Abstract
We sought to determine if infection of the colon with Entamoeba histolytica induces the expression of cyclooxygenase-2 and, if it does, to determine the contribution of prostaglandins produced through cyclooxygenase-2 to the host response to amebic infection. Human fetal intestinal xenografts were implanted subcutaneously in mice with severe combined immunodeficiency and allowed to grow; the xenografts were then infected with E. histolytica trophozoites. Infection with E. histolytica resulted in the expression of cyclooxygenase-2 in epithelial cells and lamina propria macrophages. Infection with E. histolytica increased prostaglandin E2 (PGE2) levels 10-fold in the xenografts and resulted in neutrophil infiltration, as manifested by an 18-fold increase in myeloperoxidase activity. Amebic infection also induced an 18-fold increase in interleukin 8 (IL-8) production and a >100-fold increase in epithelial permeability. Treatment of the host mouse with indomethacin, an inhibitor of cyclooxygenase-1 and cyclooxygenase-2, or with NS-398, a selective inhibitor of cyclooxygenase-2, resulted in (i) decreased PGE2 levels, (ii) a decrease in neutrophil infiltration, (iii) a decrease in IL-8 production, and (iv) a decrease in the enhanced epithelial permeability seen with amebic infection. These results indicate that amebic infection in the colon induces the expression of cyclooxygenase-2 in epithelial cells and macrophages. Moreover, prostaglandins produced through cyclooxygenase-2 participate in the mediation of the neutrophil response to infection and enhance epithelial permeability.
Entamoeba histolytica, a human intestinal protozoan parasite, is the causative agent of amebic dysentery and amebic liver abscess. E. histolytica trophozoites in the colonic lumen penetrate the mucus layer and adhere to the underlying epithelial cells (22). They enhance tissue invasion by releasing neutral cysteine proteases, which degrade components of the extracellular matrix (7). Trophozoites lyse colonic epithelial cells and penetrate the underlying tissue, coming into close contact with fibroblasts, smooth muscle cells, and inflammatory cells (11, 22).
Infection with E. histolytica trophozoites induces a host response, which includes the expression of chemotactic factors and proinflammatory cytokines by epithelial cells. Coculture of transformed human intestinal epithelial cell lines with E. histolytica trophozoites results in the secretion of interleukin 8 (IL-8), growth-related protein α (GROα), granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-1α and IL-6 (3). The host response is also marked by the infiltration of neutrophils into the mucosa. A central question in understanding the pathogenesis of amebic colitis is whether injury to the intestine results primarily from the infection with E. histolytica or from the host inflammatory response.
To dissect the mechanisms by which infection with E. histolytica damages the intestinal mucosa in vivo, we developed a severe combined immunodeficient mouse-human intestinal xenograft (SCID-HU-INT) model of E. histolytica infection (17, 18). Fetal human intestine transplanted onto the back of a SCID mouse becomes extensively vascularized and, over a 10-week period, develops into morphologically normal human intestine. Infection of these grafts by intraluminal installation of E. histolytica trophozoites results in extensive tissue damage and the development of an intense inflammatory response. Using this approach, it was previously reported that infection with E. histolytica trophozoites results in the increased production of IL-1β and IL-8 (17, 18). Intralumenal administration of an antisense oligonucleotide to the human p65 subunit of nuclear factor κB blocks the production of IL-1 and IL-8 and inhibits neutrophil influx into the E. histolytica-infected intestinal xenografts (18). Inhibition of the gut inflammatory response by this antisense oligonucleotide also blocks the increase in intestinal permeability observed with ameba infection.
Prostaglandins are thought to mediate part of the intestinal response to injury, including vasodilation, enhanced chloride secretion, and enhanced vascular permeability (1). The enzyme cyclooxygenase (Cox) constitutes the rate-limiting step in the production of prostaglandins. There are two Cox isoforms: Cox-1 is a constitutive enzyme expressed in crypt epithelial cells and a variety of other cell types in the intestine, and Cox-2 is an inducible enzyme that is found at low levels in the normal intestine but at much higher levels in a variety of models of intestinal inflammation (4, 16, 19, 20). Cox-2 is expressed in villus epithelial cells in Crohn's ileitis and in surface epithelial cells of the colon in ulcerative colitis and Crohn's colitis (19). It is induced in intestinal epithelial cell lines infected with salmonellae and in the SCID-HU-INT model when the xenograft is infected with salmonellae (4). In this study we have used the SCID-HU-INT mouse model to determine if infection with amebae is associated with the induction of Cox-2 expression and if the prostaglandins produced through Cox-2 contribute to the intestinal injury associated with ameba infections.
MATERIALS AND METHODS
Production of SCID-HU-INT mice.
Fetal human intestine sections were engrafted into the rear flanks and suprascapular regions of SCID mice (6 to 8 weeks of age) as previously described (17, 18). The fetal tissues (day 84 to 112 of gestation), which were destined for discard following prostaglandin-saline-induced therapeutic abortions, were obtained from the Central Laboratory for Human Embryology of the University of Washington. Grafts were allowed to develop for at least 8 weeks before use. Isografts were removed by blunt dissection after the skin was incised. The isografts came out easily. A portion of each isograft was sent for histology. Experimental procedures were approved by the Animal Studies Committee and Human Studies Committee of Washington University School of Medicine.
