Abstract
Mice with severe combined immunodeficiency (scid mice) and infected with the relapsing fever agent Borrelia turicatae develop manifestations that resemble those of disseminated Lyme disease. We have characterized two isogenic serotypes, A and B, which differ in their variable small proteins (Vsps) and disease manifestations. Serotype A but not serotype B was cultured from the brain during early infection, and serotype B caused more severe arthritis, myocarditis, and vestibular dysfunction than serotype A. Here we compared the localization and number of spirochetes and the severity of inflammation in scid mice, using immunostained and hematoxylin-and-eosin-stained coronal sections of decalcified heads. Spirochetes in the brain localized predominantly to the leptomeninges, and those in peripheral tissues localized mainly to the extracellular matrix. There were significantly more serotype A than B spirochetes in the leptomeninges and more serotype B than A spirochetes in the skin. The first tissue where spirochetes were observed outside the vasculature was the dura mater. Inflammation was more severe in the skin than in the brain. VspA, VspB, and the periplasmic flagellin protein were expressed in all tissues examined. These findings indicate that isogenic but antigenically distinct Borrelia serotypes can have marked differences in their localization in tissues.
Relapsing fever is a disease of humans caused by several species of the genus Borrelia (3). During infection there may be several febrile periods and spirochetemia separated by periods of well-being. The disease is also notable for involvement of the nervous system; manifestations include encephalitis, meningitis, peripheral and cranial neuritis, myelitis, and neuropsychiatric disturbances (9). These bacteria persist in the host through antigenic variation of single surface lipoproteins of two types: variable small proteins (Vsps) of about 23 kDa, and variable large proteins (Vlps) of about 38 kDa. We found that mice with severe combined immunodeficiency (scid mice) infected with Borrelia turicatae, the agent of tick-borne relapsing fever in southwestern North America, develop manifestations that resemble those of disseminated Lyme disease (14). Two serotypes, A and B, differed in the Vsps they expressed and the disease they produced in mice. Overall, serotype B was more virulent than serotype A; it killed infant mice and caused severe arthritis, myocarditis, and vestibular dysfunction (14, 33). Serotype A, on the other hand, was more neurotropic: it infected the brain early in the infection, even though its density in the blood was 10-fold lower than that of serotype B.
Serotype A is defined by the expression of VspA, and serotype B is defined by the expression of VspB. The silent and expressed genes for VspA and VspB have been cloned and characterized (13, 33). VspA and VspB are part of a larger family of proteins that includes the Vsps of Borrelia hermsii and the OspCs of the Lyme disease spirochetes B. burgdorferi, B. afzelii, and B. garinii. The sequence of the B. turicatae vsp promoter downstream of the −35 element is highly similar to that of the OspC promoter of B. burgdorferi. VspA and VspB are antigenically distinct and differ at 40% of their residues, the greatest diversity being in their C-terminal halves. VspA shows higher hydrophobicity than VspB (13), and VspB exhibits an exceptionally basic pI (33). The expressed loci for vspA and vspB are located near the center of linear plasmids and are identical to their archival counterparts from the vsp itself to at least 13 to 14 kb downstream (34).
The greater arthritogenicity and overall greater virulence of serotype B appear to be the consequence of 10-fold-higher levels of serotype B spirochetes in the blood and joints than those for serotype A infections (32, 33). Less understood is the neurotropism of serotype A cells. Investigation of the localization of serotype A and B cells in the brain and surrounding tissues during early infection may provide a basis for understanding the invasion of the brain by spirochetes. As an initial step toward this goal, we compared the localization of spirochetes using light microscopy of immunostained coronal sections of decalcified heads. The results indicate that isogenic but antigenically distinct serotypes can show marked differences in their localization during infection.
MATERIALS AND METHODS
Strains and culture conditions.
B. turicatae was isolated by injecting Swiss mice with tissues from Ornithodoros turicata collected in a cave near Ozona, Tex. (14). Serotypes A and B have been previously described (13, 14, 33). Borrelias were cultured in BSK II medium with 12% rabbit serum (2) and counted in a Petroff-Hausser chamber under phase-contrast microscopy (41). When tissue samples were cultured, rifampin (50 μg/ml) and phosphomycin (100 μg/ml) were present in the medium. Plasma samples from infected mice were either frozen with 10% dimethyl sulfoxide at −80°C or used to start broth cultures, which at cell densities of 108 per ml were aliquoted and similarly frozen until use. The purity of the populations was assessed before infection by Western blotting with serotype-specific monoclonal antibodies (13, 33).
Protein analysis.
Whole cells from harvested cultures were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) with 12.5% acrylamide (5). For Western blot analysis, proteins were transferred to nitrocellulose membranes (Millipore), which were then blocked with 3% (wt/vol) dried nonfat milk in 10 mM Tris (pH 7.4)–150 mM NaCl (milk/TS) for 4 h (37). After the membranes were washed three times with 0.3% milk/TS, they were incubated with polyclonal antisera diluted 1:50,000 in 0.3% milk/TS. Alkaline phosphatase-conjugated anti-rabbit antibody (Pierce) served as the second ligand. The blots were developed with nitroblue tetrazolium chloride–5-bromo 4-chloro-3-indolylphosphatase p-toluidine salt as the substrate (Pierce).
Mouse infections.
Four- to six-week-old female CB-17 scid mice (Charles River Laboratories, Houston, Tex.) were inoculated intraperitoneally with 103 borrelia suspensions in 300 μl of phosphate-buffered saline (PBS). Animals sham inoculated with PBS were used as controls. The housing and care of animals were in accordance with the Animal Welfare Act and federal guidelines (27a) in facilities accredited by the American Association for Accreditation of Laboratory Animal Care. For evaluation of spirochetemia, tail vein blood was mixed with an equal volume of PBS and examined by dark-field microscopy. scid mice were maintained in a germ-free environment before and after infection.
Tissue and fluid collection.
