Abstract
Clinical research regularly includes required, nontherapeutic procedures to answer research questions. Optional procedures usually offer minimal or no personal benefit and may involve harms and burdens.
Members from the Bangkok SEARCH010/RV254 HIV research cohort of individuals acutely HIV-infected are recruited to six optional procedures varying in invasiveness: leukapheresis, genital secretions collection, lumbar puncture, brain MRI/MRS/DTI, colon biopsy, and lymph node biopsy. We surveyed cohort members about their first recruitment for each procedure to examine factors associated with decision making and attitudes about compensation.
406 members (68%) completed the survey. Reported procedure participation ranged from 71% (MRI) to 27% (lymph node biopsy). Respondents underwent 0–6 procedure types (median 3). Ordinal regression indicated that lower perceived HIV impact and HIV remission trial participation were associated with more procedures completed. Reports of decision difficulty varied, and feeling pressured by research staff was low overall. Notably, those who declined procedures and those who underwent more invasive procedures reported greater decision difficulty and perceived pressure. Most respondents felt compensation amounts were appropriate, although opinions differed by procedure, and for some procedures, between people who agreed and declined.
There is limited literature regarding consent to and attitudes about optional research procedures. Researchers must consider how to best support voluntary decisions for procedures with little personal benefit, particularly in lower-income or marginalized populations. In this longitudinal research cohort, perceived pressure to participate may be a concern, although our finding of variation in participation rates corresponding to invasiveness is reassuring. Data from different research contexts would provide important comparators.
Keywords: Clinical research ethics, Research biopsies, Optional procedures, Research cohorts, HIV research, Decision-making
1. Introduction
Participation in clinical research commonly requires participants to undergo non-therapeutic study procedures that are necessary to meet the research objectives. Participants may also be asked to volunteer for procedures that are optional; i.e., that address additional research objectives and are not part of the primary study consent [1]. These optional procedures include medical and laboratory assessments that differ in invasiveness, burden, time required, and compensation offered [2]. Like many routine trial procedures, optional procedures generally do not provide direct clinical benefit to participants, and carry potential harms or burdens. Some attention has been devoted to consent for optional research biopsies in early phase oncology trials, which offer no chance of benefit [3,4]. Ethical and regulatory standards require the risks of mandatory trial procedures be justified by potential knowledge gained. It is unclear, however, whether procedures categorized as optional are held to the same standards [[5], [6], [7]]. Furthermore, while research has examined how individuals weigh risks and benefits of trial participation [8,9], very little is known about decision making for optional procedures that offer little or no personal benefit and often involve discomfort and burden.
During 2016–2020 we conducted interviews about decision making for 74 individuals in an HIV research cohort in Thailand (SEARCH010/RV254 – hereafter RV254) who were invited to participate in four remission (“cure”) trials [6,10]. The cohort is comprised of individuals diagnosed during acute HIV infection, a population of interest for HIV remission trials. Many interviewees described their experiences with optional procedures requested of RV254 cohort members. Some reported that they welcomed optional procedures as another way to contribute to science, but for others, participation had significant, perhaps unanticipated burden [10,11]. Based on these interview findings in our previous research, we initiated further investigation of this issue by developing a survey for the entire RV254 research cohort to learn about decision making for optional study procedures.
RV254 provides an informative cohort to explore this topic because cohort members are asked to undergo a range of optional procedures, including invasive biopsies. One might expect more participants to volunteer for less invasive and burdensome procedures, although compensation or perceived pressure from staff might influence participant decisions. In this exploratory, retrospective study, we examine participation rates across the six procedures offered to RV254 participants. We compare decision making in RV254 cohort participants who consented to and who declined each optional procedure. Developing a deeper understanding of these processes may identify opportunities to strengthen communication with participants about optional procedures and improve the informed consent process.
This study aimed to address the following research questions:
Research Question 1: What is the frequency of volunteering for each type of optional procedure, and what clinical and demographic factors are associated with the number of procedures done per respondent?
Research Question 2:Are there differences in how respondents rate the decision difficulty and perceived pressure from cohort staff when comparing those who undergo or decline different procedures?
Research Question 3: Are there differences in how respondents rate the adequacy of compensation when comparing those who undergo or decline different procedures?
2. Methods
RV254 is a research cohort established in 2009 by the US Military HIV Research Program (clinicaltrials.gov NCT00796146) in collaboration with the Thai Red Cross AIDS Research Centre in Bangkok [12]. Acutely-infected individuals are referred from local voluntary HIV testing and counseling centers, and placed on antiretroviral treatment (ART) immediately upon consent. The RV254 cohort is primarily young, MSM (men who have sex with men), with low HIV clinical impact and preserved immunity [13]. By 2020, 643 individuals had joined the cohort. Among those, some were recruited to sub-studies, including the HIV remission trials that were the focus of our previous interview study [6,10].
Initially, RV254 investigators used the cohort to investigate the basic biology of early infection, disease incidence, viral diversity, host genetics, and treatment outcomes. RV254 cohort members visit the study clinic at minimum every 6 months for routine follow-up and care. They undergo full neurological and neuropsychological evaluation at baseline and annually. Blood, plasma, and cerebrospinal fluid (CSF) specimens are captured and stored for virological and immunological assays. When it began, RV254 required frequent and repeated viral load testing and peripheral blood mononuclear cells (PBMCs) storage, given how little was known about HIV-host interactions in the earliest stages, and about acute HIV infection generally. Cohort investigators anticipated the research value of additional samples that required more participant burden to collect, and therefore added a set of optional procedures to the protocol.
