Abstract
Background
Angiotensin receptor neprilysin inhibition (ARNI) is superior to enalapril in reducing the risk of cardiovascular death and heart failure hospitalization (HFH). However, whether prescription pattern is associated with heart failure outcome is unknown.
Methods
This is a retrospective study of 153 patients who received ARNI in a tertiary medical center in Taiwan. We analyzed the impact of dose up-titration and prescription timing including during initial admission, within 3 months after initial HFH discharge, and at outpatient clinics without prior HFH. The primary endpoint was the composite of cardiovascular death and HFH.
Results
After a mean follow-up period of 287 ± 197 days, the primary endpoint occurred in 43 (28.1%) subjects. Patients without and with a primary endpoint significantly differed in terms of history of valvular heart disease (VHD, p = 0.006), ventricular tachyarrhythmia (VT, p = 0.043), percutaneous coronary intervention (p = 0.007), coronary artery bypass grafting (p = 0.002), chronic kidney disease (p = 0.002), age (p = 0.002), diastolic blood pressure (p = 0.025), and prescription timing (p = 0.002). Kaplan-Meier analysis showed ARNI up-titration and prescription timing had a significant association with primary endpoint-free survival (Breslow test; p = 0.032, and log-rank test; p = 0.001, respectively). Cox regression analysis showed that independent predictors for the primary endpoint were ARNI up-titration [hazard ratio (HR): 0.41, p = 0.024], non-hospital ARNI versus hospital ARNI (HR: 0.41, p = 0.009), VHD (HR: 2.71, p = 0.013), VT (HR: 3.09, p = 0.02), and age (HR: 1.03, p = 0.033).
Conclusions
The prescription pattern of ARNI could be associated with heart failure events.
Keywords: Angiotensin receptor neprilysin inhibitor, Cardiac pharmacology, Heart failure, Prescribing habit
Abbreviations
ACEI, Angiotensin-converting enzyme inhibitor
ADHF, Acute decompensated HF
ARB, Angiotensin receptor blocker
ARNI, Angiotensin receptor neprilysin inhibition
BNP, Brain natriuretic peptide
CABG, Coronary artery bypass graft
CAD, Coronary artery disease
CI, Confidence interval
CKD, Chronic kidney disease
DBP, Diastolic blood pressure
DCM, Dilated cardiomyopathy
EBM, Evidence-based medical
EKG, Electrocardiogram
HF, Heart failure
HFH, Heart failure hospitalization
HFrEF, HF with reduced ejection fraction
HR, Hazard ratio
ICD, Implantable cardioverter defibrillator
LVEF, Left ventricular ejection fraction
MRA, Mineralocorticoid receptor antagonist
NT, N-terminal
NYHA, New York Heart Association Functional Classification
PARADIGM-HF, Prospective Comparison of Angiotensin Receptor Antagonist and Neprilysin Inhibitor with Angiotensin-Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial
PCI, Percutaneous coronary intervention
SBP, Systolic blood pressure
VHD, Valvular heart disease
VT, Ventricular tachyarrhythmia
INTRODUCTION
The high rates of mortality and hospitalization in heart failure (HF) patients make HF a major public health burden.1,2 An aging population and improving survival of myocardial infarction have increased the HF population worldwide.3,4 Progression of HF is associated with an increasing frequency of hospitalization, morbidity, mortality, and medical expenditures and with a decrease in quality of life.5 Evidence-based medical (EBM) therapy, e.g., treatment with an angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), beta-blocker, or mineralocorticoid receptor antagonist (MRA), is the most effective strategy and the cornerstone treatment for HF with reduced ejection fraction (HFrEF).6,7
Sacubitril/valsartan, an angiotensin receptor antagonist and neprilysin inhibitor (ARNI), was recently shown to be superior to ACEI in reducing the risks of cardiovascular death and HF hospitalization (HFH) in The Prospective Comparison of Angiotensin Receptor Antagonist and Neprilysin Inhibitor with Angiotensin-Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial (PARADIGM-HF).8 Thus, most guidelines currently recommend ARNI as an EBM therapy for ambulatory patients with HFrEF.6,7,9
However, whether different ARNI prescription timings are associated with different HF outcomes is uncertain. The PIONEER-HF study first demonstrated the feasibility of ARNI in patients in stable condition after acute decompensated HF (ADHF).10 Additionally, the PIONEER-HF study did not determine whether the ARNI prescription pattern, including dosing and timing, is associated with a HF outcome as the clinical characteristics of the enrolled patients differed from those typically encountered in real-world practice. Therefore, the aim of this study was to investigate how different ARNI prescription patterns affect HF outcomes in patients treated for HFrEF at a tertiary medical center.