Infection of human intestinal xenografts.
Human intestinal xenografts were infected with E. histolytica trophozoites as previously described (17, 18). In brief, log-phase cultures of E. histolytica HM1:IMSS trophozoites were chilled on ice for 10 min, pelleted by centrifugation at 500 × g for 5 min, and resuspended in B1-S-33 medium at 106 trophozoites per 100 μl. One hundred microliters of the amebic suspension was injected directly into the lumen of the grafts via a 26-gauge needle. A small square of gelfoam was placed over the injection site upon removal of the needle, in order to prevent leakage.
Immunohistochemistry.
For immunohistochemical localization of human Cox-2 (19) or the serine-rich E. histolytica protein (SREHP) (21), deparaffinized sections of Bouin's fixed tissue were incubated with a 1:10,000 dilution of rabbit anti-human Cox-2 (Oxford Biomedical, Oxford, Miss.) or a 1:20,000 dilution of rabbit anti-SREHP (21). Endogenous peroxidase activity was quenched with 3% hydrogen peroxide. Nonspecific staining was blocked by incubating sections sequentially in blocking buffer (NEN, Boston, Mass.), avidin-D solution (Vector Laboratories, Burlingame, Calif.), biotin-D (Vector Laboratories), and normal donkey serum (Sigma, St. Louis, Mo.) before incubating slides overnight at 4°C with primary antibodies. Sections incubated with preimmune rabbit serum (Oxford Biomedical) or without primary antibody served as negative controls. A 1:2,000 dilution of biotinylated donkey anti-rabbit immunoglobulin G (Jackson Immuno Research Laboratories, West Grove, Pa.) was used as the secondary antibody. Signal amplification was carried out with the indirect biotin tyramide system (NEN). The amplified antibody signal was detected using 3,3′-diaminobenzidine tetrahydrochloride (Vector Laboratories).
SDS-PAGE and Western blot analysis of Cox-1 and Cox-2.
Xenografts isolated from ameba-infected and uninfected human xenografts were assayed for Cox-1 and Cox-2 by Western blotting. Samples for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were homogenized in a proteinase inhibitor cocktail containing 25 μg of antipain/ml, 25 μg of aprotinin/ml, 25 μg of leupeptin/ml, 25 μg of chymostatin/ml, 50 μmol of phenanthrolene/liter, 10 μg of pepstatin A/ml, and 2 nmol of dithiothreitol/liter in 20 mmol of N-Tris (hydroxymethyl)-methyl-2-aminoethane sulfonic acid/liter, pH 7.4. An aliquot of the homogenate was used for measuring protein concentration by the method of Lowry et al. (10). A portion of the homogenate was mixed with SDS-PAGE sample buffer (pH 6.8) containing Tris (62.5 mmol/liter), glycerol (10%), SDS (2%), bromophenol blue (1%), and β-mercaptoethanol (5%). Samples in SDS-PAGE sample buffer were snap-frozen in an acetone-dry ice bath, allowed to thaw, and then heated in a boiling water bath for 10 min. Equal amounts of protein from infected and noninfected human xenograft lysates and positive control (purified sheep Cox-1 and Cox-2) were separated by electrophoresis on SDS–8% polyacrylamide gels. After electrophoresis the separated protein were transferred to a polyvinylidene fluoride membrane (Immobilon-P; Millipore Corp., Bedford, Mass.). Rabbit polyclonal antibodies against sheep seminal vesicle Cox-1 (no. 241; Merck-Frosst Laboratories, Kirkland, Quebec, Canada) and sheep placental Cox-2 (no. 24; Merck-Frosst Corp.) were used to detect bands corresponding to Cox-1 and Cox-2, respectively. Bound antibody was visualized using a donkey anti-rabbit immunoglobulin G linked to horseradish peroxidase and an ECL kit (Amersham, Arlington Heights, Ill.) with fluorographic detection on BioMax ML film (Kodak, Rochester, N.Y.).
Cox inhibitors.
Indomethacin (Sigma) was dissolved in ethanol and diluted into sterile 5% sodium bicarbonate immediately before use. Indomethacin was administered at a dose of 1.5 mg/kg by intraperitoneal injection every 8 h. NS-398 (Biomol, Plymouth Meeting, Pa.) was dissolved as for indomethacin and administered at a dose of 1 mg/kg intraperitoneally every 8 h (12).
Measurements of PGE2 levels.
Lipids were extracted from the xenografts by homogenizing flash-frozen tissue in 70% cold ethanol–30% monobasic sodium phosphate (0.1 mol/liter), pH 4.0, followed by shaking incubation for 30 min at room temperature. Homogenates were centrifuged at 1,000 × g for 10 min. The aliquot of the supernatant was dried down under a stream of nitrogen, and the prostaglandin E2 (PGE2) concentration was determined by a PGE2-specific enzyme-linked immunoassay (Cayman Chemical, Ann Arbor, Mich.) according to the manufacturer's directions.
Myeloperoxidase (MPO) assay.
