Abstract
Background
The rapid acquisition of an electrocardiogram (ECG) plays a crucial role in the diagnosis and management decisions in patients with acute coronary syndrome (ACS).
Objectives
We determined the time-to-ECG acquisition, identified factors associated with timely acquisition, and evaluated the influence of time-to-ECG on in-hospital mortality.
Methods
We measured the door-to-ECG time for 903 of 2140 patients in the emergency department of Far Eastern Memorial Hospital with a diagnosis of ACS from January 1, 2016 to December 31, 2018, via a retrospective chart review. The primary outcome was in-hospital mortality. Outcome analysis of mortality was conducted using multivariable logistic regression. The secondary outcome was to determine which factors influenced whether or not a patient received an ECG within 10 min. The analysis was conducted using multiple logistic regression.
Results
The median time-to-ECG was 5 min (interquartile range: 4-11 min) in all patients. In multivariable logistic regression analysis, we found that older age and more severe heart-broken index were significantly related to timely ECG acquisition. In-hospital mortality was higher in those in whom ECG was performed after more than 10 min. However, in the multivariable logistic regression analysis, it did not have a significant positive correlation with ECG acquisition time.
Conclusions
Timely ECG acquisition owing to the triage protocol at our institution, the heart-broken index, led to early PCI and thus better outcomes for the ACS patients in this study. The implementation of a protocol-driven timely evaluation of patients with ACS and prompt PCI are important.
Keywords: Acute coronary syndrome, Electrocardiogram, Non-ST-segment elevation myocardial infarction and unstable angina, ST-elevation myocardial infarction
Abbreviations
ACS, Acute coronary syndrome
CABG, Coronary artery bypass graft
Cis, Confidence intervals
ECG, Electrocardiogram
ECMO, Extracorporeal membrane oxygenation
ED, Emergency department
IQR, Interquartile range
NSTE-ACS, Non-ST-segment elevation myocardial infarction and unstable angina
Ors, Odds ratios
PCI, Percutaneous coronary intervention
SD, Standard deviation
STEMI, ST-elevation myocardial infarction
INTRODUCTION
Acute coronary syndrome (ACS) is an important issue in Taiwan because of its high prevalence and fatal outcomes. The wide adoption of reperfusion strategies, including percutaneous coronary intervention (PCI), thrombolytic therapy, and the administration of antiplatelet agents has significantly reduced the adverse outcomes of ACS.1,2 A shortened time to reperfusion is associated with lower mortality and morbidity and has also been endorsed by contemporary consensus. Thus, a target 90 min door-to-balloon time is generally recommended among national guidelines.3 To achieve an optimal door-to-balloon time, several strategies have been applied, with the early recognition of ACS always the main consideration.
The identification of an ACS event, including ST-segment-elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction and unstable angina (NSTE-ACS) is mainly based on clinical symptoms, an electrocardiogram (ECG), and cardiac enzymes.4 Therefore, rapid acquisition of the ECG plays a crucial step in the diagnosis and further management decisions. A door-to-ECG time within 10 min is preferable once the patient arrives at the hospital, especially for those with ongoing chest pain.5
It is also noteworthy that the incidence of NSTE-ACS has been greater than that of STEMI since 2004 and is still increasing, and that higher rates of mortality and morbidity have been reported after discharge in patients with NSTE-ACS compared to patients with STEMI.6 Although several studies have demonstrated a correlation of prolonged door-to-ECG time with delayed reperfusion therapy in patients with STEMI, leading to worse outcomes, there is still limited evidence to support the same association in patients with NSTE-ACS. Additionally, the effect of the practical application of a 10 min door-to-ECG time is still unclear in different clinical settings, as well as the determining factors to achieve such a goal. It is important to examine this issue thoroughly to develop bet-ter protocols for optimal door-to-ECG time.
Accordingly, the aim of this study was to evaluate the current performance in obtaining an ECG within 10 min at our institution. We also identified the factors related to the door-to-ECG time and the consequences of delayed acquisition according to the recommended time interval.