2.1. Optional research procedures in RV254
During initial enrollment, all RV254 cohort members are asked to volunteer for six optional procedures: leukapheresis, collection of genital secretions, lumbar puncture, brain magnetic resonance imaging (MRI)/magnetic resonance spectroscopy (MRS)/diffusion tensor imaging (DTI), colon biopsy, and lymph node biopsy (Table 1). Consent for optional procedures was conducted in-person by RV254 study nurses. The consent forms specify that these procedures are for research purposes and not for treatment, and that volunteering has no impact on cohort membership or medical care. Each procedure includes serial data collection; participants who initially agree to participate in a procedure are asked intermittently to participate again. Thus, cohort members who joined RV254 in the early years have been asked to volunteer for optional procedures more times than those who joined recently. Risks of optional procedures are detailed within the consent forms. The primary benefit noted is contributing to knowledge about how HIV affects the body in the early, acute phase of infection, thus helping create better treatments. The possibility that procedure results could improve an individual's HIV care is also noted, and that results would only be returned in those cases.
Table 1.
Optional procedures included in the RV254 cohort study.
| Procedure | Duration (hours) | Invasiveness | Location | Compensation |
|---|---|---|---|---|
| Leukapheresis | 2–3 | Medium | Regular study clinic | 1000 THB/31 USDa |
| Genital secretion collection | 0.25 | Low | Regular study clinic | 500 THB/16 USD |
| Lumbar puncture | 1.5 | Medium | Regular study clinic | 1000 THB/31 USD |
| Brain MRI/MRS/DTI | 2 | Non-invasive | Hospital | 500 THB/16 USD |
| Lymph node biopsy | 0.5b | High | Regular study clinic | 2000 THB/63 USD |
| Colon biopsy | 1.5b | Medium | Hospital | 1500 THB/47 USD |
1 USD = 31.88 THB on May 26, 2020.
These are surgical procedures. Colon biopsy requires one day preparation and recovery time. Lymph node biopsy involves up to a week of recovery time.
The procedures vary in degree of invasiveness and burden, and in associated compensation (Table 1). Invasiveness is a subjective measure of intrusiveness and discomfort. The RV254 clinician-investigators rate that the most invasive is the lymph node biopsy, which is a surgical procedure that leaves a small scar. Least invasive are the MRI (contrast is not used) and collection of genital secretions (requiring a speculum inserted into the vagina and/or rectum, without biopsy, and masturbation for semen). The double lumen leukapheresis is classified as medium invasiveness (requiring needles to be inserted in both arms and the participant to be immobilized throughout). Compensation amounts were proposed by investigators, based on their perceptions of the amount of lost work time, food and travel costs, and the invasiveness of the procedure; amounts were ultimately determined by the local IRB at Chulalongkorn University.
2.2. Study development
In 2016, we initiated a longitudinal study of decision-making regarding HIV remission trial participation, collecting interview and survey data from 74 members of the RV254 research cohort recruited to four HIV remission trials [11,14]. Though not asked specifically about optional procedures, interviewees frequently mentioned them when queried about the most difficult aspects of remission trial participation. Some described their experiences with optional procedures to include experiencing pain or side effects, from the more invasive procedures, and/or basing their decisions on anticipated pain: “I won't get the tissue biopsy … I chose two procedures which do not seem painful.” Others mentioned burden and impact of the procedures on life and work: “It was difficult to go to work after the operations.” Based on these findings, in a recent publication we recommended to investigators: “More forecasting about optional procedures may be warranted, reinforcing that they are optional, do not impact trial participation, and may involve serial procedures” [10].
With the goal of further assessing attitudes about and participation in optional procedures in the RV254 cohort overall, we conducted a review of the information provided to cohort members in consent forms, consulted with the lead study nurse about the consent process, and used the RV254 protocols and characterization of procedure burdens to inform item development for a cohort survey. The resulting questionnaire asked respondents which types of procedures they had ever done or declined doing as part of RV254, along with a brief description of each procedure to aid recall. Questions about each procedure type collected information on various aspects of decision making, including the items described below.
2.3. Survey items
Respondents were asked to think back to the first time they were invited to do an optional procedure and respond whether they did each procedure (yes/no, do not remember). Then for each procedure, they were asked about:
-
•Decision difficulty: “How easy or difficult was your decision to do [procedure type]?”
-
o4 item response scale: very easy, somewhat easy, somewhat difficult, very difficult.
-
o
-
•Pressure from cohort staff: “How much pressure did you feel, if any, from the SEARCH010/RV254 team to do [procedure type]?”
-
o4 item response scale: none at all, a little, a moderate amount, a lot.
-
o
-
•Compensation: “Do you think the compensation of Thai Baht (THB) [amount] was?”
-
o3 item response scale: too much, just right, too little.
-
o
2.4. Other data used in analyses
We included select RV254 clinical data, with participants' consent: age, sex at birth, self-identifying as men who have sex with men (MSM), education level (dichotomized by bachelor's degree or higher: yes/no), monthly income, time enrolled in RV254, and prior participation in HIV remission trials (yes/no). Additionally, we used the Brief Illness Perception Questionnaire (IPQ) measure. It includes eight questions related to illness identity, timeline, consequences, and control, with a response scale of 0–10, where 0 = none and 10 = extreme [15]. Example questions include: how much does HIV affect your life; how much control do you feel you have over your HIV; and, how much do you think your treatment with ART can help your HIV? An overall IPQ score was calculated in accordance with published guidance, including internal consistency checks, with a higher score indicating more negative illness perceptions [15].
2.5. Survey administration
Recruitment took place at RV254 clinical research follow-up visits during September through December 2019. All RV254 cohort members who visited the clinic during this four-month period were offered participation, and those who completed the survey were compensated 300 THB (9.41 USD). The questionnaire was administered online using Qualtrics (Qualtrics, Provo, UT), with respondents able to take the survey privately on a personal or a study-provided electronic device. Respondents were informed that the survey was not anonymous. To allow linkage with other data sources and prevent duplicate responses, respondents were asked their RV254 ID number. However, the consent stated that RV254 investigators would not have access to the ID-linked survey data. The survey was approved by the Chulalongkorn University IRB on June 11, 2019 and all respondents provided electronic informed consent.