METHODS
Study design and data source
The data in this study were retrospectively collected from a tertiary medical center in Kaohsiung, Taiwan. Patients with HFrEF stage C were included if they had a history of sacubitril/valsartan therapy and data collected during HF treatment in a clinic or hospitalization between October 1, 2016, and April 30, 2018. All data used in this study, including clinical, demographic, laboratory, and medical data, were collected retrospectively from electronic medical records. An HFrEF was defined as a left ventricular ejection fraction (LVEF) less than 40%. Up-titration was defined as changing and increasing the dose of ARNI during the period of study. Clinical co-morbidities and relevant medical information collected in this study included hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation or flutter, valvular heart disease (VHD) of higher than mild severity, dilated cardiomyopathy (DCM), ventricular tachyarrhythmia (VT) or ventricular fibrillation, implantable cardioverter defibrillator (ICD), stroke, coronary artery disease (CAD), percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), chronic kidney disease (CKD), end stage renal disease, gout, and cancer. We defined VT from the medical records including electrocardiogram (EKG) or Holter EKG with 3 or more heartbeats in a row. We defined VHD according to echocardiographic reports with more than or equal to the moderate severity. The data for initial dosing and up-titration of ARNI treatment were also obtained from medical records. The study protocol was evaluated and approved by the Institutional Review Board (KMUHIRB-E(I)-20190222).
Study outcomes
Overall, 153 patients who had received ARNI in a tertiary medical center in Taiwan were examined. The primary endpoint was the composite of cardiovascular death and HFH. The three prescription timings were ARNI prescribed during a first HFH (hospital ARNI), ARNI prescribed within 3 months after a first HFH discharge (hospital discharge ARNI), and ARNI prescribed at an outpatient clinicin patients with no history of HFH (non-hospital ARNI). The impacts of dose up-titration and prescription timing on clinical outcomes were also analyzed.
Statistical analysis
The data were reported as means ± standard deviations for continuous variables and as percentages for categorical variables. Between-group comparisons of categorical and continuous variables were performed by Chi-square test, one-way ANOVA, and independent t-test. To reduce the potential for immortal time bias in the group of up-titration, we removed the period from the time of patient enrolment to the time of ARNI up-titration before survival analysis. Then, we described the cumulative event-free survival curves by the Kaplan-Meier approach with a time-fixed model and inspected the differences between the two groups by using the Breslow test. In addition, cumulative event-free survival curves between groups of ARNI prescription time were also described by the Kaplan-Meier method and compared by the log-rank test. Significant variables tested by the univariate analysis from baseline characteristics were eligible for multivariable analysis with a forced entry approach. We adopted Cox proportional hazards regression to identify significant variables and an ARNI prescription pattern associated with event-free survival. The survival models were reported by HR and showed a 95% confidence interval for heart failure outcome. All p < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software version 22 (IBM SPSS Statistics).
