Abstract
Background
Breast screening services were suspended for several months owing to the coronavirus disease 2019 (COVID-19) pandemic. We estimated the potential impact on breast cancer mortality using long-term global observations. However, the magnitude of the impact may vary across countries; therefore, we conducted an analysis and modeling study of this impact in Japan.
Patients and Methods
We compared the clinicopathological features of breast cancers between the nonpandemicgroup (April 1, 2019 to October 31, 2019) and the pandemic group (April 1, 2020 to October 31, 2020). We also compared the estimated 10-year survival rates between the two groups based on the weighted average of the 10-year survival rate by clinical stage and site (2004–2007).
Results
Results...Pandemic-related disruption decreased the number of breast cancer cases from296 to 249 during both 7-month periods. The percentage of patients with stage IIB or higher disease was significantly higher in the pandemic group than in the non-pandemic group (22.0% vs. 31.3%, P = 0.0133). The percentage of cases with a Ki-67 labeling index higher than 20% tended to be higher in the pandemic group than in the non-pandemic group (62.2% vs. 54.4%). The estimated 10-year survival rate was lower in the pandemic group than in the non-pandemic group (83.9% vs. 87.9%, 95% confidence interval of the difference: 0.87−8.8, P > 0.05).
Conclusion
We found more aggressive and advanced disease afterthe suspension of breast cancer screening services owing to the COVID-19 pandemic. This may have affected the long-term clinical outcomes of patients with breast cancer.
Keywords: Breast cancer, Breast cancer screening, COVID-19, Cancer survival outcomes
Micro-Abstract
We analysed the impact of the COVID-19 pandemic on breast cancer mortality in Japan. We compared clinicopathological features of breast cancers and estimated 10-year survival rates between non-pandemic and pandemic groups. More aggressive and advanced disease occurred after the suspension of breast cancer screening services owing to the pandemic. This may have affected the long-term clinical outcomes of these patients.
Introduction
In December 2019, the coronavirus disease 2019 (COVID-19) outbreak originating in Wuhan, China, spread worldwide; as of February 2022, the number of people infected worldwide exceeded 410 million and the number of deaths surpassed 5.81 million.1 Since the onset of lockdowns in each country, screenings for various cancers decreased by 62%–96% in April–September 2020 compared to that in April–September 2019, decreasing in all age groups.2 The number of infected people and detected cancer cases remained inversely proportional thereafter.3, 4, 5 The number of patients diagnosed with and treated for breast cancer in 2020 was 26% lower than that in 2019, representing a less significant decrease than initially predicted. Nevertheless, a survey of the uptake rate of breast cancer screenings scheduled for April and May 2020, corresponding to the time when Japan declared a new coronavirus infection emergency, found that 26.3% of screenings were postponed or canceled; however, the long-term impact is unknown.6
In this study, we aimed to investigate the effect of delay in breast cancer diagnosis due to withholding breast cancer screening and outpatient visits during the first wave of the COVID-19 pandemic. We compared the expected future changes in survival and death rates based on fluctuations in the proportion of patients with breast cancer of different stages before and after the COVID-19 pandemic. In addition, by comparing the disease duration, motive for consultation, change in the Ki-67 labeling index of each stage and change in percentage of each subtype between pre-and post-COVID pandemic data, and by examining the impact of postponing consultations during an infectious disease pandemic, we discuss countermeasures that will contribute to future reductions in breast cancer deaths.
Patients and Methods
Study Design
We conducted a retrospective, 2-group, independent-sample comparative study. The study used medical records, biopsy specimen pathology data at the time of diagnosis, and imaging data from 4 facilities: Tokyo Medical University Hospital, The Second Kawasaki Saiwai Clinic, Hachioji Medical Center, and Ibaraki Medical Center.
Patients diagnosed with breast cancer who had their first visit between April 1st, 2019, and October 31st, 2019 (non-pandemic group) and those diagnosed with breast cancer with the first visit between April 1st, 2020, and October 31st, 2020 (pandemic group) were eligible, with no age limit for either group. Patients who were required to undergo breast cancer screening and were diagnosed with breast cancer at a facility were included, even if the date of screening was outside the designated period as long as the date of the first visit to the facility was within the designated period. To estimate the impact of postponing screening and outpatient visits during the COVID-19 pandemic, patients who were instructed to undergo regular follow-up before the designated period and were diagnosed with breast cancer during the study period were excluded. Patients with stage 0 breast cancer were excluded since survival rates were not calculated by the National Cancer Institute Council. Male patients with breast cancer, those with recurrent breast cancer, and those who requested not to participate in the study were also excluded.