2.6. Statistical analysis
Quantitative analyses were conducted in SAS version 9.4 (SAS Institute Inc., Cary, NC). Survey data were cleaned and then linked with RV254 clinical data and IPQ score using unique identification numbers. Standard univariate descriptive analyses were conducted, including considerations of variable distributions. Additional analyses were conducted as follows for each research question:
Research question 1: Variables assessed for association with number of procedures were selected based on our review of the limited available literature, our prior interview data, RV254 researchers’ experiences, and variable availability. Bivariate assessment of continuous variables used Spearmen rank correlation coefficients, as all but one were not normally distributed. Bivariate association with remission trial participation and education was assessed using Mantel-Haenszel chi-square to test for association, accounting for the ordinal nature of the outcome variable. Multivariate analysis consisted of ordinal logistic regression. Regression procedures included assessing correlation between continuous variables to identify multi-collinearity concerns and ensuring the proportional odds assumption was not violated.
Research questions 2 and 3: The proportion of responses for each response option was stratified by procedure type and whether or not the respondent completed that procedure. Statistical comparisons by procedure type were done using Kruskal-Wallis testing to account for the data not being normally distributed. Respondents who reported not doing any of the optional procedures were excluded from these analyses, a decision made in advance given our assumption that decision-making may be different in this group. Our a priori assumptions also guided the selection of genital secretions collection as the comparator group for comparisons by procedure type, as we hypothesized it would be the most acceptable given that it is part of routine RV254 STI screening.
3. Results
Of 597 cohort members invited to take the survey, 406 participated, a response rate of 68%. The majority were male and MSM, mean age was 31.5 years and mean time in the RV254 cohort was 3.8 years (range 0.3–10.6 years) (Table 2). Respondents were well educated, 63% earned a university degree, with a median monthly income of 40,000 THB (IQR: 20,000–70,000) [16]. Respondents had higher income [16] and were better educated than the Thai population [17]. Survey respondents did not differ meaningfully from the entire RV254 cohort by age, sex at birth, education, sexual orientation, or proportion with detectable viral load (data not shown), but did differ by time since RV254 enrollment/ART initiation (respondents’ mean years of enrollment was 3.8, SD 2.4, versus RV254 overall of 4.4 years, SD, 2.4.)
Table 2.
Demographic characteristics and RV254 variables among survey respondents (n = 406).
| Mean ± SD | Min | Max | Range | |
|---|---|---|---|---|
| Age in years | 31.5 ± 7.1 | 19.0 | 57.0 | 38.0 |
| Sex at birth, n (%) | ||||
| Male | 397 (98) | |||
| Female | 9 (2) | |||
| Identify as MSM, n (%) | 382 (94) | |||
| Education: Bachelor's or higher, n (%) | 258 (64) | |||
| Age at RV254 enrollment | 27.7 ± 6.7 | 18.0 | 54.0 | 36.0 |
| Years in RV254 | 3.8 ± 2.4 | 0.03 | 10.6 | 10.6 |
| Participated in RV254 HIV remission (“cure”) trial, n (%) | 39 (10) | |||
3.1. Research question 1
What is the frequency of volunteering for each type of optional procedure, and what clinical and demographic factors are associated with the number of procedures done per respondent?
3.2. Frequency of volunteering
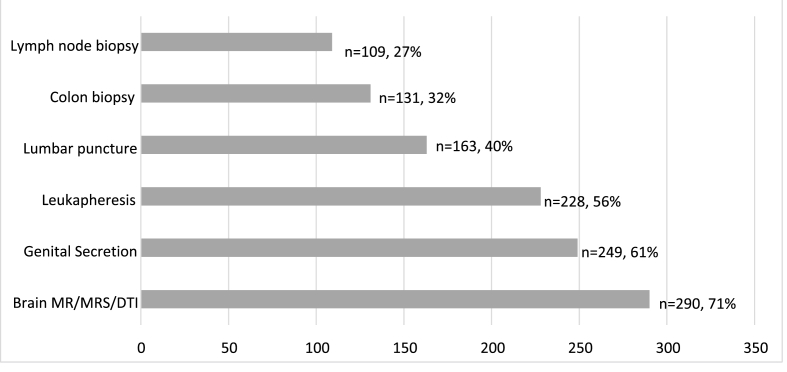
Reported participation rates (doing each procedure at least once) varied considerably by type of procedure (Fig. 1). Most frequently reported were MRI (71%) and genital secretions collection (61%). Least frequent was lymph node biopsy (27%). The proportion of respondents who said they did not know/did not remember if they had done a certain procedure type ranged from 4 to 8% across procedures, with 5 respondents reporting they did not know if they had done any of the optional procedures.
Fig. 1.
Reported participation in optional procedure types (n = 406).