RESULTS
Overall, the analysis included 153 HF patients who had received ARNI at Kaohsiung Medical University Hospital. The mean age was 61.7 ± 14.2 years; 77.2% were male; median LVEF was 27.9%; body mass index was 26.2 kg/m2; brain natriuretic peptide (BNP) level was 1405 pg/mL; median systolic blood pressure (SBP) and diastolic blood pressure (DBP) were 125.5 and 76.8 mmHg respectively. Serum potassium and estimated glomerular filtration rate were 3.99 mEq/L and 64.2 mL/min/1.73 m2, respectively. The leading comorbidities were hypertension (66%), VHD (54.2%), dyslipidemia (52.9%), diabetes (50.3%), CAD (49.7%) and atrial fibrillation or flutter (30.7%). PCI were performed in 36.6% of subjects. Administration of EBM therapy included renin-angiotensin system blockers (81.7%), beta-blockers (84.3%), and MRAs (40.5%). Loop diuretic, digoxin, ivabradine and amiodarone had been prescribed in 51.3%, 9.2%, 30.7% and 5.2% patients, respectively. The average initial dose of hospital ARNI, hospital discharge, and non-hospital ARNI prescription were 219 mg, 159 mg, and 184 mg. Final dose of hospital ARNI, hospital discharge, and non-hospital ARNI prescription were 297 mg, 228 mg, and 264 mg respectively. The final total dose in the up-titration group were 100 mg in 1 person, 200 mg in 17 persons, and 400 mg in 43 persons, which indicated that 98% patients in the up-titration group achieved a 50% ARNI target dose.
After a mean follow-up period of 287 ± 197 days, the primary endpoint had occurred in 43 (28.1%) subjects. Patients without and with the primary endpoint significantly differed in history of VHD (47.3 vs. 72.1%, respectively; p = 0.006), VT (4.5 vs. 13.9%, respectively; p = 0.043), PCI (30 vs. 53.5%, respectively; p = 0.007), CABG (3.6 vs. 18.6%, respectively; p = 0.002), CKD (16.4 vs. 39.5%, respectively; p = 0.002), age (59.6 ± 13.7 vs. 67.3 ± 14.1 years, p = 0.002), and DBP (79 ± 15 vs. 73 ± 12 mmHg, p = 0.025) (Table 1). The rate of the primary endpoint significantly (p = 0.002) differed among three prescription timings (hospital ARNI, hospital discharge ARNI, non-hospital ARNI) and between in-hospital and out-hospital prescription as well (p = 0.008).
Table 1. Baseline characteristics between patients with and without cardiovascular death and hospitalization.
| Without (N = 110) | With (N = 43) | p | |
| Age | 59.6 ± 13.7 | 67.3 ± 14.1 | 0.002* |
| Sex/male | 77 (70%) | 32 (74.4%) | 0.59 |
| SBP (mmHg) | 123 ± 19 | 126 ± 22 | 0.42 |
| DBP(mmHg) | 79 ± 15 | 73 ± 12 | 0.025* |
| Hypertension | 71 (64.6%) | 30 (69.8%) | 0.54 |
| Diabetes | 51 (46.4%) | 26 (60.5%) | 0.12 |
| Dyslipidemia | 62 (56.4%) | 19 (44.2%) | 0.18 |
| Af | 30 (27.3%) | 17 (39.5%) | 0.14 |
| VHD | 52 (47.3%) | 31 (72.1%) | 0.006* |
| Gout | 18 (16.4%) | 8 (18.6%) | 0.74 |
| VT | 5 (4.5%) | 6 (13.9%) | 0.043* |
| CAD | 51 (46.4%) | 25 (58.1%) | 0.19 |
| PCI | 33 (30.0%) | 23 (53.5%) | 0.007* |
| CABG | 4 (3.6%) | 8 (18.6%) | 0.002* |
| CKD | 18 (16.4%) | 17 (39.5%) | 0.002* |
| LVEF (%) | 27.6 ± 7.2 | 28.1 ± 7.2 | 0.67 |
| BNP (pg/mL) | 1309 ± 1514 | 1796 ± 1375 | 0.11 |
| Scr (mg/dL) | 1.2 ± 0.5 | 1.6 ± 1.3 | 0.051 |
Af, atrial fibrillation; BNP, brain natriuretic peptide; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; DBP, diastolic blood pressure; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; Scr, serum creatinine; VHD, valvular heart disease; VT, ventricular tachyarrhythmia.
* p value < 0.05.