Mice were anesthetized with methoxyflurane for euthanasia. Sodium citrate was used as anticoagulant for exanguination by heart puncture. Total-body perfusion with 30 ml of PBS was performed as described previously (10), followed by a second perfusion with 30 ml of 4% paraformaldehyde. The seven mice used to culture blood and brain were not perfused with paraformaldehyde. For brain cultures, after the skull was opened under sterile conditions, brain tissue was removed and rinsed three times with 1 ml of PBS in sterile 2-ml microcentrifuge tubes (Sarstedt, Newton, N.C.) (14). To the brain tissue sample was added twice its volume of BSK II medium. With plungers of sterile 1-ml plastic syringes (Becton Dickinson, Rutherford, N.J.), the brain tissue was homogenized in the tubes and then suspended in BSK II medium by briefly vortexing. Blood and brain suspensions were centrifuged for 5 s at 7,000 × g prior to inoculation into culture tubes. All cultures were examined for a 2-week period.
Histopathological studies.
The head and spinal cord were removed after the skin was peeled off at necropsy. The tissues were fixed in 4% paraformaldehyde for 48 h at 4°C and then decalcified in 20% EDTA in PBS (pH 7.4) for 3 weeks. The EDTA solution was changed weekly. The heads were divided in four coronal sections and embedded in paraffin. The spinal cords were divided in four axial sections and also embedded in paraffin. Paraffin-embedded sections were sectioned at 5 μm. Hematoxylin-and-eosin (H&E)-stained slides were prepared by standard technique. All tissue sections were examined with standard light microscopy by a neuropathologist masked to the infectious status. The spirochetal load was measured after immunostaining (see below) by counting the total number of spirochetes in each of 10 400× and five 200× consecutive paraffin sections in the head and spinal cord, respectively. Spirochetes were defined by their characteristic spiral morphology and positive staining with the chromogen 3,3-diaminobenzidine tetrahydrochloride. Inflammation was defined by the characteristic morphology of mononuclear inflammatory cells on H&E staining. Ten and five 200× microscopic fields were scored per tissue per mouse in the head and spinal cord, respectively, as follows: 0, no inflammation; 1, single infiltrate; 2, small multifocal infiltrates; 3, large multifocal infiltrates; 4, confluent multifocal infiltrate; or 5, diffuse inflammation.
Immunohistochemistry.
Precleaned superfrost glass slides (Fisher Scientific) were used for immunohistochemistry. A three-step streptavidin-peroxidase technique was used to determine the localization of spirochetes in tissues. All reactions were performed at room temperature manually or in an automatic immunostainer (Biogenex, San Ramon, Calif.). Nonspecific binding was reduced by blocking slides with Biogenex blocking solution for 15 min. Endogenous peroxidase activity was reduced by incubation with 3% H2O2 for 20 min at room temperature. Formalin-fixed sections were treated with protease type VIII (0.5 mg/ml; Sigma P-5380) for 10 min for antigen retrieval. A 1:103 dilution of hyperimmune rabbit serum from rabbit infected with B. burgdorferi strain N40Br was used for detection of borrelias (11, 28). This hyperimmune serum has high titers of anti-B. burgdorferi antibodies by enzyme-linked immunosorbent assay (ELISA) and Western blotting and cross-reacts with relapsing fever Borrelia spp. (not shown). Rabbit polyclonal antibody anti-recombinant VspA and VspB at a 1/10,000 dilution were used for detection of expression of VspA and VspB in tissues (43). Preliminary studies indicated that these polyclonal antibodies were specific at dilutions ≥1/10,000 but were cross-reactive at lower dilutions. Commercially available rat monoclonal antibody anti-mouse macrophages (F4/80; Serotec, Kidlington, United Kingdom) at a 1/1,000 dilution was used for identification of monocytes. For the localization of flagellin, we used mouse monoclonal antibody H9724 (4). The secondary reagent was a biotinylated goat anti-rabbit, goat anti-mouse, or rat anti-mouse polyclonal antibody (Biogenex). The tertiary reagent was horseradish peroxidase-labeled streptavidin (Biogenex). Incubation times were 30 min for the primary antibody and 20 min for the secondary and tertiary reagents. The chromogen was 3,3-diaminobenzidine tetrahydrochloride in 0.24% H2O2 for 5 to 15 min. The counterstain was Mayer's hematoxylin for 1 min. In some experiments, the counterstain was not used to facilitate visualization of spirochetes. Each incubation was separated by three washes with OptiMax wash buffer (Biogenex). Tissue sections from uninfected animals were used as negative controls.
Statistical analysis.
Results are given as mean (95% confidence interval of the mean). Nonparametric tests (Mann-Whitney Test) were used to determine whether the differences in mean number of spirochetes between tissues was significant. Chi-square analysis was used to assess the difference in the proportions of tissues where spirochetes were observed.
RESULTS
Infection of blood and brain.
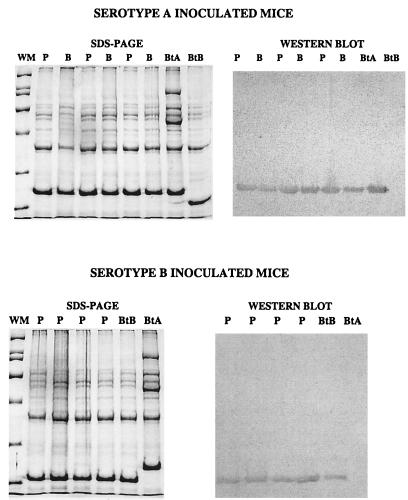
Our goal was to compare the localization of spirochetes in the nervous system and other tissues of scid mice infected with neurotropic and nonneurotropic serotypes of B. turicatae. For this, scid mice were inoculated intraperitoneally with 103 cells of serotype A (n = 9) or serotype B (n = 10) of B. turicatae and sacrificed 6 (n = 4), 18 (n = 5 for serotype A and 6 for serotype B), and 40 (n = 4) days later. Four additional mice were sham inoculated with PBS and sacrificed 6 (n = 2) and 40 (n = 2) days after inoculation as controls. Spirochetemia was confirmed prior to necropsy by dark-field examination of tail vein blood (not shown). In previous studies, serotype A but not serotype B could be cultured from the brain at 4, 8, and 11 days after inoculation (14). To extend this observation, we examined plasma and brain infection by culture in three serotype A-inoculated and four serotype B-inoculated mice at day 18. The results showed plasma infection in three of three mice inoculated with serotype A and four of four mice inoculated with serotype B but brain infection only in the mice inoculated with serotype A. The serotypes present in plasma and brain were characterized by electrophoresis of whole-cell lysates of cultured spirochetes (Fig. 1, left panels). SDS-PAGE showed that a single serotype with a Vsp of the same size of that of serotype A (23 kDa) was present in plasma and brain cultures from all serotype A-inoculated mice; similarly, a single serotype with a Vsp of the same size of that of VspB (20 kDa) was present in all plasma cultures from serotype B inoculated mice (Fig. 1). To confirm the identity of these serotypes, whole-cell lysates of cultured spirochetes were examined by Western blotting with rabbit polyclonal antibodies to recombinant VspA and VspB at 1/50,000 dilution. The results confirmed that the serotypes present in plasma and/or brain cultures were serotype A or B (Fig. 1, right panels).
