Introduction
According to the trans-lamina cribrosa (Fig. 1) gradient hypothesis, intracranial hypotension and intracranial hypertension are risk and protective factors, respectively, for normal tension glaucoma (NTG) [1]. This prediction was tested in patients with normal pressure hydrocephalus (NPH) who received CSF shunting (CSFs) to reduce their intracranial pressure (ICP) [2]. We previously reported on 22 of such patients who had been evaluated for NTG in 2016. By that time, nine patients (41%) had developed NTG, while 13 had not (Supplementary Fig. 1) [2]. Here we report the extended follow-up to monitor the possible occurrence of NTG among the patients of our initial cohort who were still free from NTG.
Fig. 1. The optic nerve at the lamina cribrosa level.
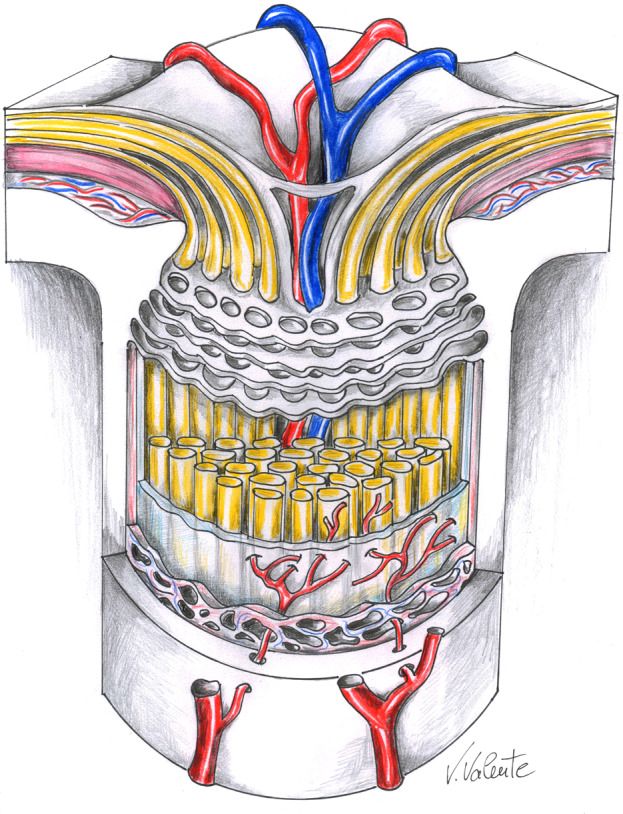
Artistic drawing by Vinicio Valente, neurosurgeon at the “Annunziata” Hospital, Cosenza, Italy, illustrating a section of the optic nerve at the lamina cribrosa level, which forms the anatomical floor of the optic nerve head and separates the intraocular and intracranial pressure compartments. Lowering of intracranial pressure, by cerebrospinal fluid shunting in idiopathic normal pressure hydrocephalus may increase the pressure gradient across the lamina cribrosa leading to glaucomatous damage.
Materials and methods
In this retro-prospective study (IRB approval OSS.16.253), patients were invited to attend ophthalmic evaluation [2]. Incidence of NTG and life status were recorded during a follow-up extended up to December 31st, 2019. We used Kaplan-Meier survival curves (with follow-up time defined as the time from CSFs until NTG diagnosis, i.e., “exposure period”, death, or loss to follow-up, whichever came first) to describe the occurrence of NTG. We applied the log-rank test to identify risk and protective factors of NTG occurrence.
Results
Results are summarized in the supplemental flow chart (Supplementary Fig. 1). Three patients died (one in the NGT group and two in non-NGT group), while seven patients (two NTG and five non-NTG) deteriorated cognitively (thus preventing their evaluation) during the extended follow-up. Two of the six non-NTG patients that could be re-evaluated developed NTG (Table 1) after 28 and 32 months from the original visit (9.5 and 4.0 years from CSFs, respectively) raising the number of patients with documented NTG to eleven (50% of the total cohort). Four patients were still NTG free. However, ganglion cell complex thickness, mean deviation and pattern standard deviation values, showed significant deterioration (Table 1).
Table 1.
Comparison of instrumental ophthalmic data obtained at 2 time points in 6 idiopathic normal pressure hydrocephalus patients who underwent cerebrospinal fluid shunting.