The baseline characteristics and background medical therapy among the three groups was also analyzed. The baseline characteristics including sex, diabetes mellitus, atrial fibrillation, VT, ICD, stroke, CAD, PCI, CABG, and CKD showed no differences among the three groups. The background medical therapy among the three prescription timing groups also showed no differences, including beta-blockers, mineralocorticoid receptor antagonists, renin-angiotensin system blockers, ivabradine, digoxin, and sodium-glucose cotransporter 2 inhibitors (Table 2).
Table 2. Baseline characteristics between three prescription timing.
| In-hospital (N = 32) | Discharge (N = 37) | Non-hospital (N = 84) | p | |
| Age | 60.3 | 65.1 | 60.7 | 0.25 |
| Sex (male) | 22 (68.8%) | 26 (70.3%) | 61 (72.6%) | 0.91 |
| Initial dose (mg) | 219 | 160 | 184 | 0.049* |
| Final dose (mg) | 296 | 228 | 264 | 0.076 |
| LVEF (%) | 26.1 | 26.5 | 28.8 | 0.09 |
| SBP (mmHg) | 123.1 | 125.8 | 126.4 | 0.76 |
| DBP (mmHg) | 75.8 | 76.4 | 77.4 | 0.85 |
| Hypertension | 20 (62.5%) | 29 (78.4%) | 52 (61.9%) | 0.19 |
| Diabetes | 21 (65.6%) | 19 (51.4%) | 37 (44.1%) | 0.11 |
| Dyslipidemia | 12 (37.5%) | 14 (37.8%) | 55 (65.5%) | 0.003* |
| Af | 11 (34.4%) | 13 (35.1%) | 23 (27.4%) | 0.61 |
| VHD | 21 (65.6%) | 24 (64.9%) | 38 (45.2%) | 0.047* |
| VT | 1 (3.1%) | 2 (5.4%) | 8 (9.5%) | 0.44 |
| ICD | 1 (3.1%) | 0 (0%) | 2 (2.4%) | 0.59 |
| CAD | 17 (53.1%) | 19 (51.4%) | 40 (47.6%) | 0.85 |
| PCI | 11 (34.4%) | 15 (40.5%) | 30 (35.7%) | 0.84 |
| CABG | 4 (12.5%) | 3 (8.1%) | 5 (6%) | 0.50 |
| CKD | 11 (34.4%) | 10 (27%) | 14 (16.7%) | 0.10 |
| Beta blocker | 25 (78.1%) | 29 (78.4%) | 75 (89.3%) | 0.18 |
| MRA | 13 (40.6%) | 17 (45.9%) | 32 (38.1%) | 0.72 |
| Ivabradine | 12 (37.5%) | 14 (37.8%) | 21 (25%) | 0.24 |
| Digoxin | 2 (6.2%) | 4 (10.8%) | 8 (9.5%) | 0.79 |
| Amiodarone | 2 (6.2%) | 0 (0%) | 6 (7.1%) | 0.26 |
| SGLT2i | 6 (18.8%) | 2 (5.4%) | 5 (6%) | 0.065 |
| RAS blocker | 26 (81.3%) | 29 (78.4%) | 70 (83.3%) | 0.81 |
| BNP (pg/mL) | 1688 | 1967 | 1079 | 0.026* |
| Scr (mg/dL) | 1.63 | 1.33 | 1.14 | 0.017* |
Af, atrial fibrillation; BNP, brain natriuretic peptide; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; DBP, diastolic blood pressure; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; PCI, percutaneous coronary intervention; RAS blocker, renin-angiotensin system blocker; SBP, systolic blood pressure; Scr, serum creatinine; SGLT2i, sodium-glucose cotransporter 2 inhibitors; VHD, valvular heart disease; VT, ventricular tachyarrhythmia.
* p value < 0.05.