We compared the clinicopathological findings and estimated the 10-year survival rates between the pandemic and non-pandemic groups.
Clinicopathological Findings
The distribution of clinical stages within each target group was determined, and changes in the percentage of cases classified as stage ⅡB or higher were compared using the χ2 test.
Estimated 10-year Survival Rates
Based on the percentage of patients in each clinical stage, we calculated and compared the 10-year overall survival rates between the 2 groups on a weighted average basis, using the relative 10-year survival rates by site-specific clinical staging (cases diagnosed in 2004–2007) published by the National Council of Cancer Centers.
Estimates of deaths are based on the 2017 national registry of patients with breast cancer (C50) and the impact of the 7-month COVID-19 pandemic.
The stage-adsisted 10-year survival proportion is estimated as:
| (1) |
| (2) |
with the proportion of the patients of stage i, , and the corresponding 10-year survival proportion , and size (i = 1, …, 4).
| (3) |
| (4) |
Statistical Analysis
The disease duration was examined in patients who underwent breast cancer screening. The median disease duration from the date of screening to the date of examination was calculated for each group and compared using the Brunner–Munzel test. In addition, the extent to which screening decreased in terms of motive for medical examination was examined using the χ2 test. The χ2 test was used to compare whether there was a difference in the distribution of breast cancer with high proliferative activity as measured using the Ki-67 labeling index and in the distribution of subtypes between the 2 groups. P-values < .05 were considered statistically significant. IBM SPSS Statistics version 27 (IBM Corp., Armonk, NY) and R-Studio (R Foundation for Statistical Computing, Vienna, Austria) were used for the statistical analysis.
Results
The overall clinicopathological characteristics of the patients are presented in Table 1 . The total number of cases during the designated period was 296 in the non-pandemic group and 249 in the pandemic group, showing a 15.9% decrease in the number of breast cancer diagnoses owing to the COVID-19 pandemic.
Table 1.
Patients Background in the Overall Population According to Year of Diagnosis
| Non-Pandemic |
Pandemic |
|||||
|---|---|---|---|---|---|---|
| n=296 | % | n=249 | % | P value | ||
| Age,yrs | Mean(SD) | 60.3(±14.6) | 61.6(±15.8) | 0.299 | ||
| BMI | ||||||
| <25 | 202 | 68.2 | 162 | 65.1 | 0.031 | |
| ≧25 | 77 | 26.0 | 57 | 22.9 | ||
| Unknown | 17 | 5.7 | 30 | 12.0 | ||
| Family history | ||||||
| Family history of breast and ovarian cancer | 55 | 18.9 | 37 | 14.9 | 0.002 | |
| No family history | 239 | 80.7 | 198 | 79.5 | ||
| Unknown | 2 | 0.7 | 14 | 5.6 | ||
| Medical history | ||||||
| Cancer | 25 | 8.4 | 15 | 6.0 | 0.536 | |
| Other | 151 | 51.0 | 132 | 53.0 | ||
| Nothing | 117 | 39.5 | 100 | 40.2 | ||
| Unknown | 3 | 1.0 | 2 | 0.8 | ||
| Parity | ||||||
| <1 | 92 | 31.1 | 91 | 36.5 | 0.080 | |
| ≧1 | 202 | 68.2 | 152 | 61.0 | ||
| Unknown | 2 | 0.7 | 6 | 2.4 | ||
Clinicopathological findings
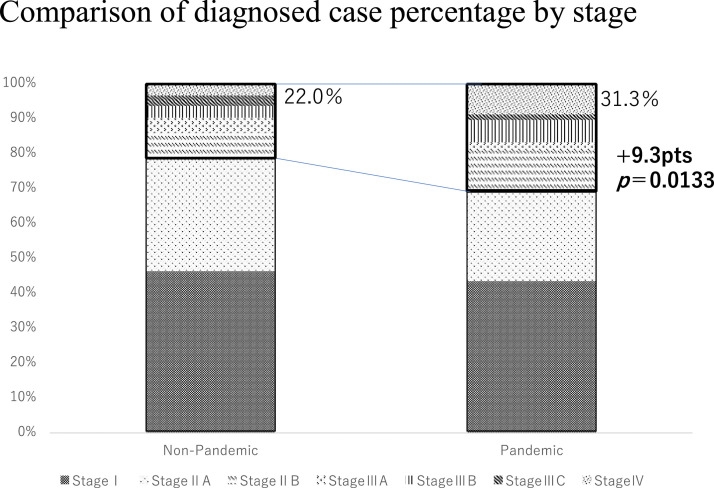
In the non-pandemic group, 137 (46.3%) patients were in stage I, 117 (39.5%) were in stage II, 32 (10.8%) were in stage III, and 10 (3.4%) were in stage IV. In contrast, in the pandemic group, 108 (43.4%) patients were in stage I, 93 (37.3%) were in stage II, 26 (10.4%) were in stage III, and 22 (8.8%) were in stage IV (Table 2 ). In the pandemic group, the percentage of patients with stage IIB or higher disease increased from 22.0% to 31.3%, corresponding to an increase of 9.3 points (95% confidence interval [CI]: 1.920627–16.81106, P = .0133) (Figure 1 ).