| Right eye | Left eye | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case no | IOP (mmHg) | Average RNFL (μm) | GCC (μm) | vCDR | MD (dB) | PSD (dB) | IOP (mmHg) | Average RNFL (μm) | GCC (μm) | vCDR | MD (dB) | PSD (dB) | ||||||||||||
| T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | |
| 4 | 20 | 14 | 102 | 100 | 122 | 116 | 0.25 | 0.33 | −2.43 | −4.93 | 1.23 | 2.78 | 20 | 12 | 80 | 110 | 149 | 135 | 0.69 | 0.42 | −5.12 | −7.36 | 2.11 | 3.13 |
| 6 | 18 | 12 | 77 | 97 | 103 | 96 | 0.56 | 0.52 | −5.45 | −7.59 | 4.33 | 7.29 | 18 | 12 | 87 | 103 | 102 | 96 | 0.63 | 0.58 | −12.34 | −15.57 | 4.22 | 5.48 |
| 10 | 15 | 15 | 95 | 87 | 98 | 86 | 0.59 | 0.45 | −3.34 | −5.02 | 2.87 | 4.38 | 15 | 13 | 99 | 86 | 103 | 89 | 0.65 | 0.64 | 1.89 | 1.0 | 0.77 | 1.78 |
| 18 | 17 | 22 | 107 | 99 | 100 | 91 | 0.57 | – | −1.12 | −2.82 | 2.22 | 3.13 | 17 | 18 | 113 | 101 | 122 | 100 | 0.48 | – | −2.34 | −4.72 | 3.34 | 4.52 |
| p-value | 0.557 | 0.945 | 0.008 | 0.652 | 0.002 | 0.028 | 0.160 | 0.656 | 0.023 | 0.307 | 0.020 | <0.001 | ||||||||||||
| 11 | 14 | 12 | 85 | 88 | 111 | 93 | 0.55 | 0.65 | −1.02 | −2.3 | 1.54 | 2.06 | 16 | 12 | 86 | 75 | 98 | 77 | 0.65 | 0.74 | −5.23 | −8.27 | 7.45 | 9.39 |
| 14 | 18 | 17 | 89 | 80 | 99 | 80 | 0.46 | 0.52 | −3.21 | −4.52 | 1.54 | 2.81 | 19 | 17 | 87 | 87 | 86 | 74 | 0.16 | 0.20 | −1.22 | −3.59 | 2.12 | 3.29 |
GCC ganglion cell complex, IOP intraocular pressure, MD mean deviation, PSD pattern standard deviation, RNFL retinal nerve fiber layer, vCDR vertical cup disc ratio.
The identification number of patients refers to that in Gallina et al. [2]. The median interval between T0 and T1 was 25 months (range 25–33 months). Changes in ophthalmic parameters for the patients who have not developed NTG were tested for statistical significance using the paired t test (p < 0.05). Corneal central thickness was ≥520 μm in every patient. Data of normal tension glaucoma (NTG) patients are shadowed. We labeled as NTG those patients with an IOP < 21 mmHg, vertical cup disc ratio >0.50 in association with mean deviation <−2 dB and pattern standard deviation >2 dB. The macular retinal nerve fiber layer and ganglion cell complex were measured by DRI OCT Triton plus, Topcon Medical Systems Inc.; mean deviation and pattern standard deviation were assessed by Humphrey Field Analyzer, Zeiss.
Bold values indicate statistical significance p < 0.05.
Discussion
NTG occurred at an approximately steady rate suggesting that it may be a common fate among shunted NPH patients. Particularly, survival analysis suggested that three quarters of such patients will be diagnosed with NTG within approximately 10 years from CSFs, assuming they survive long enough. In addition, four of 11 non-NTG patients experienced a worsening of their ophthalmological parameters that may suggest a possible future diagnosis of NTG. The occurrence of NTG could not be evaluated among the other seven patients who were non-NTG at the previous follow-up. Therefore, the already high prevalence of NTG in our series is likely to be underestimated.
We substantiated the trans-lamina cribrosa gradient hypothesis, overcoming the limit of the previous study2 and demonstrating that NTG occurred after CSFs indeed. NTG risk was not associated with the extent of ICP decrease. This may occur simply because the latter did not have sufficient variability to achieve statistical significance in this relatively small-sized population. We showed the relevance of “exposure period” in NTG occurrence and confirmed that a longer “protection period” could protect patients from developing NTG. A slightly enhanced ICP might make the optic nerve, habituated for a long time to a minor translaminar gradient, highly susceptible to barometric changes.
Supplementary information
Author contributions
PG, AlS and AnS conceived the study and were involved in diagnostic evaluation and management of the patients with support from MB, LA and AM who helped in acquisition and interpretation of data. SC designed the study and did statistical analysis. SR performed critical revision of the manuscript. BP helped in study conception and design. All authors provided intellectual content for the manuscript and approved the final version, having been involved throughout the drafting and editing process.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Pasquale Gallina, Alfonso Savastano.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-022-02064-9.
References
- 1.Jonas JB. Role of cerebrospinal fluid pressure in the pathogenesis of glaucoma. Acta Ophthalmol. 2011;89:505–14. doi: 10.1111/j.1755-3768.2010.01915.x. [DOI] [PubMed] [Google Scholar]
- 2.Gallina P, Savastano A, Becattini E, Orlandini S, Scollato A, Rizzo S, et al. Glaucoma in patients with shunt-treated normal pressure hydrocephalus. J Neurosurg. 2018;4:1078–84. doi: 10.3171/2017.5.JNS163062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