FIG. 1.
Left panels, Coomassie blue-stained proteins in whole-cell lysates of plasma (P) and brain (B) cultures from scid mice inoculated with serotype A (n = 3) (top) or serotype B (n = 4) (bottom) of B. turicatae and sacrificed 18 days later. Whole-cell lysates of serotype A (BtA) and serotype B (BtB) cells are shown to the right for comparison. The migration of the following molecular weight markers (WM) are shown to the left (in descending order): 200,000, 116,000, 66,000, 45,000, 31,000, and 21,000. Right panels, whole-cell lysates of the same P and B cultures and control BtA and BtB lysates, transferred to nitrocellulose membranes and probed with rabbit polyclonal antibody to recombinant VspA (top) and VspB (bottom) at a 1/50,000 dilution.
Localization of spirochetes in tissues.
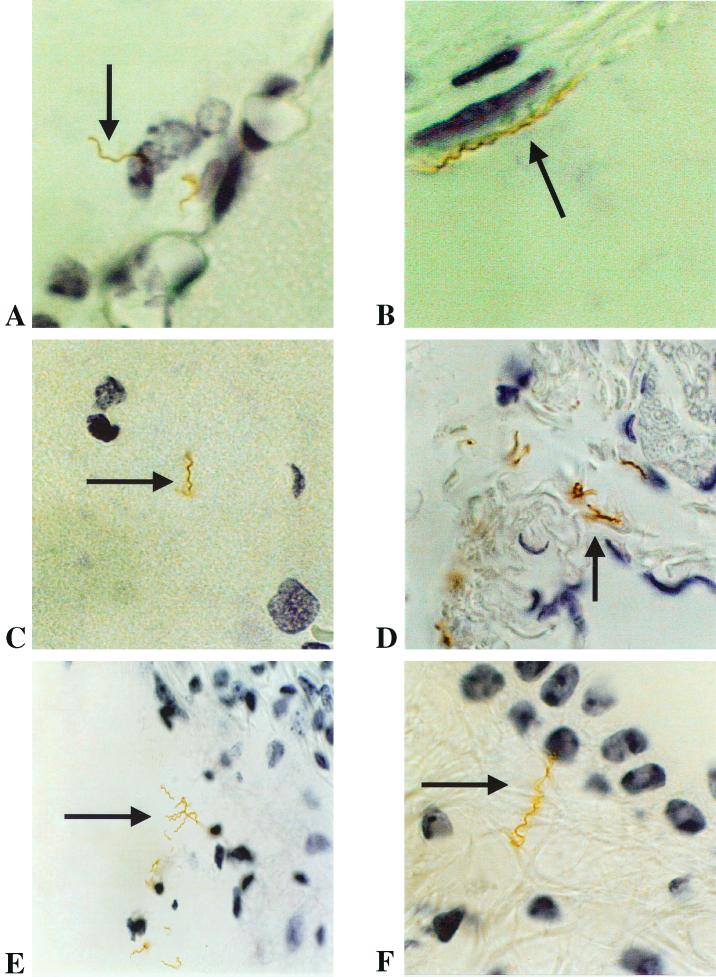
We next sought to study the localization of spirochetes in the brain and other tissues from mice infected with serotype A or B. For this, we used coronal sections of whole-decalcified heads at the level of the pituitary gland and the inner ear, immunostained with a rabbit polyclonal antibody to Borrelia spp. Multiple sections from two mice each inoculated with serotype A or serotype B and sacrificed 6, 18, and 40 days after inoculation were first examined by light microscopy for the presence of spirochetes. Spirochetes were found in several tissues at days 18 and 40 after inoculation. In the brain, the localization was primarily leptomeningeal (Fig. 2A), although rare spirochetes were also found in the brain parenchyma (Fig. 2C). The spirochetes were not limited to the central nervous system. They were also present in the dura mater in the convexity (Fig. 2B) and at the base of the brain, in the middle and inner (Fig. 2E and F) ear, in the endoneurium, in the skin, and in the bone marrow. Clumps of spirochetes were observed only in the subarachnoid space, inner ear (Fig. 2E), and skin (Fig. 3B). Spirochetes were also found in the extracellular matrix of skeletal and cardiac muscle and in peripheral and cranial nerves, notably the VII (facial) cranial nerve (Fig. 2D). No spirochetes were found in salivary glands. Intravascular spirochetes and red blood cells were infrequent, confirming that exanguination and perfusion were adequate. No spirochetes were seen in any of the PBS-inoculated controls.
FIG. 2.
B. turicatae in the leptomeninges (A; magnification, ×900), dura mater (B; ×900), brain parenchyma (C; ×750), peripheral facial (VII) nerve (D; ×750), and cochlea next to the origin of the auditory nerve (E; ×500) and in a semicircular canal at the origin of the vestibular nerve (E; ×900) in scid mice 40 days after inoculation (immunohistochemistry with anti-Borrelia polyclonal antibody).