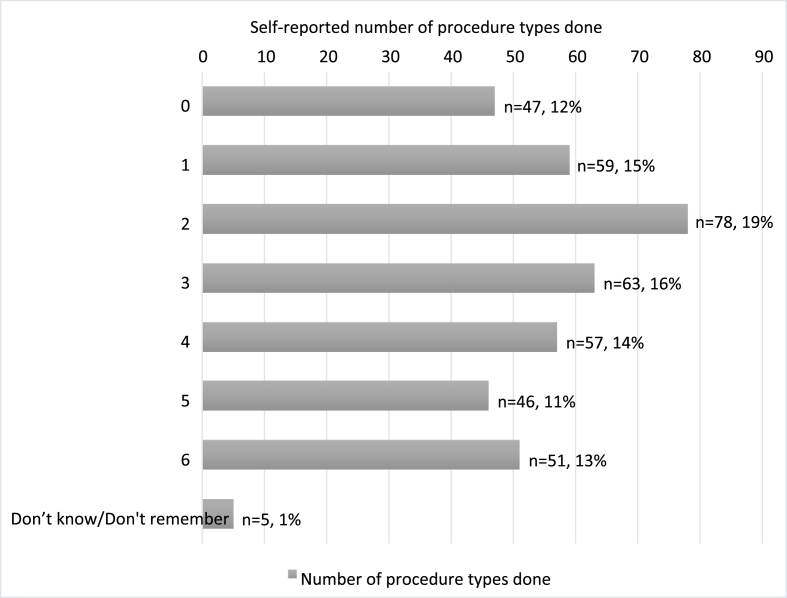
Number of unique procedure types done. The number of procedure types respondents had participated in, at least one time, had a range of 0–6 (Fig. 2). The median number of procedures done was 3.
Fig. 2.
Self-reported number of optional procedure types done by respondents (n = 406).
3.3. Clinical and demographic factors associated with the number of procedure types done
Bivariate analyses did not demonstrate an association between the number of optional procedure types reported done and age, time in RV254, or having at least a four year college degree. There was evidence of a weak, inverse association with IPQ score, and a positive association with remission trial participation (Table 3). In the multivariate analysis, ordinal logistic regression indicated that IPQ score and remission trial participation were independently associated with the number of procedure types reported done (Table 3).
Table 3.
Associations among participant characteristics and number of optional procedure types done (range: 0–6) (n = 406).
| Correlation Results | ||||
|---|---|---|---|---|
| Variable | Mean | Median | Spearman rank correlation coefficient | p-value |
| Age (years) | 31.5 | 30 | 0.07262 | 0.1490 |
| Time in RV254 (years) | 3.8 | 3.7 | 0.08984 | 0.0723 |
| HIV Burden: IPQ Score | 26 | 26 | −0.12055 | 0.0157 |
| Bachelor's degree or higher (n, %) | n = 258 | 64% | χΜΗ2 /Μ2 = 1.4132 | 0.2345 |
| Participated in HIV remission (“cure”) trial (n, %) | n yes = 39 | 10% | χΜΗ2 /Μ2 = 12.9344 | 0.0003 |
| Ordinal Logistic Regression Results | ||||
|---|---|---|---|---|
| Variable | Odds Ratio | 95% CI | Wald Chi-Square | p-value |
| Age (years) | 1.026 | (0.999, 1.055) | 3.4437 | 0.4400 |
| Time in RV254 (years) | 1.032 | (0.952, 1.119) | 0.5963 | 0.1264 |
| HIV Burden: IPQ Score | 0.980 | (0.965, 0.996) | 6.2199 | 0.0126 |
| Education: Bachelor's or higher | 0.742 | (0.507, 1.088) | 2.3367 | 0.1264 |
| Participated in a RV254 HIV remission (“cure”) trial | 2.549 | (1.374, 4.728) | 8.8041 | 0.0030 |
3.4. Research question 2
Are there differences in how respondents rate the decision difficulty and perceived pressure from cohort staff when comparing those who undergo or decline different procedures?
3.5. Decision difficulty
Table 4 presents reported ease of decision making for each procedure, stratified by decision (to do each optional procedure versus not). Those who did a procedure generally reported finding the decision easier compared to those who declined. Among those who did a certain procedure type, rating the decision as “very easy” ranged from 50% (genital secretions) to 25% (lumbar puncture), while among those who did not do that procedure type, “very easy” ranged from 32% (genital secretions) to 8% (both biopsies). Within specific procedures, decision difficulty varied greatly by participation: 39% of those who did not do lumber puncture found the decision “very difficult” compared to 5% of those who did.
Table 4.
Self-reported decision difficulty by procedure type in response to the question ‘How easy or difficult was your decision to do the [procedure]?’ (n = 406).
| Among those who did the procedure | ||||||
|---|---|---|---|---|---|---|
| Genital Secretions (n = 248) | Leukapheresisa (n = 228) | Lumbar puncturea (n = 164) | Brain MRI (n = 288) | Lymph Node Biopsya (n = 109) | Colon Biopsya (n = 131) | |
| Very easy | 50% | 40% | 25% | 53% | 33% | 29% |
| Somewhat easy | 44% | 43% | 38% | 37% | 28% | 39% |
| Somewhat difficult | 6% | 15% | 31% | 9% | 29% | 29% |
| Very difficult |
0% |
1% |
5% |
0% |
9% |
3% |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 |
|
Among those who did not do the specified procedure, but did at least one other procedure | ||||||
|---|---|---|---|---|---|---|
| Genital Secretions (n = 99) | Leukapheresisa (n = 119) | Lumbar puncturea (n = 192) | Brain MRI (n = 60) | Lymph Node Biopsya (n = 239) | Colon Biopsya (n = 211) | |
| Very easy | 32% | 9% | 9% | 23% | 8% | 8% |
| Somewhat easy | 27% | 21% | 8% | 32% | 11% | 10% |
| Somewhat difficult | 34% | 48% | 44% | 38% | 47% | 49% |
| Very difficult |
6% |
22% |
39% |
7% |
33% |
34% |
| Missingb | n = 31 | n = 31 | n = 33 | n = 31 | n = 31 | n = 31 |
Compare to genital secretions p < .05.
Respondents who said they did not do these procedures but did not answer the degree of difficulty questions for each procedure type.
Comparing across procedure types, MRI and genital secretions collection were the easiest decisions to make, both for those who did and did not do the procedures. Decisions regarding other procedures (leukapheresis, lumbar puncture, and the two biopsies) were significantly more difficult than deciding about collection of genital secretions.