Tissue samples were homogenized for 30 s at a concentration of 50 mg/ml in a solution of phosphate-buffered saline (PBS) containing 1 μg each of aprotinin, leupeptin, and pepstatin A/ml. Samples were spun at 12,000 × g for 15 min, and the pellet was resuspended in the same volume of 80 mM sodium phosphate–1% hexadecyltrimethylammonium bromide (Sigma)–5 mM EDTA, pH 5.4. Samples were subjected three times to a freeze-thaw cycle and spun at 2,000 × g for 15 minutes, and the supernatants were frozen until the time of assay. A 25-μl aliquot of the supernatant was combined with 125 μl of 80 mM sodium phosphate, pH 5.4, and 25 μl of 1.28 mM 3,3′,5,5′-tetramethylbenzidine dihydrochloride (Sigma) in dimethyl sulfoxide. Twenty-five microliters of H2O2 in 80 mM sodium phosphate was added immediately prior to analysis to yield a final concentration of 0.24 mM and a final reaction volume of 200 μl. Conversion of the substrate was read at 650 nm. Dilutions of purified myeloperoxidase (Sigma) were used as standards.
IL-8 assay.
Protein samples for enzyme-linked immunosorbent assay for IL-8 were prepared by homogenizing tissue at 50 mg/ml in a solution of PBS containing 1 μg each of aprotinin, leupeptin, and pepstatin A (Sigma)/ml (17). The homogenized samples were centrifuged at 12,000 × g for 15 min. Supernatants were processed for enzyme-linked immunosorbent assay according to the manufacturer's protocol (Endogen, Woburn, Mass.). The sensitivity was 2 pg/ml for IL-8.
Measurement of intestinal permeability.
Dextrans of approximately 4,000 Da, labeled with either fluorescein isothiocyanate (FITC) or tetramethylrhodamine isothiocyanate (TRITC), were purchased from Sigma and resuspended in endotoxin-free PBS at a concentration of 10 mg/ml (18). Four hours prior to sacrifice, SCID-HU-INT mice were anesthetized and the renal pedicle was tied off to prevent excretion of the fluorophore. Subsequently, 50 μl of the FITC-dextran or TRITC-dextran solution was injected directly into the lumen of the human intestinal xenograft. At various times following injection of the fluorophore, animals were bled, and 20 μl of blood was diluted in 400 μl of 150 mM NaCl–50 mM Tris, pH 10.3, and spun at 2,000 × g for 15 min. The supernatants were analyzed on a Cytofluor 23000 fluorescent plate reader (Millipore).
RESULTS
Infection of human intestinal xenografts with E. histolytica induces the expression of Cox-2.
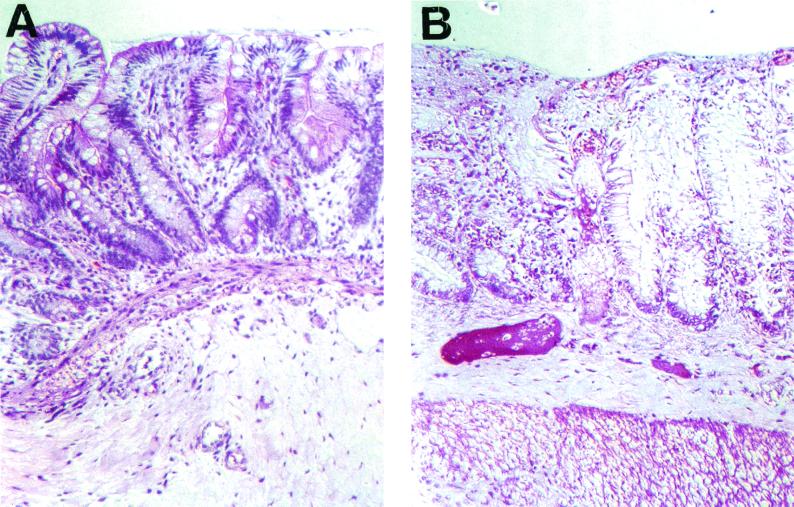
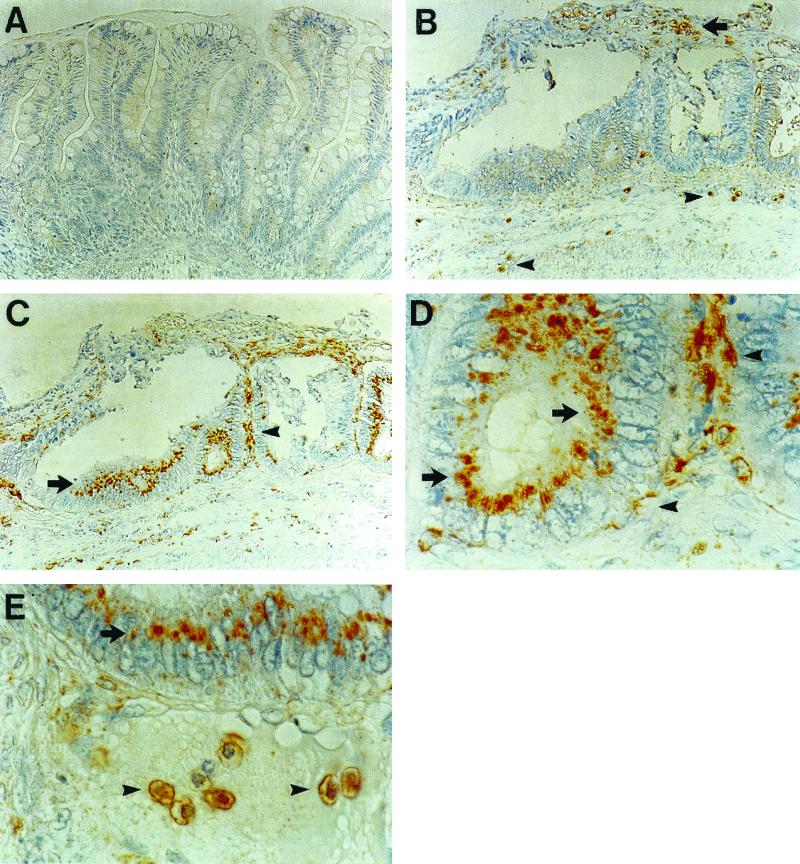
The uninfected xenograft shows an intact epithelium, regular crypt villus structure, and mucous droplets in the enterocytes (Fig. 1A). We used xenografts injected with medium or isografts that were never injected as uninfected controls. We did not see a difference in any parameter (IL-1 production, IL-8 production, MPO levels, or intestinal permeability) with medium injected and uninjected isografts. Infection of fetal human intestinal xenografts with E. histolytica trophozoites results in superficial ulceration, mucus depletion, disruption of crypt architecture, edema in the lamina propria and submucosa, and dilated veins (Fig. 1B). Uninfected human intestinal xenografts express little, if any, Cox-2 (Fig. 2A). Twenty-four hours after infection of the isograft, E. histolytica trophozoites were seen in the