The Kaplan-Meier analysis indicated that the ARNI prescription timing, 50% ARNI target dose attainment, and up-titration had a significant association with primary endpoint-free survival [log-rank test; p = 0.001 for ARNI timing (Figure 1), p = 0.001 for 50% ARNI target dose and 0.024 for ARNI up-titration respectively]. Further time-fix model after removing immortal time from ARNI up-titration group revealed a significant association between ARNI prescription timing with primary endpoint-free survival (Breslow test; p = 0.032) (Figure 2). Factors associated with the primary endpoint in univariable analysis included age, DBP, VHD, VT, PCI, CABG, CKD, ARNI up-titration, and ARNI timing. Further multivariable analyses were performed to investigate whether those factors were associated with the primary endpoint. In the Cox regression hazard model, significant independent predictors were ARNI up-titration [hazard ratio (HR): 0.41; 95% confidence interval (CI): 0.19-0.89; p = 0.024], non-hospital ARNI versus hospital ARNI (HR: 0.41; 95% CI: 0.21-0.80; p = 0.009), VHD (HR: 2.71; 95% CI: 1.23-5.98; p = 0.013), VT (HR: 3.09; 95% CI: 1.19-7.97; p = 0.02), and age (HR: 1.03; 95% CI: 1.00-1.06; p = 0.033) (Table 3).
Figure 1.
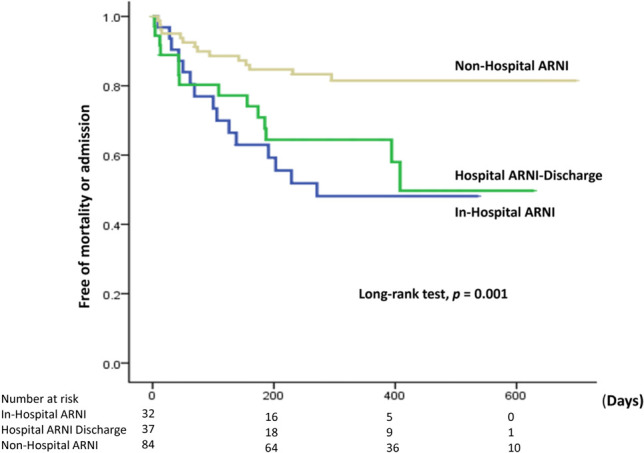
Kaplan-Meier analysis of primary endpoint-free survival in three prescription timing. ARNI, angiotensin receptor neprilysin inhibitor; in-hospital ARNI; ARNI prescribed during hospitalization, Hospital ARNI discharge; ARNI prescribed within 3 months after hospitalization, non-hospital ARNI; ARNI prescribed outpatient clinics without prior heart failure hospitalization.
Figure 2.
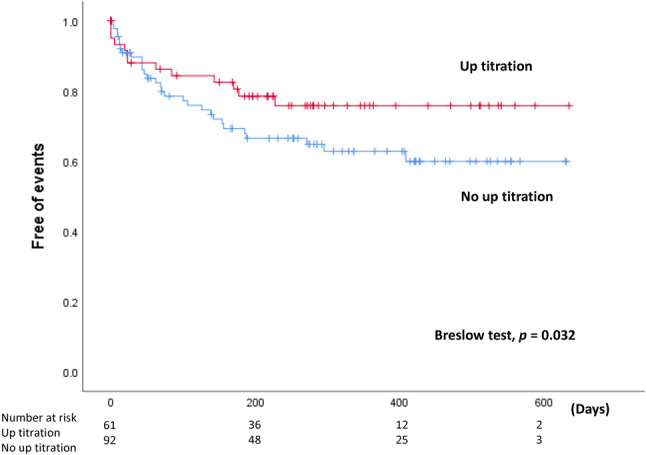
Kaplan-Meier analysis of primary endpoint-free survival with removing immortal time from up-titration group.