3.6. Perceived pressure to participate
Table 5 presents how much pressure from cohort staff respondents reported feeling to do each procedure, from “none at all” to “a lot”. Most who volunteered for procedures reported feeling little or no pressure to participate. This result was consistent across procedure types when compared to genital secretions, except for lumbar puncture, where significantly greater pressure was reported.
Table 5.
Perception of pressure from study staff to do a procedure by procedure type in response to the question ‘How much pressure did you feel, if any, from the SEARCH 010 clinicians to do the [procedure]?’ (n = 406).
| Among those who did the procedure | ||||||
|---|---|---|---|---|---|---|
| Genital Secretions (n = 248) | Leukapheresis (n = 228) | Lumbar puncturea (n = 164) | Brain MRI (n = 288) | Lymph Node Biopsy (n = 109) | Colon Biopsy (n = 131) | |
| None at all | 81% | 84% | 71% | 85% | 77% | 75% |
| A little | 17% | 16% | 25% | 14% | 17% | 21% |
| A moderate amount | 1% | 0% | 3% | 0% | 5% | 3% |
| A lot |
0% |
0% |
1% |
1% |
2% |
1% |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 |
|
Among those who did not do the specified procedure, but did at least one other procedure | ||||||
|---|---|---|---|---|---|---|
| Genital Secretions (n = 99) | Leukapheresis (n = 119) | Lumbar puncturea (n = 192) | Brain MRI (n = 60) | Lymph Node Biopsya (n = 239) | Colon Biopsya (n = 211) | |
| None at all | 73% | 68% | 65% | 68% | 63% | 63% |
| A little | 22% | 18% | 16% | 22% | 21% | 20% |
| A moderate amount | 4% | 8% | 9% | 8% | 9% | 9% |
| A lot |
1% |
6% |
10% |
2% |
8% |
8% |
| Missingb | n = 31 | n = 31 | n = 33 | n = 31 | n = 31 | n = 31 |
Compare to genital secretions p < .05.
Respondents who said they did not do these procedures but did not answer the perceived pressure questions for each procedure type.
The proportion of respondents reporting they felt “a moderate amount” or “a lot” of pressure was higher among those who did not do procedures versus those who did, for every procedure type. Though uncommon, reports of feeling “a lot” of pressure were almost entirely made by those who declined procedures. Across procedures, significantly greater pressure was reported by those who chose not to do lumbar puncture and the two biopsies, compared to those who chose not to do genital secretions. Based on these findings, in a post-hoc analysis we examined all pressure item data (from those who did the procedure and those who declined the procedure) to determine whether a subset of respondents reported “a lot” of pressure across many or all of the procedures, or whether individuals reported pressure differently for different procedures. We found that reports of "a lot" of pressure were not from the same people. Overall, 31 of the respondents (8% of the total) reported “a lot” of pressure for at least one of the six procedure types. We found that none reported “a lot” of pressure for all six procedures, one reported “a lot” for 5 procedures, six reported “a lot” for 4 procedures, five reported “a lot” for 3 procedures, six reported “a lot” for 2 procedures, and thirteen of the respondents reported “a lot” of pressure for 1 procedure.
3.7. Research question 3
Are there differences in how participants rate the adequacy of compensation when comparing those who undergo or decline different procedures?
3.8. Amount of compensation
Table 6 presents respondents’ assessment of the amount of compensation for optional procedures. The majority of respondents reported that the amount was “just right” for every procedure, regardless of whether they did the procedure or not. The 1000 THB compensation for lumbar puncture was reported as “too little” for about a quarter of respondents, with similar proportions among those who did and did not do the procedure. For the two biopsies, there were more reports that compensation was “too little” from respondents who did not do the procedures (13% and 17%) than respondents who did them (6% and 9%). Very few reported compensation was “too much” for any of the optional procedures.
Table 6.
Assessment of compensation for each procedure type in response to the question ‘Do you think the compensation of THB [X,XXX] was too much, just right, or too little?’ (n = 406).
| Among those who did the procedure | ||||||
|---|---|---|---|---|---|---|
| Genital Secretions |
Leukapheresis |
Lumbar Puncturea |
Brain MRIa |
Lymph Node Biopsy |
Colon Biopsy |
|
| 500 THB |
1000 THB |
1000 THB |
500 THB |
2000 THB |
1500 THB |
|
| n = 248 | n = 228 | n = 164 | n = 288 | n = 109 | n = 131 | |
| Too much | 4% | 4% | 2% | 2% | 2% | 2% |
| Just right | 87% | 81% | 73% | 82% | 93% | 89% |
| Too little | 9% | 15% | 25% | 16% | 6% | 9% |
|
Among those who did not do the specified procedure, but did at least one other procedure | ||||||
|---|---|---|---|---|---|---|
| Genital Secretions |
Leukapheresisa |
Lumbar, Puncturea, b |
Brain MRIa |
Lymph Node Biopsya |
Colon Biopsya |
|
| 500 THB |
1000 THB |
1000 THB |
500 THB |
2000 THB |
1500 THB |
|
| n = 99 | n = 119 | n = 192 | n = 60 | n = 239 | n = 211 | |
| Too much | 2% | 0% | 0% | 1% | 1% | 0% |
| Just right | 94% | 85% | 76% | 84% | 86% | 82% |
| Too little | 4% | 15% | 23% | 15% | 13% | 17% |
Compare to genital secretions p < .05.
2 missing.
4. Discussion
In this investigation, we present what is to our knowledge the first analysis of decision making for optional research procedures within a large research cohort. Our study has several important strengths. Our investigaton arose from interview data with a subset of cohort members who were invited to HIV remission (“cure”) trials during 2016–2019. These prior data suggested a mix of attitudes and experiences, from altruistic motivations to help HIV research, interest in what might potentially be revealed about their own health, and concerns about pain, short-term burden, and unanticipated side effects from the procedures [10,11]. Over 400 RV254 cohort members responded to our survey, creating a sample that did not differ in demographic characteristics from the entire cohort except in time enrolled in RV254, probably because those who have recently joined visit the clinic more often and thus were more likely to be recruited to this survey.