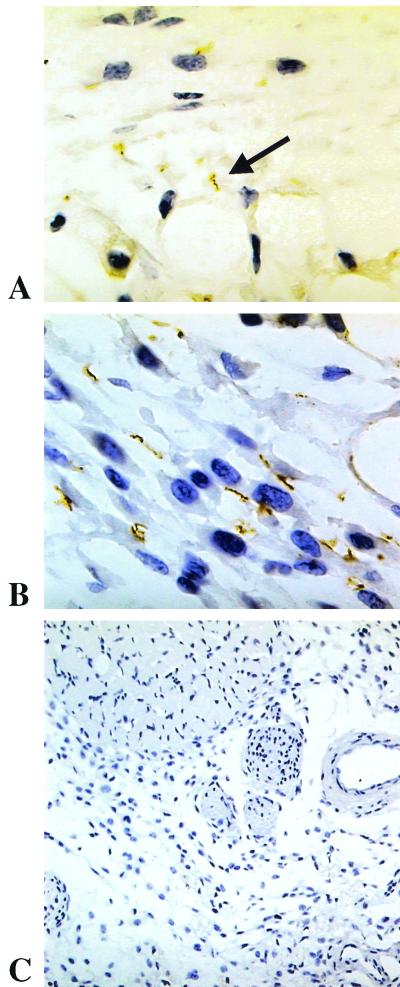
FIG. 3.
Immunostaining for Vsp in skin from scid mice infected with B. turicatae serotypes A (A; anti-VspA rabbit polyclonal antibody; magnification, ×750), serotype B (B; anti-VspB rabbit polyclonal antibody; ×750), and PBS as a control (C; anti-VspB rabbit polyclonal antibody; ×400).
We next determined the number of spirochetes in 20 consecutive 400× microscopic fields of different tissues of mice infected with serotype A or B at days 6, 18, and 40 after inoculation (Table 1). Rare spirochetes were seen in the brain parenchyma, leptomeninges, dura mater, and skin 6 days after inoculation. The numbers were higher in the dura mater at day 6. Eighteen days after inoculation, the number of spirochetes was significantly higher in the leptomeninges for serotype A-infected than for serotype B-infected mice and significantly higher in the skin for serotype B-infected than for serotype A-infected mice. The tissues with the highest and lowest spirochetal numbers 18 days after inoculation were the skin and the brain parenchyma, respectively. Forty days after inoculation, there were still about four times as many serotype A as serotype B spirochetes in the leptomeninges, while there was not a statistical difference between serotype A- and B-infected mice in the numbers of the spirochetes in the skin. There was no significant difference in the number of spirochetes in the dura mater at any time.
TABLE 1.
Number of spirochetes in tissues of scid mice examined 6, 18, and 40 days after inoculation with B. turicatae serotype A or serotype B or PBS as a control
| Groupa | Mean (SD) no. of spirochetes/400× microscopic field
|
Ratio
|
||||
|---|---|---|---|---|---|---|
| Tissues
| ||||||
| Brain parenchyma | Leptomeninges | Dura mater | Skin | Leptomeninges/skin | Leptomeninges/dura | |
| Day 6 | ||||||
| A | 0.0 | 0.01 (0.1) | 0.19 (0.58) | 0.0 | NAb | NA |
| B | 0.09 (0.58) | 0.21 (0.74) | 0.8 (2.2) | 0.02 (0.16) | NA | 0.26 |
| C | 0.0 | 0.0 | 0.0 | 0.0 | NA | NA |
| Day 18 | ||||||
| A | 0.03 (0.18) | 2.1 (3.2) | 0.72 (2.0) | 4.7 (10.1) | 0.45c | 2.91 |
| B | 0.07 (0.31) | 0.45 (0.89) | 0.27 (0.71) | 26.3 (27.3)c | 0.017 | 1.66 |
| Day 40 | ||||||
| A | 0.04 (0.25) | 1.8 (3.0)c | 0.36 (0.78) | 8.5 (7.1) | 0.2c | 5.0c |
| B | 0.06 (0.28) | 0.42 (1.46) | 0.45 (0.82) | 10.8 (12.1) | 0.04 | 0.93 |
| C | 0.0 | 0.0 | 0.0 | 0.0 | NA | NA |
Twenty fields were examined from two mice in each group (A, serotype A; B, serotype B; C, control) by a masked examiner.
NA, Not applicable.
P < 0.001 for the difference between groups A and B in each tissue (Mann-Whitney test).
Characterization of early infection.
The previous results suggested that the largest number of spirochetes in tissues during early infection was found in the dura mater. To further investigate this, we infected new groups of scid mice with serotype A (n = 5) or serotype B (n = 5) and sacrificed them 3 (n = 2 each) and 4 (n = 3 each) days later. Several coronal sections from whole-decalcified heads immunostained with anti-Borrelia antibody were examined for the presence of spirochetes in the bone marrow, skin, dura mater, leptomeninges, and brain parenchyma. The results showed spirochetes present in the bone marrow and dura mater in 9 of 10 and 8 of 10 mice, respectively, compared with spirochetes in the skin and the brain (leptomeninges and parenchyma) in 1 of 10 and none of 10 mice, respectively. There were more spirochetes in the dura mater than in the bone marrow in all mice. These results indicated that the dura mater was the first tissue in the head where spirochetes disseminate from the blood.
Expression of spirochetal proteins in tissues.
To determine if the variable surface proteins of serotype A (VspA) and serotype B (VspB) and the flagellar protein of Borrelia spp. (flagellin) were expressed by spirochetes in the nervous system and other tissues, we studied coronal sections from whole-decalcified heads by immunohistochemistry with antibodies to VspA, VspB, and flagellin. The results showed expression of VspA by spirochetes in all tissues examined from serotype A-inoculated mice and of VspB in all tissues examined from serotype B-inoculated mice (Fig. 3). Flagellin was expressed in all tissues examined (not shown).
Inflammatory response to the infection.
We compared the presence and severity of inflammation in infected tissues from serotype A- and B-inoculated mice and uninfected controls using light microscopy of H&E-stained paraffin sections. For this, the severity of inflammation was scored from 0 to 5 in 10 consecutive 200× microscopic fields (Table 2). No inflammation was found in the meninges or skin during early infection. At days 18 and 40 after inoculation, there was severe inflammation in the skin of mice infected with either serotype (Fig. 4B). Minimal although significant inflammation was also found in the meninges at days 18 and 40, with no significant differences between the two serotypes. Examination with H&E staining revealed that the inflammatory infiltrate in the skin was mainly mononuclear. Immunostaining of the skin revealed that the majority of the inflammatory cells were macrophages (Fig. 4C).