Table 2.
Distribution of Patients in Each Group by Stage and 10-year Survival Rate. And Expected Additional Deaths
| Non-pandemic |
Pandemic |
The 10-year Survival Rate (%) (The Relative Values) | |||
|---|---|---|---|---|---|
| n=296 | % | n=249 | % | ||
| Stage Ⅰ | 137 | 46.3 | 108 | 43.4 | 98.0 |
| Stage Ⅱ | 117 | 40.0 | 93 | 37.3 | 88.4 |
| Stage Ⅲ | 32 | 10.8 | 26 | 10.4 | 63.8 |
| Stage Ⅳ | 10 | 3.4 | 22 | 8.8 | 19.2 |
| Total | 296 | 100.0 | 249 | 100.0 | 86.8 |
| The weighted average | 0.8785(SE=0.0164) | 0.8388(SE=0.0184) | |||
| Difference in survival rate | 3.96points (95%CI: -0.87—8.80 P>0.05) | ||||
| Expected increase in number of deaths | 2134 (-470−4738) | ||||
Figure 1.
Comparison of the percentage of patients in the pandemic and non-pandemic groups according to the clinical stage.
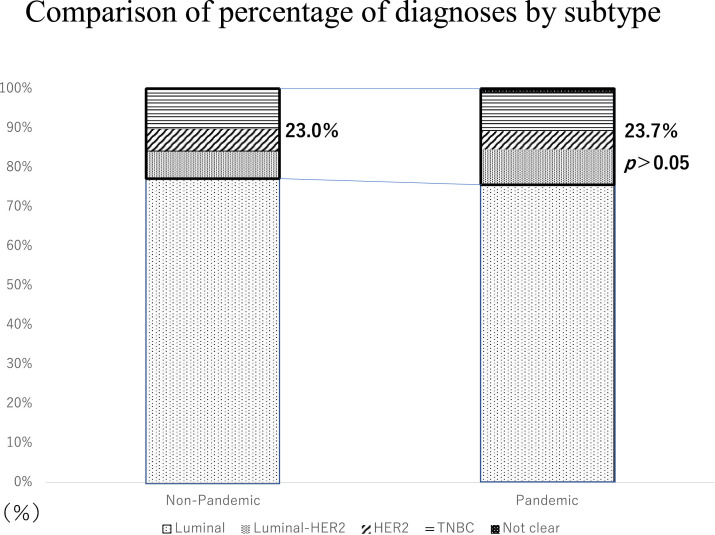
Regarding the cancer subtype, in the non-pandemic group, there were 228 (77.0%) and 68 (23.0%) cases of luminal and non-luminal disease (HER2+, Luminal-HER2+, triple negative type), respectively, while in the pandemic group, the corresponding values were 189 (75.9%) and 59 (23.7%). There was no difference in the percentage of patients in each stage between the 2 groups within each subtype. Moreover, there was no increase in the non-luminal type, which is generally considered highly malignant (Figure 2 ).
Figure 2.
Comparison of patients by subtype between the pandemic and non-pandemic groups, with patients classified as non-luminal type boxed in bold.