All of the optional procedures in RV254 have been performed safely with infrequent and mostly mild adverse events [[18], [19], [20]] and yet we found wide variability in frequency of volunteering by procedure type, ranging from 71% for MRI to 27% for lymph node biopsy. The rates of participation were consistent with the cohort investigators’ prior assessments for procedure invasiveness and burden, as shown in Table 1, which guided the compensation provided. Our findings regarding decisions made by participants suggest that the assessments made by clinicians and approved by the IRB appear reasonable. In addition, the declining rate of participation with greater procedure invasiveness and burden is a sign that voluntariness does not appear to be significantly compromised. Our results indicate that respondents can and did say no to optional procedure requests, and those who participated in some optional procedures refused others.
We found that having lower perceived illness burden and having participated in a remission trial were associated with volunteering for more procedures, independent of age, education, or time in RV254. Those who perceived themselves to have lower HIV burden may have felt more equipped to deal with the burden of the optional procedures. Those who participated in remission trials may be particularly willing to enroll in a wide range of research, including optional prcoedures. In addition, those participants may have been invited to optional procedures as part of the trial and may not have differentiated those optional trial procedures from the optional RV254 procedures.
Our analyses of those who volunteered for some procedures yet declined others revealed that while most report the decision to be relatively easy, with little or no staff pressure, the decision not to participate in optional procedures may be more difficult and associated with feeling greater pressure. Data from interviews with cohort members revealed similar difficulties declining participation in “cure” trials [10]. Cohorts like RV254 offer a special context of optional procedures requests—even serial volunteering [21] —in which trust and reciprocity may enter into decision making [22]. This may explain why decliners report feeling more pressure from the research team than those who agree, in that for those who agree, any feelings of obligation and subtle perception of pressure may be released upon consenting to participate. In this way “pressure” may not be purely external, from something the research team is doing or saying. It may derive from a feeling that one ought to volunteer for reasons of reciprocity and maintaining good relations. On the other hand, the fact that some participants reported greater pressure to undergo a lumbar puncture and ultimately agreed to the procedure is a potential point of concern, as it may signal that some decisions are unduly influenced by unequal power relationships between researcher and participant. Internal and external pressure are hard to disentangle. This calls attention to the complicated psychosocial dynamic between participants and research teams in cohort studies, and also the need for consideration of the social and cultural contexts in which trials are carried out [21].
Lastly, we explored respondents’ perceptions of compensation adequacy. First, our data indicate that respondents took into consideration the characteristics of the procedure when assessing compensation. They were more likely to report that compensation for lumbar puncture was “too little” compared to leukapheresis—even though the same amount was offered, and both were rated “medium” invasiveness. Further investigation is merited regarding how individuals perceive or experience invasiveness and burden related to specific procedures. Second, perception that compensation was “too little” was more common, though still a minority, among those who decided against procedures than those who decided to do them. It is also possible compensation served as an excuse for other reasons to decline, or that perceptions of compensation are mediated by altruistic motivations for those who agree. Regardless, we do not have evidence of undue influence of compensation.
The ethics of paying for research participation has been addressed extensively in the literature. Grady [23] identified compensation as a common practice, dating back 100 years, but that its application has been “uneven and contentious.” It can be conceptualized as wage, incentive, reimbursement for time and inconvenience, or reward; and variation can be tied to disease severity, sociodemographic characteristics, the availability of treatment alternatives, and the culture of the medical subspecialty [23]. In the US [23], federal guidelines specify compensation for time and inconvenience, but there is no industry standard to provide payment guidelines. To address this gap, Dominguez and colleagues [24] assembled four years of investigator-generated payment data from the NIH Clinical Center, based on “inconvenience units,” which include time and discomfort. The highest was for lumbar puncture which was three times greater than for MRI. (In RV254, compensation for lumbar puncture was twice that for MRI.)
Studies have demonstrated that potential participants assume that the magnitude of risks and incentives are related [25,26]. In a longitudinal study of healthy volunteers in the US, Fisher and Walker [27] analyzed what participants believed they were being paid for. Responses varied, including contributing to scientific value, allowing their body to be used, and compensating for time and inconvenience. They conclude that “view[ing] studies that offer greater compensation as higher risk signals ongoing problems both in the ethical oversight of payment and how compensation is explained in the informed consent process.” (p.545). While most participants in RV254 optional procedures report compensation as “just right” or “too much,” further attention is needed to understand why those who felt it was “too little” still participated, particularly for those procedures judged as most burdensome.
Our study has several limitations. The first is recall bias related to the amount of time elapsed since respondents made their decisions. Recall bias may differentially impact our participants due to the rolling recruitment for RV254 and changes to the recruitment approach over time. Additionally, though we asked participants to think back to their first decision to participate in an optional procedure, some individuals who agreed to participate more than once may have difficulty separating the initial decision from subsequent decisions.
Though including those who agreed to optional procedures and those who declined is a strength of our study, our results must be interpreted with an understanding of potential differences in attitudes among those who have had an experience and those who have not. The responses of those who experienced a procedure will undoubtedly be influenced by that experience, even though we asked participants to think back to the time of decision making. Those who have not experienced the procedure will draw on a different set of inputs when answering attitude questions.
Finally, generalizability to research participants in other settings, studies, or cohorts may be limited. RV254 is conducted in Thailand, with mostly young, relatively well-educated MSM, and also reflects the nature of a longitudinal research cohort, with participant decision making possibly influenced by staff-participant relationships [21], and given this close relationship, there is potential for social desirability bias.