debris over an ulcer and in the submucosa (Fig. 2B). After infection with E. histolytica trophozoites there was expression of Cox-2 in intestinal epithelial cells and in cells in the lamina propria (Fig. 2C). Epithelial cell Cox-2 expression was patchy; there were crypts in which almost all of the epithelial cells expressed Cox-2 and other crypts with sparse Cox-2 expression. There was not a precise correlation between amebic infection and epithelial Cox-2 expression. Although there was epithelial Cox-2 expression adjacent to most invading amebae, there were some amebae with no Cox-2-expressing epithelial cells in the immediate area. In intestinal epithelial cells, Cox-2 was expressed in the cytoplasm between the nucleus and the apical portion of the plasma membrane; this distribution is consistent with expression in the Golgi (Fig. 2D). Some of the Cox-2-expressing cells in the lamina propria resembled myofibroblasts; these cells were long and flat and situated directly below the epithelial cells. Other Cox-2-expressing cells in the lamina propria looked more like macrophages. These cells were rounder and not immediately adjacent to the epithelial cells. There were areas in which amebae were found directly below the basement membrane of crypts in which epithelial cells expressed Cox-2 (Fig. 2E). In these cases, all of the identified amebae were situated below the basement membrane associated with the epithelium; there were no amebae identified either in or between epithelial cells.
FIG. 1.
Light microscopy of hematoxylin-and-eosin-stained uninfected fetal lumen intestinal xenograft (A) and a xenograft 24 h after infection with E. histolytica trophozoites (B).
FIG. 2.
Immunohistochemistry for Cox-2 (A, C, D, and E) and SREHP (B and E). (A) Cox-2 immunohistochemistry in uninfected xenograft. (B) Immunohistochemistry for SREHP 24 h after infection with E. histolytica trophozoites. Trophozoites are in debris over an ulcer (arrow) and in the submucosa (arrowheads). (C) Cox-2 immunohistochemistry in a xenograft 24 h after infection with trophozoites. There is staining of crypt epithelial cells (arrow) and of cells in the lamina propria (arrowhead). (D) High-power view of an area in panel C showing Cox-2 staining in crypt epithelial cells (arrow) and lamina propria cells (arrowheads). (E) Staining for Cox-2 in crypt epithelial cells (arrow) and staining for SREHP (arrowheads). Magnification, ×200 (A through C) and ×1,000 (D and E).
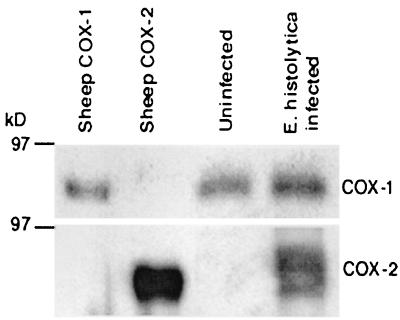
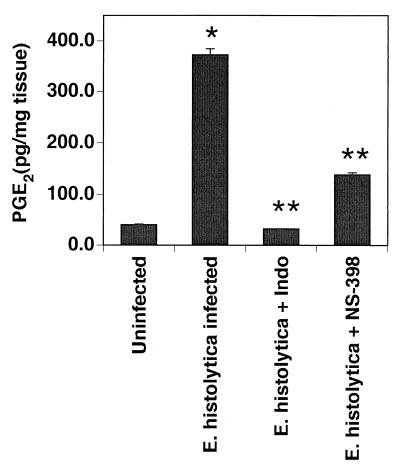
Western blots for Cox-1 demonstrated equivalent levels of Cox-1 in uninfected and ameba-infected xenografts (Fig. 3). Uninfected isografts did not express Cox-2, but there was strong staining for Cox-2 in ameba-infected xenografts. The increase in Cox-2 expression was associated with a 10-fold increase in PGE2 levels in ameba-infected isoografts compared with uninfected xenografts (Fig. 4). Administration of indomethacin, which inhibits both Cox-1 and Cox-2, resulted in a >90% reduction in PGE2 levels in ameba-infected xenografts. Administration of NS-398, which inhibits only Cox-2, resulted in a 70% reduction in PGE2 levels. This suggests that most but not all of the increased PGE2 production associated with ameba infection is produced through Cox-2.