METHODS
Study design
This was a retrospective cohort study conducted at Far Eastern Memorial Hospital, a tertiary medical hospital in New Taipei City in northern Taiwan with an annual census of approximately 130,000 emergency department (ED) visits. The incidence of ACS at the ED is around 500 cases a year, with approximately 300 cases of primary PCI a year. We retrospectively enrolled patients older than 18 years who were admitted through the ED with a main diagnosis of ACS from January 1, 2016 to December 31, 2018. The diagnosis of ACS was based on the ESC/ACCF/AHA/WHF (European Society of Cardiology, American College of Cardiology Foundation, American Heart Association, and World Heart Federation) Third Universal Definition. The main diagnosis was identified using ICD codes (ICD 9: 410.0-410.9, 411.1, 413.9, ICD 10 I21.0-I21.A9, I20.0). We excluded patients who were referred from the outpatient department, were transferred from other hospitals, or who had an out-of-hospital cardiac arrest. This study was approved by the Institutional Research Ethics Review Committee of Far Eastern Memorial Hospital, and the committee waived the requirement for informed consent.
Data collection and outcomes
Data on the patient baseline characteristics, mode and timing of ED visit, symptoms and signs, heart-broken index, door-to-ECG time, door-to-balloon time, intervention, disease severity on presentation (Killip score), length of hospital stay, discharge date, in-hospital mortality, and readmission were collected via chart review. Baseline characteristics included age, sex, and past medical history (diabetes, hypertension, coronary artery disease, end-stage renal disease). Mode and timing of the ED visit included transport to the ED by the patient themselves or by ambulance, and three shifts (0:00-8:00, 8:00-16:00, 16:00-24:00) of the hospital staff. Symptoms and signs included the patient’s symptoms, heart rate, systolic blood pressure, and diastolic blood pressure at triage. Heart-broken index is a scoring system conducted at triage in our hospital, which is calculated using typical symptoms of ACS1 and composed of four items: chest pain, cold sweat, epigastric pain, and shortness of breath. Each item is scored as one, except for chest pain which is scored as two. If the heart-broken index is greater than or equal to two, then triage would immediately perform ECG. The index has shown a high diagnostic sensitivity for STEMI of 97%7 and is regularly used during ED triage at Far Eastern Memorial Hospital. Door-to-ECG time was defined as the interval from the patient registration at the hospital to when the ECG was obtained. Door-to-balloon time was defined as the interval from the patient registration at the hospital to first balloon inflation or device deployment in the procedure. Interventions included PCI, application of extracorporeal membrane oxygenation (ECMO), and coronary artery bypass graft (CABG) surgery. In-hospital mortality was defined as any mortality during hospitalization. Both cardiovascular and non-cardiovascular deaths were included in the analysis. The enrolled patients were first divided into two groups: those with STEMI and those with NSTE-ACS. We then further divided the patients into four groups according to whether the door-to-ECG time was > 10 min or ≤ 10 min. The primary outcome of this study was in-hospital mortality. We aimed to determine whether the length of door-to-ECG time would influence in-hospital mortality. The secondary outcome was to determine which factors influenced whether or not a patient received an ECG within 10 min.
Statistical analysis
Continuous variables were summarized using the mean and standard deviation (SD) or median values with interquartile range (IQR) based on their distributions. Categorical variables were summarized as number and percentage. The patient baseline characteristics, mode and timing of the ED visit, symptoms and signs, heart-broken index, door-to-ECG time, door-to-balloon time, intervention, disease severity on presentation (Killip score), length of hospital stay, discharge date, and mortality were compared between groups using the Student’s t test or Wilcoxon rank sum test for continuous variables based on the distributions, and chi-square test or Fisher’s exact test for categorical variables when appropriate. Outcome analysis of in-hospital mortality was conducted by multivariable logistic regression using all-possible regression. Age, sex, past medical history (diabetes, hypertension, coronary artery disease, end-stage renal disease), heart-broken index, door-to-balloon time, intervention (PCI, CABG, ECMO), and Killip score were included in the regression model. To evaluate which factors influenced whether the door-to-ECG time was within a 10-min period or not, multiple logistic regression was used to analyze patient variables. The results were expressed as odds ratios (ORs) and 95% confidence intervals (CIs). A p-value of 0.05 or less was considered to be statistically significant. Statistical analysis was conducted with IBM SPSS software, version 25.0.