5. Conclusion
Decision making for optional procedures is an under-recognized issue in clinical research ethics. We found that participation varied by procedure type—being higher for less invasive procedures, despite the higher compensation for procedures that were more invasive and burdensome. The majority of those who volunteered did not find these decisions difficult to make, though our data demonstrate that investigators should not assume equal decision making ease across all conditions. Additionally, the majority of those who participated reported feeling little or no pressure and that the amount of compensation offered was “just right.” These findings indicate that common threats to voluntariness (e.g., external pressure, money) were not reported to be driving decision making for optional procedures.
More empirical research into these decisions is needed, as IRB members, clinical investigators, and those asked to undergo optional research procedures all may have somewhat different views of degree of invasiveness, burden, and risk for common procedures. Optional procedures may have received less ethical attention in the past because they typically involve interventions that are familiar and commonly used in clinical contexts, often for diagnostic purposes. However, these procedures usually have little or no personal benefit in the research context, raising potential exploitation concerns, particularly in lower-income settings. Enhanced focus on optional procedures within trials may be needed, regarding whether any should be mandatory, how to improve education and informed consent, and how to ensure individuals feel able to decline without pressure or recourse. Careful assessments should be made by research teams, ideally involving community input, regarding whether, which and how many procedures are needed.
Acknowledgements
We thank the participants who contributed their time to this research, as well as the RV254 staff for their assistance. We acknowledge the support from NIH, NIAID R01AI127024, “Integrating Decision-Making Studies into HIV Cure Trials: A Real-Time Longitudinal Assessment” (PI G. Henderson), and University of North Carolina at Chapel Hill Center for AIDS Research (P30 AI50410). We thank Donn Colby for providing background information on SEARCH010/RV254. We would also like to acknowledge Dr. Sandhya Vasan and the US Military HIV Research Program.
References
- 1.Kimmelman J., Resnik D.B., Peppercorn J., Ratain M.J. Burdensome research procedures in trials: why less is more. J. Natl. Cancer Inst. 2017;109:djw315. doi: 10.1093/jnci/djw315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominguez D., Jawara M., Martino N., Sinaii N., Grady C. Commonly performed procedures in clinical research: a benchmark for payment. Contemp. Clin. Trials. 2012;33:860–868. doi: 10.1016/j.cct.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimmelman J., Lemmens T., Kim S.Y.H. Analysis of consent validity for invasive, nondiagnostic research procedures. IRB. 2012;34:1–7. [PubMed] [Google Scholar]
- 4.Abadie R., Kimmelman J., Lafleur J., Lemmens T. Consent for nondiagnostic research biopsies: a pilot study of participant recall and therapeutic orientation. IRB. 2014;36:9–15. [PubMed] [Google Scholar]
- 5.Helft P.R., Daugherty C.K. Are we taking without giving in return? The ethics of research-related biopsies and the benefits of clinical trial participation. J. Clin. Oncol. 2006;24:4793–4795. doi: 10.1200/JCO.2006.05.7125. [DOI] [PubMed] [Google Scholar]
- 6.Overman M.J., Ellis L.M., Joffe S. Ethics and the underreporting of research biopsy findings in clinical trials. JAMA Oncol. 2018;4:1041–1042. doi: 10.1001/jamaoncol.2018.1002. [DOI] [PubMed] [Google Scholar]
- 7.Parseghian C.M., Raghav K., Wolff R.A., Ensor J., Yao J., Ellis L.M., Tam A.L., Overman M.J. Underreporting of research biopsies from clinical trials in oncology. Clin. Cancer Res. 2017;23:6450. doi: 10.1158/1078-0432.CCR-17-1449. LP – 6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson G.E., Waltz M., Meagher K., Cadigan R.J., Jupimai T., Isaacson S., Ormsby N.Q., Colby D.J., Kroon E., Phanuphak N., Ananworanich J., Peay H.L. Going off antiretroviral treatment in a closely monitored HIV “cure” trial: longitudinal assessments of acutely diagnosed trial participants and decliners. J. Int. AIDS Soc. 2019;22 doi: 10.1002/jia2.25260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubé K., Evans D., Sylla L., Taylor J., Weiner B.J., Skinner A., Thirumurthy H., Tucker J.D., Rennie S., Greene S.B. Willingness to participate and take risks in HIV cure research: survey results from 400 people living with HIV in the US. J Virus Erad. 2017;3:40–50.e21. [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson G.E., Waltz M., Meagher K., Cadigan R.J., Jupimai T., Isaacson S., Ormsby N.Q., Colby D.J., Kroon E., Phanuphak N., Ananworanich J., Peay H.L. Going off antiretroviral treatment in a closely monitored HIV “cure” trial: longitudinal assessments of acutely diagnosed trial participants and decliners. J. Int. AIDS Soc. 2019;22 doi: 10.1002/jia2.25260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson G.E., Peay H.L., Kroon E., Cadigan R.J., Meagher K., Jupimai T., Gilbertson A., Fisher J., Ormsby N.Q., Chomchey N., Phanuphak N., Ananworanich J., Rennie S. Ethics of treatment interruption trials in HIV cure research: addressing the conundrum of risk/benefit assessment. J. Med. Ethics. 2018;44:270–276. doi: 10.1136/medethics-2017-104433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Souza M.S., Phanuphak N., Pinyakorn S., Trichavaroj R., Pattanachaiwit S., Chomchey N., Fletcher J.L., Kroon E.D., Michael N.L., Phanuphak P., Kim J.H., Ananworanich J., Teeratakulpisarn N., Colby D., Sutthichom D., Rattanamanee S., Mangyu P., Ubolyam S., Eamyoung P., Puttamaswin S., Tipsuk S., Sangtawan P., Ngauy V., O'Connell R., Akapirat S., Tantibul N., Savadsuk H., Assawadarachai V., Robb M., Tovanabutra S. Impact of nucleic acid testing relative to antigen/antibody combination immunoassay on the detection of acute HIV infection. AIDS. 2015 doi: 10.1097/QAD.0000000000000616. [DOI] [PubMed] [Google Scholar]