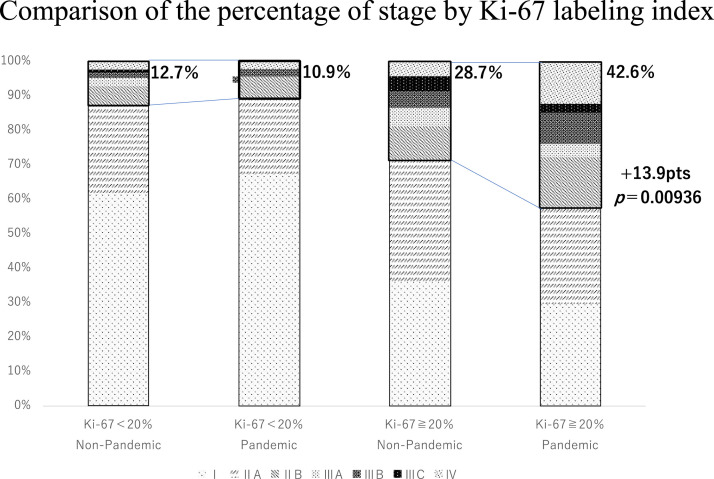
Regarding the Ki-67 labeling index in the non-pandemic group, 164 (55.4%) and 126 (42.6%) patients had a Ki-67 labeling index of 20% or more and less than 20%, respectively. In the pandemic group, the corresponding values were 155 (62.2%) and 92 (36.9%). Among patients with a Ki-67 index below 20%, the proportion of patients who progressed to stage IIB or higher was 16 (12.7%) in the non-pandemic group and 10 (10.9%) in the pandemic group, which was not significantly different. However, among patients with a Ki-67 index higher than 20%, the pandemic group had a significantly higher percentage (66 cases [42.6%] vs. 47 cases (28.7%), 13.9 pts, P = .00936) (Figure 3 ).
Figure 3.
Comparison of patients with a low (< 20%) and high (≥ 20%) Ki67 labeling between the pandemic and non-pandemic groups, with patients classified as stage IIb or higher boxed in bold.
Estimated 10-year Survival Rates
The 10-year survival rate (relative value) of the National Cancer Institute was used to calculate the 10-year survival rate from the stage-weighted mean for each group. The expected 10-year survival rates were 87.9% and 83.9% in the non-pandemic and pandemic groups, respectively. There was a difference in survival of 3.96 points (95% CI: -0.87 - 8.8, one .05) between the 2 groups; assuming the number of patients affected was the same as the 92253 count in 2017, the estimated number of deaths would be 2134 (min. -470 to max. 4738). Although there was a trend towards a more advanced clinical stage in the pandemic group, there was no statistically significant difference (Table 2). Similarly, the 10-year survival rate for each group, restricted to the high Ki-67 group, was calculated to be 6.3 points lower and the number of deaths higher in the pandemic group.
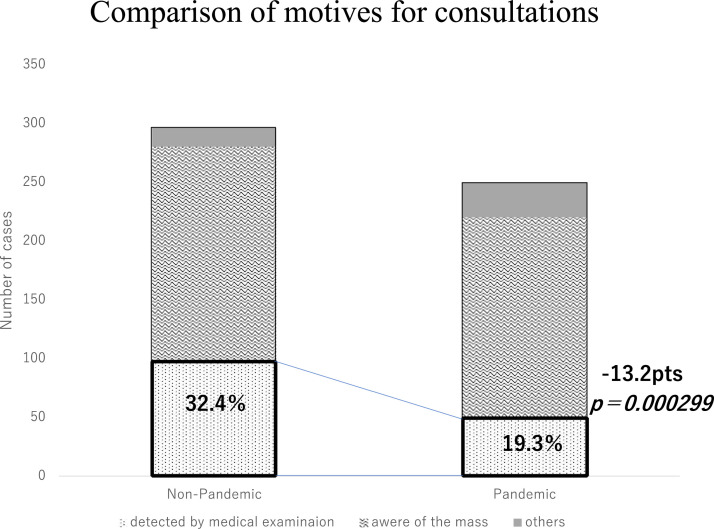
The median disease duration from mass awareness was 30 days (0–2190) for the non-pandemic group and 30 days (0–2555) for the pandemic group, with no difference in the distribution between the 2 groups (P = .385). As for the motive for consultation, in the non-pandemic group, 96 patients (32.4%) were screened for diagnosis, 184 (62.2%) were mass-aware, and 16 (5.4%) were referred by other departments. In the pandemic group, the corresponding values were 48 (19.3%), 172 (69.1%), and 29 (11.6%). The number of cases of screening diagnoses in the pandemic group decreased by 50%, while the number of patients with mass awareness decreased by 6.5%. A significant decrease in the number of screening diagnoses was observed (P = .000299) (Figure 4 ).