TABLE 2.
Severity of inflammation over time in different tissues from scid mice infected with serotype A or B of B. turicatae or PBS as a control
| Group | Severity of inflammationa
|
|
|---|---|---|
| Meninges | Skin | |
| Day 6 | ||
| Serotype A | 0.15 (0.02–0.3) | 0.05 (0–0.11) |
| Serotype B | 0.1 (0–0.2) | 0.15 (0.04–0.2) |
| PBS | 0.2 (0.02–0.4) | 0.2 (01–0.4) |
| Day 18 | ||
| Serotype A | 0.7 (0.5–0.9) | 5 |
| Serotype B | 1.0 (0.7–1.3) | 5 |
| Day 40 | ||
| Serotype A | 0.2 (0.02–0.37) | 5 |
| Serotype B | 0.25 (0.1–0.4) | 5 |
| PBS | 0 | 0 |
Ten 200× microscopic fields per tissue per mouse (two mice per group) were scored as 0 (no inflammation), 1 (single infiltrate), 2 (small multifocal infiltrates), 3 (large multifocal infiltrates), 4 (confluent multifocal infiltrate), or 5 (diffuse infiltrate). Results are given a mean (95% confidence interval).
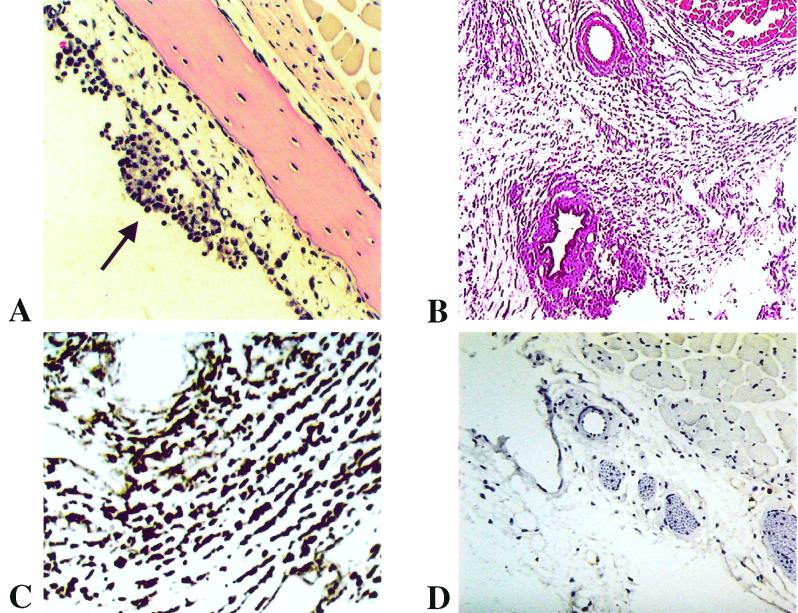
FIG. 4.
Inflammation in scid mice 40 days after inoculation of B. turicatae. (A and B) Mononuclear inflammatory exudate in the middle ear (A; magnification ×300) and the skin (B; ×100) of a scid mouse infected with serotype B (H&E stain). (C and D) Immunostaining for mouse macrophages in the skin of scid mice inoculated with B. turicatae serotype B (C; ×800) or PBS as a control (D; ×400; F4/80 antibody and hematoxylin counterstain).
We had previously found that a peripheral-type vestibular disorder began 3 to 4 weeks after inoculation and occurs more often in scid mice infected with serotype B than serotype A (14). To investigate the etiology of this vestibular disorder, we examined H&E-stained coronal sections from decalcified heads at the level of the inner ear for the presence of inflammation. Eight mice infected with serotype A (n = 4) or serotype B (n = 4) and sacrificed at 18 (n = 2 each) and 40 (n = 2 each) days after inoculation were examined. Inflammation in both the middle and inner ear was observed in all cases; the greatest amount of exudate was in the mucosa of the middle ear (Fig. 4A). At day 40 after inoculation, the number of spirochetes was significantly higher in the inner (Fig. 2E) than in the middle ear with both serotypes: the mean (95% confidence interval of the mean) numbers of spirochetes in the middle and inner ear, respectively, were 0.5 (0.06 to 0.9) and 5.96 (3.4 to 8.5) for serotype A, 0.12 (0 to 0.24) and 7.5 (3.6 to 11.4) for serotype B, and 0 and 0 for uninfected controls. In contrast, no spirochetes or inflammation were observed in the cerebellum or brainstem. These experiments revealed that the vestibular disorder was caused by infection and inflammation of the vestibular apparatus in the inner ear (laberynthitis).
Infection of the spinal cord.
To determine if the differences found in the brain of scid mice infected with serotype A or B also occurred in the spinal cord, a different examiner counted the number of spirochetes and estimated the severity of inflammation in five 200× microscopic fields at various levels of the spinal cord (Table 3). The mean (95% confidence interval) number of spirochetes during early infection was very low in all mice examined. The mean number of spirochetes at day 18 was significantly higher in the skin of serotype B than serotype A-infected mice, as was the case for the head. Meningeal inflammation was also mild in both groups. In contrast, the number of leptomeningeal spirochetes in the spinal cord was very low in both serotype A- and serotype B-infected mice. Also different from the heads was the finding of mild inflammation in the skin surrounding the spinal cord during early infection.
TABLE 3.
Infection of the spinal cord in scid mice inoculated with serotype A or B of B. turicatae at various times after inoculationa
| Group (n) | No. of spirochetes
|
Severity of inflammationb
|
||
|---|---|---|---|---|
| Meninges | Skin | Meninges | Skin | |
| Day 3 | ||||
| Serotype A (2) | 0c | 0 | 0.1 (0–0.29) | 0.7 (0.19–1.2) |
| Serotype B (2) | 0 | 0 | 0 | 1 (0.23–1.8) |
| Day 4 | ||||
| Serotype A (3) | 0 | 0 | 0.47 (0.0–0.97) | 2.07 (1.3–2.9) |
| Serotype B (3) | 0.07 (0.03–0.1) | 0.13 (0.0–0.31) | 0.13 (0.0–0.31) | 1.47 (0.84–2.1) |
| Day 18 | ||||
| Serotype A (6) | 0.07 (0.04–0.09) | 25.2 (16.2–34.0) | 1.13 (0.74–1.53) | 4.63 (4.43–4.83) |
| Serotype B (6) | 0.1 (0.06–0.14) | 53.3 (34.2–72.4) | 1.4 (0.99–1.81) | 4.92 (4.81–5.02) |
Five 200× microscopic fields were scored per mouse at cervical, thoracic, and lumbosacral levels. Results are given as mean (95% confidence interval) per 200× microscopic field.
