulation (10). In order to reduce the impact of tumor burden on the PK and PD results, this study was performed on the CD20-positive B-cell NHL patients who had achieved CR or CRu after standard treatment. Nevertheless, a rapid drop in serum drug concentration and early recovery of B-cells were observed in the two CRu patients with high baseline tumor burden in this study, and hence these two patients were subsequently excluded from the PK analysis set to reduce the impact of tumor burden on PK evaluation. In addition, because the ADA level may influence the serum study drugs clearance and the recovery of peripheral blood B-cells, sensitivity analyses were performed on the ADA-negative patients, which showed that 90% CIs of the geometric mean ratios for PK parameters, AUC0−85 d, AUC0−inf and Cmax also fell within the pre-defined bioequivalence range of 80.0%−125.0% in the study protocol.
Ripertamab and rituximab exhibited similar immunogenic characteristics with comparable positive rates of ADA. In total, four patients developed ADA in the ripertamab group and two patients in the rituximab group after infusion of study drugs, and no significant differences were observed in the levels of ADA at any sampling time point between the ripertamab group and the rituximab group.
The incidences of TEAEs and TRAEs were similar between the ripertamab group and the rituximab group. No treatment-related deaths were observed in either group and incidence of SAEs was <5% in both groups. The types and incidence of TEAEs were consistent with those reported in other literatures of rituximab, and no new safety signals were observed in this study (17,18,22).
Conclusions
In this study, the PK, PD, immunogenicity, and safety profile of ripertamab (SCT400) were similar to rituximab (MabThera®) in Chinese patients with CD20-positive B-cell NHL.
Footnote
Conflicts of Interest: Wenlin Gai and Liangzhi Xie are employees of Sinocelltech Ltd. and have ownership in the company. The authors have no other conflicts of interest to declare.
Acknowledgements
This study was funded by Sinocelltech Ltd, Beijing China, and partly supported by China National Major Project for New Drug Innovation (No. 2012ZX09303012 and No. 2017ZX09304015).
References
- 1.GLOBOCAN 2020: Non-Hodgkin lymphoma. Available online: https://gco.iarc.fr/today/home
- 2.Aisenberg AC Coherent view of non-Hodgkin’s lymphoma. J Clin Oncol. 1995;13:2656–75. doi: 10.1200/JCO.1995.13.10.2656. [DOI] [PubMed] [Google Scholar]
- 3.Leget GA, Czuczman MS Use of rituximab, the new FDA-approved antibody. Curr Opin Oncol. 1998;10:548–51. doi: 10.1097/00001622-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 4.FDA. Rituxan: highlights of prescribing information. 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/10370 5s5454lbl.pdf
- 5.EMA. MabThera: summary of product characteristics. 2019. Available online: https://www.ema.europa.eu/en/documents/product-information/mabth era-epar-product-information_en.pdf
- 6.Salles G, Seymour JF, Offner F, et al Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 7.Foran JM, Rohatiner AZ, Cunningham D, et al European phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. J Clin Oncol. 2000;18:317–24. doi: 10.1200/JCO.2000.18.2.317. [DOI] [PubMed] [Google Scholar]
- 8.Hainsworth JD, Litchy S, Burris HA 3rd, et al Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20:4261–7. doi: 10.1200/JCO.2002.08.674. [DOI] [PubMed] [Google Scholar]
- 9.Hainsworth JD, Litchy S, Barton JH, et al Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21:1746–51. doi: 10.1200/JCO.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Mohammed R, Milne A, Kayani K, et al How the discovery of rituximab impacted the treatment of B-cell non-Hodgkin’s lymphomas. J Blood Med. 2019;10:71–84. doi: 10.2147/JBM.S190784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coiffier B, Lepage E, Briere J, et al CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 12.Pfreundschuh M, Trümper L, Osterborg A, et al CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–91. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 13.Gui L, Han X, He X, et al Phase I study of chimeric anti-CD20 monoclonal antibody in Chinese patients with CD20-positive non-Hodgkin’s lymphoma. Chin J Cancer Res. 2016;28:197–208. doi: 10.21147/j.issn.1000-9604.2016.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, Zhang Q, Hong X, et al. Comparison of efficacy and safety of ripertamab (SCT400) versus rituximab (Mabthera®) in combination with CHOP in patients with previously untreated CD20-positive diffuse large B-cell lymphoma: A randomized, single-blind, phase III clinical trial. Hematol Oncol 2022. [Online ahead of print]
- 15.Cheson BD, Horning SJ, Coiffier B, et al Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 16.Rituximab: Summary of product characteristic. 2021. Available online: https://www.medicines.org.uk/emc/medicine/2570
- 17.Tobinai K, Igarashi T, Itoh K, et al Japanese multicenter phase II and pharmacokinetic study of rituximab in relapsed or refractory patients with aggressive B-cell lymphoma. Ann Oncol. 2004;15:821–30. doi: 10.1093/annonc/mdh176. [DOI] [PubMed] [Google Scholar]
- 18.Maloney DG, Grillo-López AJ, White CA, et al IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–95. doi: 10.1182/blood.V90.6.2188. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Zhang Q, Han X, et al Phase 1 studies comparing safety, tolerability, pharmacokinetics and pharmacodynamics of HLX01 (a rituximab biosimilar) to reference rituximab in Chinese patients with CD20-positive B-cell lymphoma. Chin J Cancer Res. 2021;33:405–16. doi: 10.21147/j.issn.1000-9604.2021.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang B, Ke X, Zhang Q, et al Pharmacokinetics and safety of IBI301 versus rituximab in patients with CD20+ B-cell lymphoma: a multicenter, randomized, double-blind, parallel-controlled study. Sci Rep. 2020;10:11676. doi: 10.1038/s41598-020-68360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Candelaria M, Gonzalez D, Fernández Gómez FJ, et al. Comparative assessment of pharmacokinetics, and pharmacodynamics between RTXM83™, a rituximab biosimilar, and rituximab in diffuse large B-cell lymphoma patients: a population PK model approach. Cancer Chemother Pharmacol. 2018;81:515–27. doi: 10.1007/s00280-018-3524-9. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin P, Grillo-López AJ, Link BK, et al Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–33. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]