FIG. 3.
Detection of Cox-1 and Cox-2 in uninfected and E. histolytica-infected human intestinal xenografts by Western blotting. A total of 106 E. histolytica HM1:IMSS trophozoites (106/100 μl) were injected into the lumens of xenografts. Host mice were killed 24 h later, and the human xenograft tissue was harvested and homogenized. Cox-1 and Cox-2 proteins were detected using rabbit anti-sheep antibodies. Purified sheep Cox-1 and sheep Cox-2 proteins served as positive controls.
FIG. 4.
Production of PGE2 by human intestinal xenografts in response to infection with E. histolytica is largely blocked by pretreatment of host mice with indomethacin (Indo; 1.5 mg/kg every 8 h) or NS-398 (1 mg/kg every 8 h). Mice received indomethacin, NS-398, or vehicle beginning 1 h prior to infection of the xenograft with trophozoites; 24 h after infection, the mice were killed and the PGE2 content of human intestinal xenograft tissue was determined by enzyme immunoassay. Data are means ± standard errors of the means for 5 to 12 xenografts. ∗, P < 0.001 compared with uninfected xenograft alone; ∗∗, P < 0.001 compared with E. histolytica alone.
Inhibition of Cox-2 diminishes the level of neutrophil infiltration induced by amebic infection.
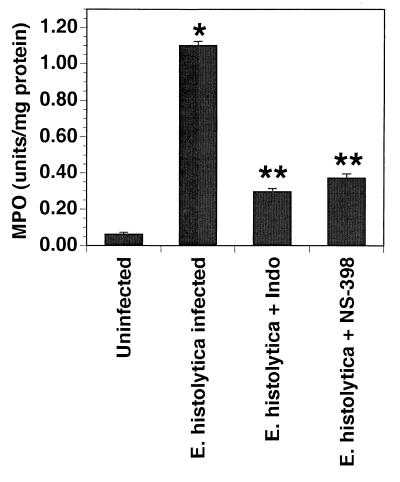
We have previously demonstrated that amebic infection in the SCID-HU-INT system is associated with a neutrophil infiltration. We quantified neutrophil influx into E. histolytica-infected human intestinal xenografts by measuring the MPO activity of the grafts. The enzyme MPO is present almost exclusively in the azurophilic granules of neutrophils; therefore, MPO activity in a given tissue can be used to quantify the number of neutrophils present in that tissue. Infection with amebae is associated with an 18-fold increase in MPO activity compared with uninfected xenografts (Fig. 5). Administration of indomethacin or NS-398 results in 77 and 66% reductions, respectively, in MPO activity in ameba-infected xenografts. These data suggest that the increase in neutrophil infiltration is mediated by prostaglandins produced through Cox-2.
FIG. 5.
Inflammatory response of human intestinal xenografts to E. histolytica is inhibited by treatment with indomethacin (Indo; 1.5 mg/kg every 8 h) or NS-398 (1 mg/kg every 8 h). Xenografts were harvested 24 h after infection with E. histolytica or sham infection with media alone. MPO activity was measured as described in Materials and Methods. Data are means ± standard errors of the means for 4 to 12 xenografts. ∗, P < 0.001 compared with uninfected xenograft; ∗∗, P < 0.001 compared with E. histolytica alone.
Inhibition of Cox-2 blocks the increase in IL-8 associated with amebic infections.
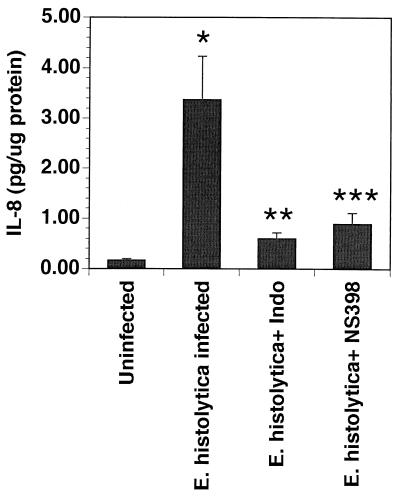
We had previously demonstrated that amebic infection induces increased production of IL-8 in the xenografts. In these experiments (Fig. 6), amebic infections induced an 18-fold increase in IL-8. Pretreatment of the host mice with either indomethacin or NS-398 resulted in an 80 to 90% inhibition of the increase in IL-8 induced by amebic infection.
FIG. 6.
Production of IL-8 by human intestinal xenografts in response to infection with E. histolytica is reduced by pretreatment of host mice with indomethacin (Indo; 1.5 mg/kg every 8 h) or NS-398 (1 mg/kg every 8 h). Twenty-four hours after infection, mice were killed and the IL-8 content of human intestinal xenograft tissue was determined by enzyme immunoassay. Data are means ± standard errors of the means for 6 to 11 xenografts. ∗, P < 0.01 compared with uninfected xenograft; ∗∗, P < 0.01 compared with E. histolytica alone; ∗∗∗, P < 0.03 compared with E. histolytica alone.