Figure 4.
Comparison of the motive to receive a medical examination between the pandemic and non-pandemic groups.
Discussion
This study revealed that the 10-year breast cancer survival rate was lower in the pandemic group than that in the non-pandemic group. A study from the United Kingdom comparing breast cancer-related deaths before and after the COVID-19 lockdown found a 7.9%–9.6% increase up to 5 years after diagnosis, corresponding to 281 (95% CI: 266–295) to 344 (95% CI: 329–358) additional deaths.7 A study in the United States study also reported a 0.52% cumulative increase in deaths over the next 10 years for a 6-month COVID-19 pandemic impact and indicated that this would almost double if the pandemic impact was extended to 12 months.6 Since the incidence of breast cancer is much higher in Europe and the United States than that in Japan, and the breast cancer screening uptake proportion is more than 30% higher than in Japan, the effect of withholding or not undergoing screening was considered to be substantial.8
We also found a significant reduction in early-stage cancers in the pandemic group. This is consistent with results from other countries, including some in Asia, which showed a 15.9%–51% reduction in early-stage cancers.9, 10, 11, 12 Additionally, the proportion of more advanced cancers was significantly increased in the pandemic group in cases with a high Ki-67 labeling index, which may be due to the fact that most interval breast cancers are of high grade and have high proliferative activity.13, 14, 15 High proliferative cancers (MIB-1 labeling index ≥ 20%) have been reported to have increased lymph node positivity and are more likely to be in stage III than those with low proliferative activity in a short period of time, a trend similar to that noted in the present study.16 If the impact of refraining from receiving medical examinations due to the COVID-19 pandemic is prolonged, these highly proliferative breast cancers may cause an increase in deaths in the future. Breast cancer screening and awareness are considered important to counteract high-grade, highly proliferative breast cancers.
Delayed diagnosis affects not only the prognosis but also the patient's quality of life. In the presence of axillary lymph node metastases, additional axillary dissection and radiation therapy may be performed, which may be accompanied by postoperative disability such as upper extremity numbness and edema.17 Alternatively, advanced breast cancer may lead to additional pre- and postoperative chemotherapy and adjuvant therapy that would have been unnecessary had it been detected earlier.
Our study has several limitations, including a small sample size and the possibility that regional characteristics may be involved; therefore, further large-scale studies in Japan are needed.
However, the suspension of breast cancer screening services has led to the development of more advanced and aggressive breast cancers, which may have affected the long-term clinical outcomes of these patients. Therefore, it is necessary to establish appropriate medical triage and screening systems. Additionally, it is important to educate patients not to delay the timing of consultations by raising awareness.
Conclusion
We found more aggressive and advanced disease after the suspension of breast cancer screening services owing to the COVID-19 pandemic. This may have affected the long-term clinical outcomes of patients with breast cancer.
Clinical Practice Points
-
•
What is already known about this subject?
The COVID-19 pandemic is expected to increase the number of deaths in the U.S. and Europe due to patients withholding screening, refraining from outpatient visits, and stopping medical services, delaying the diagnosis of breast cancer.
-
•
What are the new findings?
In this study, an increase in breast cancer stage was observed in the Japanese COVID-19 pandemic, indicating a delay in diagnosis. However, the increase in deaths due to this was expected to be less than in the United States or Europe. This may be due to the fact that the breast cancer screening uptake proportion in Japan has always been low, and even if the COVID-19 pandemic did in fact cause a delay in health examinations, the impact would be smaller than in the United States or Europe. In addition, the COVID-19 pandemic did not increase the time from mass awareness to medical examination, indicating that the impact of refraining from medical checkups was greater than that of refraining from medical examinations.
-
•
How might it impact on clinical practice in the foreseeable future?
In the event of another new infectious disease pandemic, an appropriate screening system must be established to avoid delay in diagnosis of breast cancer.