Scored as 0 (no inflammation), 1 (single infiltrate), 2 (small multifocal infiltrates), 3 (large multifocal infiltrates), 4 (confluent multifocal infiltrate) or 5 (diffuse infiltrate).
DISCUSSION
Switching between serotypes provides relapsing fever borrelias with a strategy not only to avoid the host's antibody response but also to exploit different microenvironments, including the brain. For further insight into the mechanism responsible for brain infection by Borrelia spp., we compared the localization of two serotypes of B. turicatae in the brain and other tissues of scid mice. For this, we examined coronal sections of whole-decalcified heads by immunohistochemistry and used H&E-stained sections to compare the inflammatory response to the infection. The major findings were the following: (i) the localization of spirochetes in the brain and spinal cord was primarily leptomeningeal; (ii) there were significantly more serotype A than serotype B cells in the leptomeninges 18 and 40 days after inoculation; (iii) there were significantly more serotype B than serotype A cells in the skin 18 but not 40 days after inoculation; (iv) The tissue with the most severe inflammation was the skin; (v) the dura mater was the first tissue where spirochetes were observed outside of the vasculature; and (vi) the predominant surface lipoproteins VspA and VspB and the periplasmic protein flagellin were expressed at all times in all tissues examined.
Prior studies using cultured brains had demonstrated that serotype A but not serotype B was present in the brain of scid mice 4, 8, and 11 days after inoculation (14). The present study extends this observation to 18 days after inoculation. The localization of serotype A cells in the brain primarily to the leptomeninges was expected. Meningitis is a prominent clinical manifestation of neuroborreliosis in tick-borne relapsing fever and Lyme disease (9, 29). The presence of relapsing fever spirochetes in the brain of experimental animals was known as early as 1922 (7). We knew from our own studies of infection of irradiated mice with B. hermsii that spirochetes cross the blood-brain barrier and are not merely present in the intravascular space of the brain (10). Using nonspecific silver impregnation techniques, the pioneers in the field observed relapsing fever borrelias in the gray matter between neurons and glias (7) and within cerebral capillaries, leptomeningeal vessels, and choroid plexus (24). Relapsing fever borrelias from Africa were observed more commonly in the medulla and deeper parts of the brain than in the cortex of mice and rats (38, 40). The localization was extracellular (20). We were surprised by the small number of spirochetes found in the brain parenchyma compared with the leptomeninges (21). It is possible that examination of brains infected for longer periods of time will reveal increasing localization of borrelias to the brain parenchyma. We confirmed that all spirochetes found in tissues from serotype A- or B-infected mice expressed VspA or VspB, respectively. In a prior study, we showed that VspB is expressed by spirochetes in the blood, joints, and heart of mice (33). Expression of flagellin in infected tissues has been demonstrated in the nonhuman primate model of Lyme disease (11). Down regulation of major surface proteins during infection, known to occur with B. burgdorferi (11, 16), was not observed in these studies.
The localization of spirochetes in the brain has been studied in other spirochetal diseases. In the nonhuman primate model of Lyme disease, spirochetes were found in the leptomeninges, nerve roots, dorsal root ganglia, endoneurium, and extracellular matrix of peripheral nerves, skeletal muscle, heart, and bladder in immunosuppressed animals (11). In contrast, no spirochetes were found in tissues from immunocompetent animals, even those positive by PCR-ELISA (11, 36). B. burgdorferi has been reported intracellularly in vitro in human umbilical vein endothelial cells (22) and macrophages (26) but not in vivo (11). In neurosyphilis, Treponema pallidum was found in the cortex of 25 to 40% of paretic brains examined at autopsy, mainly in the frontal areas, and is difficult to find after treatment (12). In untreated syphilitic lesions in the skin, the majority of treponemes are extracellular (42).
Our studies suggest that borrelias first enter the central nervous system in the subarachnoid space and localize mainly to the leptomeninges, with only a few moving to the brain parenchyma early on. There are two possible routes to reach the subarachnoid space from the circulation: crossing the microvessels of the leptomeninges, and crossing the dura mater-arachnoidal barrier. In support of the first route is the frequent observation of spirochetes located partially in the microvascular lumen and partially in the subarachnoid space of the scid mice. In support of the second route is the observation that the dura mater is the first tissue where spirochetes were found outside of the vasculature. The reason why serotype A cells move into the subarachnoid space significantly better than serotype B cells is unknown. One possibility could be differences in the hydrophobicity of their Vsps: VspA has higher hydrophobicity than VspB (13), and VspB has a more basic pI (33). Another possibility is differences in their binding to glycosaminoglycans or other components of the extracellular matrix. A recent study found that serotype B cells bound to glycoaminoglycans significantly better than serotype A cells, and recombinant VspB but not VspA bound heparin and dermatan sulfate (23). Other bacteria have been found to have variable proteins associated with brain infection. These include the outer membrane protein A of Escherichia coli (25, 35), internalin B of Listeria monocytogenes (31), and the pili and class 5 outer membrane proteins of Neisseria meningitidis (27).
The high number of spirochetes in the skin indicates this tissue favors the multiplication and dissemination of spirochetes. A skin lesion, erythema migrans, characterizes early Lyme disease (6). There is no evidence that B. burgdorferi produces collagenase, elastase, hyaluronidase, or other enzymes that digest extracellular matrix components. However, both B. burgdorferi and the relapsing fever borrelias have been shown to bind human plasmin, plasminogen, and urokinase-type plasminogen activator (15, 19). The binding of plasminogen allows the formation of a bioactive extracellular matrix protease, which facilitates their dissemination through the extracellular matrix of infected tissues (18).