RESULTS
The study sample comprised 2140 patient visits to the ED between January 1, 2016 and December 31, 2018. Patients referred from outpatient departments (87 visits), transferred from other hospitals (277 visits), who arrived with out-of-hospital cardiac arrest (188 visits), were discharged against medical advice (1 visit), and who had duplicate data (673 visits) were excluded, leaving 903 patients with 914 patient visits (Figure 1). Of the 914 patient visits, 475 visits (52%) involved STEMI and 439 visits (48%) involved NSTE-ACS. The demographics are shown in Table 1. Those who had ECG performed within 10 min more frequently presented with typical symptoms such as chest pain, epigastric pain, cold sweat, dyspnea and a higher heart-broken index. The door-to-balloon time was significantly shorter in the STEMI patients who had ECG performed within 10 min.
Figure 1.
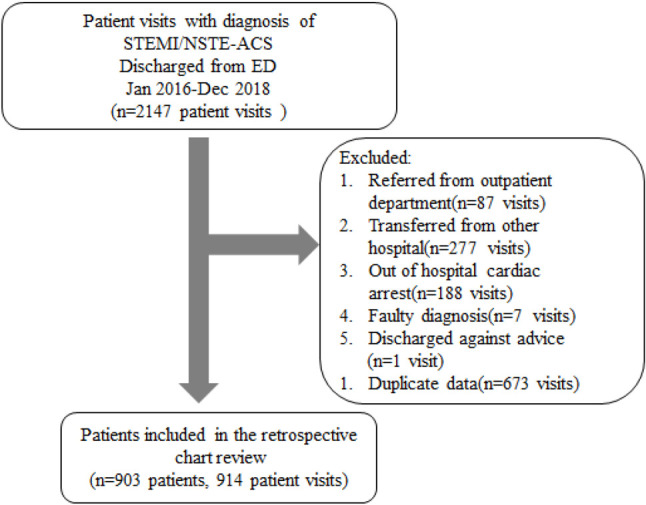
Data collection flow chart. NSTE-ACS, non-ST-segment-elevation myocardial infarction and unstable angina; STEMI, ST-segment-elevation myocardial infarction.
Table 1. Patient demographics.
| All (n = 914) | STEMI | p | NSTE-ACS | p | |||
| ECG > 10 min (n = 89) | ECG ≤ 10 min (n = 386) | ECG > 10 min (n = 159) | ECG ≤ 10 min (n = 280) | ||||
| Mean age (± SD) | 60.86 ± 12.7 | 61.39 ± 14.8 | 58.2 ± 11.1 | 0.06 | 67.27 ± 12.9 | 60.63 ± 12.7 | < 0.01 |
| Sex | 0.09 | 0.06 | |||||
| Male | 743 (81.3%) | 70 (78.7%) | 331 (85.8%) | 116 (73%) | 226 (80.7%) | ||
| Female | 171 (18.7%) | 19 (21.3%) | 55 (14.2%) | 43 (27%) | 54 (19.3%) | ||
| Time to visit ED | 0.75 | 0.35 | |||||
| Night | 230 (25.5%) | 21 (23.6%) | 89 (23.1%) | 50 (31.4%) | 70 (25%). | ||
| Day | 362 (39.6%) | 41 (46.1%) | 164 (42.5%) | 54 (34%) | 103 (36.8%) | ||
| Evening | 322 (35.2%) | 27 (30.3%) | 133 (34.5%) | 55 (34.6%) | 107 (38.2%) | ||
| SBP (± SD) | 137 ± 32.5 | 137.2 ± 35.7 | 134.3 ± 31.4 | 0.48 | 140.1 ± 35.2 | 141.7 ± 30.6 | 0.63 |
| DBP (± SD) | 85 ± 21.7 | 86.26 ± 23.4 | 85.45 ± 21.83 | 0.76 | 83 ± 21.7 | 85 ± 21 | 0.36 |
| MAP (± SD) | 102.6 ± 23.9 | 103.24 ± 26.1 | 101.7 ± 23.7 | 0.60 | 102 ± 24.7 | 103.9 ± 22.8 | 0.43 |
| HR (± SD) | 84.1 ± 27.5. | 87.44 ± 25.7 | 78.5 ± 31.5 | 0.01 | 93.4 ± 55.5 | 85.3 ± 22.6 | < 0.01 |