Table 3. Independent factors associated with cardiovascular death and hospitalization.
| HR | 95% CI | p | |
| Age | 1.03 | 1.00-1.06 | 0.033* |
| VHD | 2.71 | 1.23-5.98 | 0.013* |
| VT | 3.09 | 1.19-7.97 | 0.020* |
| PCI | 1.60 | 0.76-3.39 | 0.22 |
| CABG | 1.21 | 0.45-3.21 | 0.71 |
| CKD | 1.59 | 0.81-3.15 | 0.18 |
| DBP | 0.999 | 0.97-1.02 | 0.95 |
| Optimal dose titration | 0.88 | 0.39-1.96 | 0.76 |
| ARNI up-titration | 0.41 | 0.19-0.89 | 0.024* |
| Non-hospital vs. hospital | 0.41 | 0.21-0.80 | 0.009* |
CABG, coronary artery bypass grafting; CI, confidence interval; CKD, chronic kidney disease; DBP, diastolic pressure; HR, hazard ratio; PCI, percutaneous coronary intervention; VT, ventricular tachyarrhythmia. Optimal dose titration, achieving 50% target dose for drug up-titration. ARNI, angiotensin receptor neprilysin inhibitor, hospital; ARNI prescribed during hospitalization or within 3 months after hospitalization, non-hospital; ARNI prescribed outpatient clinics without prior heart failure hospitalization.
* p value < 0.05.
DISCUSSION
This study had three major findings. First, ARNI up-titration may be associated with HF outcomes. Second, ARNI treatment delivered at an outpatient clinic could result in a better outcome than that of ARNI treatment delivered in hospitalization. Third, even HFrEF patients who undergo ARNI treatment, valvular heart disease, and ventricular arrhythmia were risk factors in the HFrEF population for the primary endpoint.
Doses of EBM therapy should be up-titrated to the target doses applied in randomized controlled trials if they are tolerable.11 However, the target doses recommended in clinical guidelines are considered too low in real-world practice, especially in Asian populations.12,13 In PARADIGM-HF, the primary event risk was higher in low-dose participants compared to full-dose participants.14 Limited data are available for the role of ARNI up-titration in clinical outcomes of HF. After adjusting for co-morbidities, our study found that up-titrating ARNI might be associated with better outcomes in the mid-term follow-up.
The PARADIGM-HF trial demonstrated that ARNI is beneficial for ambulatory HFrEF patients,8 and PIONEER-HF further demonstrated the feasibility of prescribing ARNI in stable patients hospitalized for ADHF.10 In stable patients hospitalized for ADHF, sacubitril/valsartan is more effective than enalapril in reducing N-terminal (NT) pro-BNP. This exploratory analysis further revealed that, in stable patients hospitalized for ADHF, sacubitril/valsartan outperformed enalapril in terms of reducing HF rehospitalization or cardiovascular death.15 These data indicate the value of in-hospital initiation of sacubitril/valsartan after ADHF with reduced ejection fraction is stabilized, which further extends the PARADIGM-HF trial results. However, the optimal timing for initiating ARNI remains to be determined. The PARADIGM-HF subgroup analysis revealed larger benefits of ARNI in New York Heart Association Functional Classification (NYHA) II patients compared to NYHA III patients, which implies that the benefits of ARNI are greater when initiated at an early stage. Our results are consistent with previous reports that, in patients without prior HFH, non-hospital ARNI delivered in an outpatient clinic may achieve better outcomes compared to hospital ARNI and compared to hospital discharge ARNI.
The PROVE-HF study and the EVALUATE-HF trial reported that ARNI therapy can promote cardiac reverse remodeling in patients with HFrEF.16,17 Reducing myocardial remodeling may enhance the effectiveness of ARNI in HFrEF patients.18,19 A meta-analysis also showed that ARNI administered in patients with HFrEF obtained larger improvements in left ventricle size and hypertrophy compared with ACEIs/ARBs in HFrEF, even in short-term follow up.20 Apparently, the benefit to cardiac reverse remodeling is largest when ARNI is initiated as early as possible after HF and continued for at least 3 months. Furthermore, ARNI reduces the risk of sudden cardiac death in patients who have received an ICD or those who have been fitted for such an implant.21 Our study found that, even under ARNI treatment, both VHD and VT are still risk factors for a HF event. Selecting the appropriate intervention, whether it be a device implant, surgery, or anti-arrhythmia medication, is mandatory in these high-risk populations.