- 13.de Souza M.S., Pinyakorn S., Akapirat S., Pattanachaiwit S., Fletcher J.L.K., Chomchey N., Kroon E.D., Ubolyam S., Michael N.L., Robb M.L., Phanuphak P., Kim J.H., Phanuphak N., Ananworanich J., for the R.S. Group. Teeratakulpisarn N., Colby D., Sutthichom D., Rattanamanee S., Prueksakaew P., Eamyoung P., Puttamaswin S., Tipsuk S., Karnsomlap P., Trichavaroj R., Nuntapinit B., Panjapornsuk P., Tongchanakarn B., Kobchit W., Ngauy V., O'Connell R., Tantibul N., Savadsuk H., Assawadarachai V., Tovanabutra S., for the R.S. Group Initiation of antiretroviral therapy during acute HIV-1 infection leads to a high rate of nonreactive HIV serology. Clin. Infect. Dis. 2016;63:555–561. doi: 10.1093/cid/ciw365. [DOI] [PubMed] [Google Scholar]
- 14.Peay H.L., Henderson G.E. What motivates participation in HIV cure trials? A call for real-time assessment to improve informed consent. J Virus Erad. 2015;1:51–53. [PMC free article] [PubMed] [Google Scholar]
- 15.Broadbent E., Petrie K., Main J., Weinman J. The brief illness perception questionnaire (BIPQ) J. Psychosom. Res. 2006;60:631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 16.World Bank . 2021. GDP Per Capita (Current US$) - Thailand.Www.Worldbank.Orghttps://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=TH [Google Scholar]
- 17.CEIC, Thailand Education Statistics Ceid.Com. 2021 https://www.ceicdata.com/en/thailand/education-statistics/th-educational-attainment-at-least-bachelors-or-equivalent-population-25-years-total--cumulative [Google Scholar]
- 18.Chan P., Hellmuth J., Colby D., Kroon E., Sacdalan C., Fletcher J., Patel P., Pinyakorn S., Valcour V., Ananworanich J., Spudich S. Safety of lumbar puncture procedure in an international research setting during acute HIV infection. J Virus Erad. 2018;4:16–20. [PMC free article] [PubMed] [Google Scholar]
- 19.Chintanaphol M., Sacdalan C., Pinyakorn S., Rerknimitr R., Ridtitid W., Prueksapanich P., Sereti I., Schuetz A., Acrowell T., Colby D.J., Lrobb M., Phanuphak N., Ananworanich J., Sspudich S., Kroon E. Feasibility and safety of research sigmoid colon biopsy in a cohort of Thai men who have sex with men with acute HIV-1. Journal of Virus Eradication. 2020;6 doi: 10.1016/s2055-6640(20)30011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chintanaphol M., Sacdalan C., Chottanapund S., Pinyakorn S., Buranapraditkun S., Crowell T.A., Kroon E., Manasnayakorn S., Chipman J.G., Schacker T.W., Michael N., Phanuphak N., Spudich S.S., Colby D.J., Ananworanich J., Phanuphak P., Teeratakulpisarn N., De Souza M., Fletcher J., Tantivitayakul P., Ubolyam S., Eamyoung P., Intasan J., Sutthichom D., Prueksakaew P., Rattanamanee S., Puttamaswin S., Tipsuk S., Benjapornpong K., Ratnaratorn N., Munkong C., Kamonkan Tanjnareel, O'Connell R.J., Akapirat S., Trichavaroj R., Nuntapinit B., Robb M., Ouellette M., Butterworth O. Safety and tolerability of inguinal lymph node biopsy in individuals with acute HIV infection in Thailand. J. Acquir. Immune Defic. Syndr. 2018;79 doi: 10.1097/QAI.0000000000001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson G.E., Rennie S., Corneli A., Peay H.L. Cohorts as collections of bodies and communities of persons: insights from the SEARCH010/RV254 research cohort. International Health. 2020;12:584–590. doi: 10.1093/inthealth/ihaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nobile H., Bergmann M.M., Moldenhauer J., Borry P. Participants' accounts on their decision to join a cohort study with an attached biobank: a qualitative content analysis study within two German studies. Journal of Empirical Research on Human Research Ethics. 2016;11:237–249. doi: 10.1177/1556264616657463. [DOI] [PubMed] [Google Scholar]
- 23.Grady C. Payment of clinical research subjects. J. Clin. Investig. 2005;115:1681–1687. doi: 10.1172/JCI25694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominguez D., Jawara M., Martino N., Sinaii N., Grady C. Commonly performed procedures in clinical research: a benchmark for payment. Contemp. Clin. Trials. 2012;33:860–868. doi: 10.1016/j.cct.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.L G., Cryder C.E. John london A, volpp KG, informative inducement: study payment as a signal of risk, social science and medicine. Feb. 2010;70(3):455–464. doi: 10.1016/j.socscimed.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 26.Fisher J.A., Monahan T., Walker R.L. Picking and choosing among phase I trials : a qualitative examination of how healthy volunteers understand study risks. J. bioeth. Inq. 2019;16:535–549. doi: 10.1007/s11673-019-09946-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher J.A., Monahan T., Walker R.L. Picking and choosing among phase I trials : a qualitative examination of how healthy volunteers understand study risks. J. bioeth. Inq. 2019;16:535–549. doi: 10.1007/s11673-019-09946-w. [DOI] [PMC free article] [PubMed] [Google Scholar]