| Symptoms | |||||||
| Chest pain | 711 (77.8%) | 41 (46.1%) | 359 (93.0%) | < 0.01 | 43 (27%) | 268 (95.7%) | < 0.01 |
| Epigastric pain | 22 (2.4%) | 8 (9%) | 7 (1.8%) | < 0.01 | 2 (1.3%) | 5 (1.8%) | 1.00 |
| Cold sweat | 192 (21%) | 14 (15.7%) | 124 (32.1%) | < 0.01 | 7 (4.4%) | 47 (16.8%) | < 0.01 |
| Dyspnea | 222 (24.3%) | 31 (34.8%) | 76 (19.7%) | < 0.01 | 54 (34%) | 61 (21.8%) | < 0.01 |
| Heart-broken index | < 0.01 | < 0.01 | |||||
| 0 | 114 (12.5%) | 22 (24.7%) | 15 (3.9%) | 69 (43.4%) | 8 (2.9%) | ||
| 1 | 88 (9.6%) | 25 (28.1%) | 12 (3.1%) | 46 (28.9%) | 5 (1.8%) | ||
| 2 | 433 (47.4%) | 24 (27%) | 196 (50.8%) | 31 (19.5%) | 182 (65%) | ||
| 3 | 214 (23.4%) | 10 (11.2%) | 131 (33.9%) | 11 (6.9%) | 62 (22.1%) | ||
| 4 | 64 (7%) | 8 (9%) | 32 (8.3%) | 2 (1.3%) | 22 (7.9%) | ||
| Arrival at ED | |||||||
| By patient | 635 (69.5%) | 64 (71.9%) | 253 (65.5%) | 0.25 | 111 (69.8%) | 207 (73.9%) | 0.35 |
| ECG time (IQR) | 5 (4-11) | 31 (13-57) | 4 (3-5) | 38 (24-68.5) | 4 (3-6) | ||
| Door-to-balloon time | < 0.01 | 0.54 | |||||
| ≤ 90 min | 356 (38.9%) | 37 (41.6%) | 317 (82.1%) | 0 (0%) | 2 (0.7%) | ||
| > 90 min | 558 (61.1%) | 52 (58.4%) | 69 (17.9%) | 159 (100%) | 278 (99.3%) | ||
| Medical history | |||||||
| DM | 327 (35.8%) | 37 (41.6%) | 112 (29%) | 0.02 | 77 (48.4%) | 101 (36.1%) | 0.01 |
| HTN | 594 (65%) | 56 (62.9%) | 224 (58%) | 0.39 | 121 (76.1%) | 193 (68.9%) | 0.11 |
| CAD | 171 (18.7%) | 7 (7.9%) | 55 (14.2%) | 0.10 | 45 (28.3%) | 64 (22.9%) | 0.20 |
| ESRD | 52 (5.7%) | 3 (3.4%) | 8 (2.1%) | 0.44 | 18 (11.3%) | 23 (8.2%) | 0.28 |
| Admission days (SD) | 7.28 ± 8.3 | 6.94 ± 7.19 | 5.84 ± 7.69 | 0.22 | 11.24 ± 11.13 | 7.13 ± 6.71 | < 0.01 |
| Killip score | |||||||
| Missing | 241 (26.4%) | 6 (6.7%) | 35 (9.1%) | 79 (49.7%) | 121 (43.2%) | ||
| I | 439 (48%) | 52 (58.4%) | 239 (61.9%) | 33 (20.8%) | 115 (41.1%) | ||
| II | 97 (10.6%) | 10 (11.2%) | 56 (14.5%) | 17 (10.7%) | 14 (5%) | ||
| III | 50 (5.5%) | 6 (6.7%) | 9 (2.3%) | 18 (11.3%) | 17 (6.1%) | ||
| IV | 87 (9.5%) | 15 (16.9%) | 47 (12.2%) | 12 (7.5%) | 13 (4.6%) | ||
| Disposition procedure | |||||||
| Death | 64 (7%) | 9 (10.1%) | 16 (4.1%) | 0.02 | 24 (15.1%) | 15 (5.4%) | < 0.01 |