Limitations
This study had two limitations. First, this was a retrospective study. The up-titration strategy and prescription pattern were clinical judgments. Therefore, some confounding factors and selection bias can not be excluded in a non-randomized study. However, the data obtained in our study are complementary to data from previous randomized control trials that had investigated the roles of prescription timing and dose up-titration in HF patients treated in real-world practice. A second limitation is that the number of patients investigated in our study was comparatively small. Investigations of larger patient cohorts may reveal other factors associated with HF events.
CONCLUSIONS
The ARNI prescription pattern could be associated with HF events. In real-world practice, up-titrating and initiating ARNI in those without a prior heart failure admission might be associated with better outcomes in mid-term follow-up in HFrEF patients.
Acknowledgments
The authors would like to thank the Department of Internal Medicine and Statistical Analysis Laboratory, Department of Medical Research, Kaohsiung Medical University Hospital for their help.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22:1342–1356. doi: 10.1002/ejhf.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol. 2013;61:1510–1517. doi: 10.1016/j.jacc.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker DW. Prevention of heart failure. J Card Fail. 2002;8:333–346. doi: 10.1054/jcaf.2002.0805333. [DOI] [PubMed] [Google Scholar]
- 5.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 6.Wang CC, Wu CK, Tsai ML, et al. 2019 focused update of the guidelines of the Taiwan Society of Cardiology for the diagnosis and treatment of heart failure. Acta Cardiol Sin. 2019;35:244–283. doi: 10.6515/ACS.201905_35(3).20190422A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maddox TM, Januzzi JL, Jr., Allen LA, et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77:772–810. doi: 10.1016/j.jacc.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 8.McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 9.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 10.Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2018;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 12.Ouwerkerk W, Teng TK, Tromp J, et al. Effects of combined renin-angiotensin-aldosterone system inhibitor and beta-blocker treatment on outcomes in heart failure with reduced ejection fraction: insights from BIOSTAT-CHF and ASIAN-HF registries. Eur J Heart Fail. 2020;22:1472–1482. doi: 10.1002/ejhf.1869. [DOI] [PubMed] [Google Scholar]
- 13.Wang CC, Chang HY, Yin WH, et al. TSOC-HFrEF registry: a registry of hospitalized patients with decompensated systolic heart failure: description of population and management. Acta Cardiol Sin. 2016;32:400–411. doi: 10.6515/ACS20160704A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vardeny O, Claggett B, Packer M, et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18:1228–1234. doi: 10.1002/ejhf.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrow DA, Velazquez EJ, DeVore AD, et al. Clinical outcomes in patients with acute decompensated heart failure randomly assigned to sacubitril/valsartan or enalapril in the PIONEER-HF trial. Circulation. 2019;139:2285–2288. doi: 10.1161/CIRCULATIONAHA.118.039331. [DOI] [PubMed] [Google Scholar]
- 16.Januzzi JL, Butler J, Fombu E, et al. Rationale and methods of the prospective study of biomarkers, symptom improvement, and ventricular remodeling during sacubitril/valsartan therapy for heart failure (PROVE-HF). Am Heart J. 2018;199:130–136. doi: 10.1016/j.ahj.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Desai AS, Solomon SD, Shah AM, et al. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2019;322:1077–1084. doi: 10.1001/jama.2019.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21:365–371. doi: 10.1016/j.carpath.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Udelson JE, Konstam MA. Ventricular remodeling fundamental to the progression (and regression) of heart failure. J Am Coll Cardiol. 2011;57:1477–1479. doi: 10.1016/j.jacc.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Zhou R, Lu C, et al. Effects of the angiotensin-receptor neprilysin inhibitor on cardiac reverse remodeling: meta-analysis. J Am Heart Assoc. 2019;8:e012272. doi: 10.1161/JAHA.119.012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohde LE, Chatterjee NA, Vaduganathan M, et al. Sacubitril/valsartan and sudden cardiac death according to implantable cardioverter-defibrillator use and heart failure cause: a PARADIGM-HF analysis. JACC Heart Fail. 2020;8:844–855. doi: 10.1016/j.jchf.2020.06.015. [DOI] [PubMed] [Google Scholar]