Ethical Approval
We conducted this experiment in accordance with the Declaration of Helsinki.This study was conducted under the ethical approval of the following Research Ethics Committees: Medical Ethics Review Committee of Tokyo Medical University (T2021-0100) and Medical Ethics Review Committee of the Second Kawasaki Saiwai Clinic (R2-9).
Authors' Contributions
Kayo Adachi: data collection, formal analysis, validation, writing - original draft preparation. Fuyo Kimura: conceptualization, data collection, formal analysis, validation, writing - original draft preparation. Hideto Takahashi: formal analysis, review and editing. Hiroshi Kaise: validation, review and editing. Kimito Yamada: validation, review and editing. Ei Ueno: validation, review and editing. Takahiko Kawate: validation, review and editing. Kana Miyahara: review and editing. Ai Ueda: review and editing. Saeko Sato: review and editing. Mariko Asaoka: review and editing. Natsuki Uenaka: review and editing. Miki Okazaki: review and editing. Kyoko Orimoto: review and editing. Rongrong Wu: review and editing. Yoichi Koyama: review and editing. Takashi Ishikawa: supervision. The manuscript was read and approved by all authors.
Disclosure
The authors have no conflicts of interest to declare.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.World Health Organization. COVID-19. Accessed 10 July 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 2.Croswell JM, Corley DA, Lafata JE, et al. Cancer screening in the U.S. through the COVID-19 pandemic, recovery, and beyond. Prev Med. 2021;151 doi: 10.1016/j.ypmed.2021.106595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yabroff KR, Wu XC, Negoita S, et al. Association of the COVID-19 pandemic with patterns of statewide cancer services. J Natl Cancer Inst. 2022;114:907–909. doi: 10.1093/jnci/djab122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen RC, Haynes H, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7:878–884. doi: 10.1001/jamaoncol.2021.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Japan cancer Society. Accessed 24 March 2021. https://www.jcancer.jp/news/11952.
- 6.Japan cancer Society. Accessed 07 July 2022.https://www.jcancer.jp/news/12899.
- 7.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.OECD. OECD statistics. Accessed on 24 March 2021. https://www.stats.oecd.org/index.aspx?queryid=30159#; 2022/01/25.
- 9.Chou CP, Lin HS. Delayed breast cancer detection in an Asian country (Taiwan) with low COVID-19 incidence. Cancer Manag Res. 2021;13:5899–5906. doi: 10.2147/CMAR.S314282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kara H, Arikan AE, Dulgeroglu O, Tutar B, Tokat F, Uras C. Has the COVID-19 pandemic affected breast cancer stage and surgical volume? Front Surg. 2022;9 doi: 10.3389/fsurg.2022.811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou JZ, Kane S, Ramsey C, et al. Comparison of early- and late-stage breast and colorectal cancer diagnoses during vs before the COVID-19 pandemic. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2021.48581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eijkelboom AH, de Munck L, Vrancken Peeters MTFD, et al. Impact of the COVID-19 pandemic on diagnosis, stage, and initial treatment of breast cancer in the Netherlands: a population-based study. J Hematol Oncol. 2021;14:64. doi: 10.1186/s13045-021-01073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowsett M, Nielsen TO, A'Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the international Ki67 in breast cancer working group. J Natl Cancer Inst. 2011;103:1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmberg LH, Tabar L, Adami HO, Bergström R. Survival in breast cancer diagnosed between mammographic screening examinations. Lancet. 1986;2:27–30. doi: 10.1016/s0140-6736(86)92569-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Ding Y, Zhou Q, Wang C, Wu P, Dong J. Correlation factors analysis of breast cancer tumor volume doubling time measured by 3D-ultrasound. Med Sci Monit. 2017;23:3147–3153. doi: 10.12659/MSM.901566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toss A, Isca C, Venturelli M, et al. Two-month stop in mammographic screening significantly impacts on breast cancer stage at diagnosis and upfront treatment in the COVID era. ESMO Open. 2021;6 doi: 10.1016/j.esmoop.2021.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tandra P, Kallam A, Krishnamurthy J. Identification and management of lymphedema in patients with breast cancer. J Oncol Pract. 2019;15:255–262. doi: 10.1200/JOP.18.00141. [DOI] [PubMed] [Google Scholar]