Inhibition of cyclooxygenase-2 in human intestinal xenografts blocks the increases in intestinal permeability with E. histolytica infection.
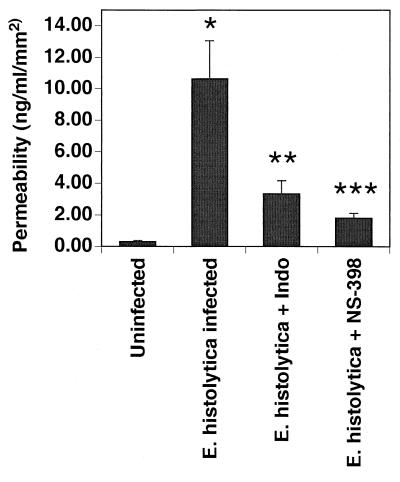
To quantify damage to the intestinal barrier we measured the permeability of the intestinal xenograft to a fluorescently labeled macromolecule, FITC-dextran. Uninfected human intestinal xenografts show little permeability to FITC-dextran as measured by the amount of FITC-dextran detected in the systemic circulation 2 to 4 h after intraluminal inoculation of the compound. In contrast, E. histolytica-infected human intestinal xenografts show an increased permeability to FITC-dextran that can be detected as early as 2 h after FITC injection. There is a >100-fold increase in permeability to FITC-dextran in ameba-infected xenografts compared with uninfected xenografts (Fig. 7). Administration of indomethacin or NS-398 results in 69 and 83% reductions, respectively, in the permeability to FITC-dextran induced by amebic infection. These data suggest that the increase in permeability to FITC-dextran induced by amebic infection is mediated in part through prostaglandins produced through Cox-2.
FIG. 7.
Epithelial barrier integrity is maintained in E. histolytica-infected human intestinal xenografts treated with indomethacin (Indo; 1.5 mg/kg every 8 h) or NS-398 (1 mg/kg every 8 h). The integrity of the epithelial barrier, as measured by the flux of fluorescently labeled dextrans from the lumen of the human intestinal xenograft into the systemic circulation, is shown for SCID-HU-INT mice whose intestinal xenografts were infected for 24 h with E. histolytica or sham infected with medium alone. Infection with E. histolytica resulted in a marked increase flux of dextran. Treatment with either indomethacin (1.5 mg/kg every 8 h) or NS-398 (1 mg/kg every 8 h) resulted in a flux of dextran that was significantly lower than that seen in xenografts receiving E. histolytica alone. Data are means ± standard errors of the means for five xenografts. ∗, P < 0.001 compared with uninfected xenograft; ∗∗, P < 0.02 compared with E. histolytica alone; ∗∗∗, P < 0.01 compared with E. histolytica alone.
DISCUSSION
In this study we have used the SCID-HU-INT model to demonstrate that infection with E. histolytica results in the induction of Cox-2 expression in epithelial cells and macrophages. Moreover, selective inhibition of Cox-2 reduces both the neutrophil infiltration and the enhanced intestinal permeability seen in E. histolytica infection. These findings suggest that the prostaglandins produced through Cox-2 participate in the mediation of the host inflammatory response to E. histolytica and that blocking the prostaglandin component of the host inflammatory response diminishes the tissue damage observed with amebic infection. A study published in 1990, prior to the identification of Cox-2, reported that incubation of amebic lysates with rat colonic strips resulted in PGE2 production, but the source of the PGE2 was not assessed (13).
There are striking similarities in the epithelial cell responses to infection with E. histolytica and infection with salmonellae and other invasive bacteria (2, 6). Cox-2 is also induced by infection of epithelial cells with Cryptosporidium parvum (9). Infection with each of the invasive bacteria results in a similar epithelial response with the upregulated expression of a group of genes, including those for proinflammatory cytokines (IL-1α and IL-6), chemokines (IL-8 and GROα), growth factors (GM-CSF), and Cox-2 (2, 6). The similarity in the genes induced by salmonella and ameba infections suggests that there is a programmed epithelial response to infection with invasive organisms and that there is a common signaling mechanism controlling the expression of these genes. Each of these upregulated genes is a target gene of the transcription factor NF-κB (5). The SCID-HU-INT model has been used previously to demonstrate that intraluminal administration of an antisense oligonucleotide to the human p65 subunit of NF-κB in xenografts infected with E. histolytica trophozoites resulted in diminished production of IL-1β and IL-8 (18). These findings raise the possibility that the induction of epithelial expression of each of these genes, including Cox-2, by amebic infection is mediated by NF-κB. Whether induction of Cox-2 expression in an epithelial cell occurs as a direct result of amebic invasion in that cell or as an indirect result of amebic invasion of neighboring cells is unclear. Cox-2 expression in epithelial cells is patchy, and there are amebae adjacent to some of the Cox-2-expressing epithelial cells; however, most of the Cox-2-expressing cells have no amebae in the immediate vicinity. This raises the possibility that an intermediate is involved in the induction of Cox-2. It is possible, for example, that amebic invasion induces the affected cell to release IL-1 (3, 18). The released IL-1 could then induce neighboring cells to express Cox-2. This model would explain the patchy nature of epithelial Cox-2 expression and would also explain the expression of Cox-2 by macrophages, as IL-1 induces Cox-2 in macrophages.