We found only mild inflammation in the meninges of the brain and spinal cord of infected animals. Early in the infection of rats with B. turicatae, there was severe congestion of leptomeningeal vessels and parenchymal capillaries, foci of cortical hemorrhages, and intense microglia reaction in the cerebral and cerebellar cortex and hippocampus (24). Later in the infection, there was only lymphocytic infiltration of the leptomeninges. Guinea pigs infected with B. persica had perivascular hemorrhage and infiltration with lymphocytes and macrophages in the brain (1). Selective damage to neurons in the upper part of the spinal cord and posterior columns was found in rats infected with relapsing fever strains from Russia (8). Leptomeningeal inflammation was observed only rarely in the nonhuman primate of Lyme disease (11, 30). Two recent studies of mice infected with B. crocidurae (39) and with a relapsing fever Borrelia from Spain (17, 18) showed meningitis and brain parenchymal microgliosis. B. crocidurae but not the relapsing fever Borrelia from Spain were observed in the brain parenchyma.
Starting with syphilis and relapsing fever and continuing with Lyme disease, spirochetes remain as important neurological pathogens. A better understanding of the mechanisms by which they cause neurological disease is needed. Our studies suggest that the proteins present in the surface of the spirochetes provide a mechanism for differential localization in tissue during infection. Future studies may elucidate the mechanisms by which spirochetes enter and persist in the brain and cause neurological disease.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grant AI24424 to A.G.B. and a National Institute of Mental Health grant to A.R.P. D.C. was supported by a Méndez Fellowship for Clinicians from the American Registry of Pathology and by a grant to the Hispanic Center of Excellence at UMDNJ-New Jersey Medical School from the Bureau of Health Professions, Health Resources and Services Administration.
REFERENCES
- 1.Ashbel R. Observations on some strains of Spirochaeta persica in Palestine. Ann Trop Med Parasitol. 1942;36:97–101. [Google Scholar]
- 2.Barbour A. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour A G, Hayes S F. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour A G, Hayes S F, Heiland R A, Schrumpf M E, Tessier S L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986;52:549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour A G, Tessier S L, Stoenner H G. Variable major proteins of Borrellia hermsii. J Exp Med. 1982;156:1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger B W. Erythema chronicum migrans of Lyme disease. Arch Dermatol. 1984;120:1017–1021. [PubMed] [Google Scholar]
- 7.Buschke A, Kroo H. Experimental recurrentis infection. Klin Wochenschr. 1922;1:2470. [Google Scholar]
- 8.Buschke A, Kroo H. Spinale strangdegeneration nach experimenteller rekurrens. Dtsch Med Wochenschr. 1923;49:1435–1436. [Google Scholar]
- 9.Cadavid D, Barbour A G. Neuroborreliosis during relapsing fever: review of the clinical manifestations, pathology, and treatment of infections in humans and experimental animals. Clin Infect Dis. 1998;26:151–164. doi: 10.1086/516276. [DOI] [PubMed] [Google Scholar]
- 10.Cadavid D, Bundoc V, Barbour A G. Experimental infection of the mouse brain by a relapsing fever Borrelia species: a molecular analysis. J Infect Dis. 1993;168:143–151. doi: 10.1093/infdis/168.1.143. [DOI] [PubMed] [Google Scholar]
- 11.Cadavid D, O'Neill T, Schaefer H, Pachner A. Localization of Borrelia burgdorferi in the nervous system and other tissues in a non-human primate model of Lyme disease. Lab Investig. 2000;80:1043–1054. doi: 10.1038/labinvest.3780109. [DOI] [PubMed] [Google Scholar]
- 12.Cadavid D, Pachner A R. Neurosyphilis. In: Griggs R C, Joynt R J, editors. Clinical neurology. Philadelphia, Pa: Lippincott-Raven; 1998. pp. 1–43. [Google Scholar]
- 13.Cadavid D, Pennington P M, Kerentseva T A, Bergstrom S, Barbour A G. Immunologic and genetic analyses of VmpA of a neurotropic strain of Borrelia turicatae. Infect Immun. 1997;65:3352–3360. doi: 10.1128/iai.65.8.3352-3360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cadavid D, Thomas D D, Crawley R, Barbour A G. Variability of a bacterial surface protein and disease expression in a possible mouse model of systemic Lyme borreliosis. J Exp Med. 1994;179:631–642. doi: 10.1084/jem.179.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman J L, Sellati T J, Testa J E, Kew R R, Furie M B, Benach J L. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect Immun. 1995;63:2478–2484. doi: 10.1128/iai.63.7.2478-2484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fikrig E, Feng W, Aversa J, Schoen R T, Flavell R A. Differential expression of Borrelia burgdorferi genes during erythema migrans and Lyme arthritis. J Infect Dis. 1998;178:1198–1201. doi: 10.1086/515684. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Monco J C, Miller N S, Backenson P B, Anda P, Benach J L. A mouse model of Borrelia meningitis after intradermal injection. J Infect Dis. 1997;175:1243–1245. doi: 10.1086/593681. [DOI] [PubMed] [Google Scholar]
- 18.Gebbia J A, Monco J C, Degen J L, Bugge T H, Benach J L. The plasminogen activation system enhances brain and heart invasion in murine relapsing fever borreliosis. J Clin Investig. 1999;103:81–87. doi: 10.1172/JCI5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klempner M S, Noring R, Epstein M P, McCloud B, Hu R, Limentani S A, Rogers R A. Binding of human plasminogen and urokinase-type plasminogen activator to the Lyme disease spirochete, Borrelia burgdorferi. J Infect Dis. 1995;171:1258–1265. doi: 10.1093/infdis/171.5.1258. [DOI] [PubMed] [Google Scholar]
- 20.Levaditi C, Anderson T, Selbie F, Schoen R. Presence du spirille de la fievre recurrente (Sp. duttoni) dans le cerveau des animaux immuns. Bull Acad Med. 1929;102:705–710. [Google Scholar]