| ECMO | 40 (4.4%) | 5 (5.6%) | 15 (3.9%) | 0.56 | 9 (5.7%) | 11 (3.9%) | 0.40 |
| PCI | 905 (99%) | 89 (100%) | 383 (99.2%) | 1.00 | 155 (97.5%) | 278 (99.3%) | 0.20 |
| CABG | 43 (4.7%) | 1 (1.1%) | 11 (2.8%) | 0.71 | 17 (10.7%) | 14 (5%) | 0.03 |
CABG, coronary artery bypass graft; CAD, coronary artery disease; DBP, diastolic blood pressure; DM, diabetes mellitus; ECG, electrocardiogram; ECMO, extracorporeal membrane oxygenation; ED, emergency department; EMS, emergency medicine system; ESRD, end-stage renal disease; HR, heart rate; HTN, hypertension; IQR, interquartile range; MAP, mean arterial pressure; NSTE-ACS, non-ST-segment-elevation myocardial infarction and unstable angina; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SD, standard deviation; STEMI, ST-segment-elevation myocardial infarction.
The median time-to-ECG was 5 min (IQR: 4-11 min) in all patients, 4 min (IQR: 3-5 min) in the STEMI patients with a door-to-ECG time < 10 min, 31 min (IQR: 13-57 min) in the STEMI patients with a door-to-ECG time > 10 min, 4 min (IQR: 3-6 min) in the NSTE-ACS patients with a door-to-ECG time < 10 min, and 38 min (24-68.5 min) in the NSTE-ACS patients with a door-to-ECG time > 10 min. We found that the patients with a door-to-ECG time > 10 min usually presented with atypical symptoms such as dizziness, abdominal pain and cough. They were often initially diagnosed with vertigo, gastritis and respiratory tract infections. Whether or not a patient had an ECG performed within 10 min was significantly associated with age, sex, diagnosis, first heart rate at the ED, heart-broken index, and medical history. We conducted multivariable logistic regression analysis of these factors (Table 2), and found that younger age and more severe heart-broken index were significantly related to an early ECG acquisition. In the subgroup analysis, early ECG acquisition time was associated with younger age and more severe heart-broken index in the patients with NSTE-ACS, but only heart-broken index was a predictor of a timely ECG acquisition in the patients with STEMI.
Table 2. Multivariable logistic regression analysis of factors which influence door-to-ECG time.