Amebic infection results in neutrophil infiltration, but the neutrophils and macrophages in this model are of mouse origin. Amebic infection induces epithelial cells to produce IL-8, which is a chemotactic agent for both human neutrophils and murine neutrophils (14, 23). It is possible that there is also induction of neutrophil chemotactic factors such as macrophage inflammatory protein-1 in the mouse macrophage and that these factors also promote neutrophil chemotaxis. We found that inhibition of Cox-2 resulted in diminished IL-8 production. We do have evidence that the host mouse macrophages are activated by amebic infection in this model in that they express Cox-2. It was somewhat surprising that administration of cyclooxygenase inhibitors markedly reduced neutrophil infiltration in that cyclooxygenase products are not chemotactic for neutrophils and PGE2 has been reported to inhibit the production of cytokines, particularly IL-1 (8). However, PGE2 stimulates IL-8 production in T84 cells, a human intestinal epithelial cell line, by a posttranslational mechanism (24). Thus, it is possible that inhibition of cyclooxygenase reduces neutrophil infiltration by reducing chemokine levels. The histology in the ameba-infected grafts in the animals treated with indomethacin or NS-398 demonstrated diminished inflammation but not a decrease in amebic infection or restitution of epithelial integrity.
We used the uptake of dextran (approximate mass, 4,000 Da), a measure of intestinal barrier function, as a marker of intestinal tissue damage in E. histolytica infection. Under normal conditions in adult animals, uptake of high-molecular-weight compounds from the intestine is extremely limited. Amebic infection results in an increase in permeability to dextran, and that increase is blocked by the administration of cyclooxygenase inhibitors. Intestinal barrier function can be compromised either by the loosening of the tight junctions between epithelial cells or by gross destruction of epithelial cells as is seen in amebic infection. Loosening of the tight junctions can occur in response to physiologic agents such as acetylcholine or in response to injury as in ischemia. In these systems, prostaglandins tend to diminish permeability. In contrast, in amebic infection, where the mechanism of increased permeability is enterocyte destruction, inhibition of prostaglandin synthesis decreases permeability.
The data presented here do not establish whether the increase in permeability to dextran and the neutrophil infiltration occur independently or if one mediates the other. However, in an earlier study we demonstrated that treatment with a neutrophil-depleting antibody blocked the increase in permeability to dextran in amebic infection in the SCID-HU-INT system (18). This suggests that damage caused by activated neutrophils mediates the increase in permeability to dextran. This also raises the possibility that the decrease in permeability to dextran seen with indomethacin and NS-398 may be mediated by the decrease in neutrophil infiltration induced by these drugs. As noted above, we previously reported that intraluminal administration of an antisense nucleotide to NF-κB in the SCID-HU-INT model blocked the expression of IL-1 and IL-8 in response to amebic infection (18). The NF-κB antisense nucleotide also blocked the neutrophil infiltration and the increased permeability to dextran associated with amebic infection. These findings combined with the results of the current study suggest that the effects of NF-κB activation on neutrophil influx and enhanced permeability in this model may be mediated through induction of Cox-2. PGE2 induces chloride secretion by colonic epithelium (15). It is likely that in amebic colitis, PGE2 produced through Cox-2 induces epithelial chloride secretion and contributes to the diarrhea characteristic of this disease.
The data presented here, combined with the results of earlier studies, suggest this sequence of events in the induction of tissue injury in amebic colitis. (i) Amebic invasion activates NF-κB in epithelial cells. (ii) Activation of NF-κB induces the expressions of proinflammatory cytokines (IL-1β and IL-6), chemokines (IL-8 and GRO-α), growth factors (GM-CSF), and Cox-2. (iii) Enhanced Cox-2 expression results in increased prostaglandin production. (iv) Increased prostaglandin production, presumably through increased chemokine production, results in neutrophil infiltration. (v) Neutrophil infiltration mediates epithelial injury, as demonstrated by increased passage of dextran out of the lumen. (vi) PGE2 produced through Cox-2 induces epithelial chloride secretion, contributing to the diarrhea associated with amebic colitis.
The data presented here suggest that the induction of Cox-2 mediates much of the host response to amebic infection, including enhanced epithelial permeability, chemokine production, and neutrophil recruitment. These findings raise a question as to whether pharmacologic inhibition of Cox-2 would block the host response to amebic infection. The consequences of this inhibition could be detrimental (delay in clearing the amebae) or beneficial (decreased inflammatory response and decreased tissue injury).
ACKNOWLEDGMENTS
This study was supported by NIH grants DK33165 and DK55753 (W.E.S.) AI30084 and AI01231 (S.L.S.), grant DK52574 from the Washington University Digestive Diseases Research Core Center, and the Center for Birth Defects Research at University of Washington HD00836. S.L.S. is a Burroughs Wellcome Scholar in molecular parasitology.