- 21.Levaditi J, Balouet G, Juminer B, Corcos A. Borreliose experimentale du raton nouveau-ne. Etude histologique. Bull Soc Pathol Exot. 1966;59:310–316. [PubMed] [Google Scholar]
- 22.Ma Y, Sturrock A, Weis J J. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect Immun. 1991;59:671–678. doi: 10.1128/iai.59.2.671-678.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magoun L, Zuckert W R, Robbins D, Parveen N, Alugupalli K R, Schwan T G, Barbour A G, Leong J M. Variable small protein (Vsp)-dependent and vsp-independent pathways for glycosaminoglycan recognition by relapsing fever spirochaetes. Mol Microbiol. 2000;36(4):886–897. doi: 10.1046/j.1365-2958.2000.01906.x. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Baez M, Villasana A. Sobre la histopatologia de la fiebre recurrente experimental. Rev Inst Salub Enferm Trop. 1945;6:185–194. [PubMed] [Google Scholar]
- 25.Meier C, Oelschlaeger T A, Merkert H, Korhonen T K, Hacker J. Ability of Escherichia coli isolates that cause meningitis in newborns to invade epithelial and endothelial cells. Infect Immun. 1996;64:2391–2399. doi: 10.1128/iai.64.7.2391-2399.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montgomery R R, Nathanson M H, Malawista S E. The fate of Borrelia burgdorferi, the agent for Lyme disease, in mouse macrophages. Destruction, survival, recovery. J Immunol. 1993;150:909–915. [PubMed] [Google Scholar]
- 27.Nassif X, So M. Interaction of pathogenic neisseriae with nonphagocytic cells. Clin Microbiol Rev. 1995;8:376–388. doi: 10.1128/cmr.8.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.National Institutes of Health. Guide for the care and use of laboratory animals, rev. ed. Department of Health and Human Services publication no. (NIH) 85–23. Bethesda, Md: National Institutes of Health; 1985. [Google Scholar]
- 28.Pachner A R, Braswell S T, Delaney E, Amemiya K, Major E. A rabbit model of Lyme neuroborreliosis: characterization by PCR, serology, and sequencing of the OspA gene from the brain. Neurology. 1994;44:1938–1943. doi: 10.1212/wnl.44.10.1938. [DOI] [PubMed] [Google Scholar]
- 29.Pachner A R, Cadavid D. Lyme neuroborreliosis. In: Griggs R C, Joynt R J, editors. Clinical neurology. Philadelphia, Pa: Lippincott-Raven; 1998. pp. 1–19. [Google Scholar]
- 30.Pachner A R, Delaney E, O'Neill T, Major E. Inoculation of nonhuman primates with the N40 strain of Borrelia burgdorferi leads to a model of Lyme neuroborreliosis faithful to the human disease. Neurology. 1995;45:165–172. doi: 10.1212/wnl.45.1.165. [DOI] [PubMed] [Google Scholar]
- 31.Parida S K, Domann E, Rohde M, Muller S, Darji A, Hain T, Wehland J, Chakraborty T. Internalin B is essential for adhesion and mediates the invasion of Listeria monocytogenes into human endothelial cells. Mol Microbiol. 1998;28:81–93. doi: 10.1046/j.1365-2958.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- 32.Pennington P M, Allred C D, West C S, Alvarez R, Barbour A G. Arthritis severity and spirochete burden are determined by serotype in the Borrelia turicatae-mouse model of Lyme disease. Infect Immun. 1997;65:285–292. doi: 10.1128/iai.65.1.285-292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pennington P M, Cadavid D, Barbour A G. Characterization of VspB of Borrelia turicatae, a major outer membrane protein expressed in blood and tissues of mice. Infect Immun. 1999;67:4637–4645. doi: 10.1128/iai.67.9.4637-4645.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pennington P M, Cadavid D, Bunikis J, Norris S J, Barbour A G. Extensive interplasmidic duplications change the virulence phenotype of the relapsing fever agent Borrelia turicatae. Mol Microbiol. 1999;34:1120–1132. doi: 10.1046/j.1365-2958.1999.01675.x. [DOI] [PubMed] [Google Scholar]
- 35.Prasadarao N V, Wass C A, Stins M F, Shimada H, Kim K S. Outer membrane protein A-promoted actin condensation of brain microvascular endothelial cells is required for Escherichia coli invasion. Infect Immun. 1999;67:5775–5783. doi: 10.1128/iai.67.11.5775-5783.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts E D, Bohm R P, Jr, Lowrie R C, Jr, Habicht G, Katona L, Piesman J, Philipp M T. Pathogenesis of Lyme neuroborreliosis in the rhesus monkey: the early disseminated and chronic phases of disease in the peripheral nervous system. J Infect Dis. 1998;178:722–732. doi: 10.1086/515357. [DOI] [PubMed] [Google Scholar]
- 37.Sadziene A, Rosa P A, Thompson P A, Hogan D M, Barbour A G. Antibody-resistant mutants of Borrelia burgdorferi: in vitro selection and characterization. J Exp Med. 1992;176:799–809. doi: 10.1084/jem.176.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schauder H. Zur frage der spirochatenpersistenz in zentralnervensystem und ihrer chemotherapeutischen beeinflussbarkeit bei experimenteller rekurrens. Arch Schiffs Trop Hyg. 1928;32:1–13. [Google Scholar]
- 39.Shamaei-Tousi A, Martin P, Bergh A, Burman N, Brannstrom T, Bergstrom S. Erythrocyte-aggregating relapsing fever spirochete Borrelia crocidurae induces formation of microemboli. J Infect Dis. 1999;180:1929–1938. doi: 10.1086/315118. [DOI] [PubMed] [Google Scholar]
- 40.Steiner G, Steinfeld J, Schauder H. Zur frage der spirochatenpersistenzim zentralnervensystem bei experimenteller recurrens. Klin Wochenschr. 1925;4:2288–2289. [Google Scholar]
- 41.Stoenner H, Dodd T, Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982;156:1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wecke J, Bartunek J, Stuttgen G. Treponema pallidum in early syphilitic lesions in humans during high-dosage penicillin therapy. An electron microscopical study. Arch Dermatol Res. 1976;257:1–15. doi: 10.1007/BF00569109. [DOI] [PubMed] [Google Scholar]
- 43.Zuckert W R, Meyer J, Barbour A G. Comparative analysis and immunological characterization of the Borrelia Bdr protein family. Infect Immun. 1999;67:3257–3266. doi: 10.1128/iai.67.7.3257-3266.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]