REFERENCES
- 1.Eberhart C E, Dubois R N. Eicosanoids and the gastrointestinal tract. Gastroenterology. 1995;109:285–301. doi: 10.1016/0016-5085(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 2.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckmann L, Reed S L, Smith J R, Kagnoff M F. Entamoeba histolytica trophozoites induce an inflammatory cytokine response by cultured human cells through the paracrine action of cytolytically released interleukin-1α. J Clin Investig. 1995;96:1269–1279. doi: 10.1172/JCI118161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckmann L, Stenson W F, Savidge T C, Lowe D L, Barrett K E, Fierer J, Smith J R, Kagnoff M F. Role of intestinal epithelial cells in the host secretory response to infection by invasive bacteria. J Clin Investig. 1997;100:296–309. doi: 10.1172/JCI119535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elewaut D, DiDonato J A, Kim J M, Truong F, Eckmann L, Kagnoff M F. NF-κB is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J Immunol. 1999;163:1457–1466. [PubMed] [Google Scholar]
- 6.Kagnoff M F, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Investig. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keene W E, Petitt M G, Allen S, McKerrow J H. The major neutral proteinase of Entamoeba histolytica. J Exp Med. 1986;163:536–549. doi: 10.1084/jem.163.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knudsen P J, Dinarello C A, Strom T B. Prostaglandins posttranscriptionally inhibit monocyte expression of interleukin 1 activity by increasing intracellular cyclic adenosine monophosphate. J Immunol. 1986;137:3189–3194. [PubMed] [Google Scholar]
- 9.Laurent F, Kagnoff M F, Savidge T C, Naciri M, Eckmann L. Human intestinal epithelial cells respond to Cryptosporidium parvum infection with increased prostaglandin H synthase 2 expression and prostaglandin E2 and F2α production. Infect Immun. 1998;66:1787–1790. doi: 10.1128/iai.66.4.1787-1790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 11.Martinez-Palomo A, Tsutsumi V, Anaya-Velazquez F, Gonzalez-Robles A. Ultrastructure of experimental intestinal invasive amebiasis. Am J Trop Med Hyg. 1989;41:273–279. [PubMed] [Google Scholar]
- 12.Masferrer J L, Zweifel B S, Manning P T, Hauser S D, Leahy K M, Smith W G, Isakson P C, Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci USA. 1994;91:3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGowan K, Piver G, Stoff J S, Donowitz M. Role of prostaglandins and calcium in the effects of entamoeba histolytica on colonic electrolyte transport. Gastroenterology. 1990;98:873–880. doi: 10.1016/0016-5085(90)90010-x. [DOI] [PubMed] [Google Scholar]
- 14.Pilewski J M, Yan H C, Juhasz I, Christofidou-Solomidou M, Williams J, Murphy G F, Albelda S M. Modulation of adhesion molecules by cytokines in vivo using human/severe combined immunodeficient (SCID) mouse chimeras. J Clin Immunol. 1995;15:122S–129S. doi: 10.1007/BF01540902. [DOI] [PubMed] [Google Scholar]
- 15.Racusen L C, Binder H J. Effect of prostaglandin on ion transport across isolated colonic mucosa. Dig Dis Sci. 1980;25:900–904. doi: 10.1007/BF01308038. [DOI] [PubMed] [Google Scholar]
- 16.Riehl T, Cohn S, Tessner T, Schloemann S, Stenson W F. Lipopolysaccharide is radioprotective in the mouse intestine through a prostaglandin-mediated mechanism. Gastroenterology. 2000;118:1106–1116. doi: 10.1016/s0016-5085(00)70363-5. [DOI] [PubMed] [Google Scholar]
- 17.Seydel K B, Li E, Swanson P E, Stanley S L., Jr Human intestinal epithelial cells produce proinflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect Immun. 1997;65:1631–1639. doi: 10.1128/iai.65.5.1631-1639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seydel K B, Li E, Zhang Z, Stanley S L., Jr Epithelial cell-initiated inflammation plays a crucial role in early tissue damage in amebic infection of human intestine. Gastroenterology. 1998;115:1446–1453. doi: 10.1016/s0016-5085(98)70023-x. [DOI] [PubMed] [Google Scholar]
- 19.Singer I I, Kawka D W, Schloemann S, Tessner T, Riehl T, Stenson W F. Cyclooxygenase-2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297–306. doi: 10.1016/s0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- 20.Smith W L, Dewitt D L. Prostaglandin endoperoxidase H synthases-1 and -2. Adv Immunol. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- 21.Stanley S L, Jr, Tian K, Koester J P, Li E. The serine rich Entamoeba histolytica protein (SREHP) is a phosphorylated membrane protein containing O-linked terminal N-acetylglucosamine (O-GlcNAc) residues. J Biol Chem. 1995;270:4121–4126. doi: 10.1074/jbc.270.8.4121. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi A, Phillips B P. Electron microscope studies of experimental Entamoeba histolytica infections in the guinea pig. I. Penetration of the intestinal epithelium by trophozoites. Am J Trop Med Hyg. 1975;24:34–48. doi: 10.4269/ajtmh.1975.24.34. [DOI] [PubMed] [Google Scholar]
- 23.Yan H C, Juhasz I, Pilewski J, Murphy G F, Herlyn M, Albelda S M. Human/severe combined immunodeficient mouse chimeras. An experimental in vivo model system to study the regulation of human endothelial cell-leukocyte adhesion molecules. J Clin Investig. 1993;91:986–996. doi: 10.1172/JCI116320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Y, Chadee K. Prostaglandin E2 stimulates IL-8 gene expression in human colonic epithelial cells by a posttranscriptional mechanism. J Immunol. 1998;161:3746–3752. [PubMed] [Google Scholar]