| Independent | All | STEMI | NSTE-ACS | ||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Male | 0.93 | (0.57-1.51) | 0.76 | 0.77 | (0.37-1.60) | 0.48 | 1.06 | (0.52-2.14) | 0.88 |
| Age | 0.98 | (0.97-0.99) | 0.02 | 0.99 | (0.97-1.02) | 0.73 | 0.97 | (0.95-0.99) | 0.01 |
| HR | 0.99 | (0.99-1.00) | 0.10 | 0.99 | (0.98-1.01) | 0.27 | 0.99 | (0.98-1.01) | 0.15 |
| DM | 0.90 | (0.59-1.36) | 0.62 | 0.78 | (0.43-1.40) | 0.41 | 0.98 | (0.54-1.82) | 0.98 |
| HTN | 0.71 | (0.46-1.09) | 0.12 | 0.76 | (0.43-1.35) | 0.35 | 0.81 | (0.41-1.59) | 0.54 |
| CAD | 0.86 | (0.53-1.41) | 0.55 | 1.87 | (0.69-5.07) | 0.216 | 0.66 | (0.34-1.27) | 0.21 |
| ESRD | 1.12 | (0.51-2.49) | 0.78 | 0.80 | (0.14-4.52) | 0.80 | 1.46 | (0.52-4.11) | 0.47 |
| Heart-broken index* | < 0.01 | < 0.01 | < 0.01 | ||||||
| Heart-broken index (1) | 1.15 | (0.56-2.36) | 0.71 | 0.73 | (0.27-1.98) | 0.54 | 1.24 | (0.37-4.19) | 0.73 |
| Heart-broken index (2) | 26.8 | (15.5-46.5) | < 0.01 | 11.2 | (5.1-24.9) | < 0.01 | 52.1 | (22.2-121) | < 0.01 |
| Heart-broken index (3) | 33.9 | (17.6-65.4) | < 0.01 | 16.4 | (6.42-41.8) | < 0.01 | 56.6 | (20.3-157) | < 0.01 |
| Heart-broken index (4) | 19.8 | (8.6-45.2) | < 0.01 | 4.88 | (1.79-13.8) | < 0.01 | 116 | (21.9-614) | < 0.01 |
CAD, coronary artery disease; CI, confidence interval; DM, diabetes mellitus; ECG, electrocardiogram; ESRD, end-stage renal disease; HR, heart rate; HTN, hypertension; NSTE-ACS, non-ST-segment elevation myocardial infarction and unstable angina; OR, odds ratio; STEMI, ST-segment-elevation myocardial infarction.
* The reference group of heart-broken index is score 0.
The death rate was higher in those who had ECG performed after more than 10 min. The in-hospital mortality rate was 7% in all patients, 5.3% in the patients with STEMI, and 8.9% in the patients with NSTE-ACS. It was higher in those who had ECG performed after more than 10 min (Table 1). However, in the multivariable logistic regression analysis, it did not have a significant positive correlation with ECG acquisition time (OR: 1.25; 95% CI: 0.54-2.89; p = 0.61) (Table 3). In the subgroup analysis, in-hospital mortality rate was also not significantly associated with ECG acquisition time in both the patients with STEMI (OR: 1.31; 95% CI: 0.38-4.55; p = 0.67) and NSTE-ACS (OR: 1.56; 95% CI: 0.07-34.77; p = 0.78) (Table 3).
Table 3. Multivariable logistic regression analysis of in-hospital mortality.
| Independent | Reference category | All | STEMI | NSTE-ACS | |||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |||||
| ECG time < 10 min | ECG time > 10 min | 1.25 | 0.54 | 2.89 | 0.61 | 1.31 | 0.38 | 4.55 | 0.67 | 1.56 | 0.07 | 34.77 | 0.78 |
| DM | 0.72 | 0.32 | 1.63 | 0.43 | 0.7 | 0.21 | 2.38 | 0.57 | 1.08 | 0.2 | 6.16 | 0.93 | |
| HTN | 0.88 | 0.41 | 1.9 | 0.74 | 1.15 | 0.33 | 3.99 | 0.82 | 0.13 | 0.01 | 1.43 | 0.1 | |
| CAD | 2.08 | 0.84 | 5.14 | 0.11 | 1.49 | 0.33 | 6.74 | 0.61 | 11.48 | 1.51 | 87.03 | 0.02 | |
| ESRD | 0.98 | 0.17 | 5.74 | 0.98 | 1.35 | 0.15 | 12.6 | 0.79 | 56.72 | 0.8 | 392.4 | 0.062 | |
| ECMO | 0.99 | 0.99 | 89.52 | 60.5 | 774.4 | 0.001 | |||||||
| PCI | 0.25 | 0.02 | 2.93 | 0.27 | 12.4 | 0.3 | 39.6 | 0.99 | 54.99 | 0.1 | 289.2 | 0.999 | |
| CABG | 1.36 | 0.29 | 6.33 | 0.7 | 10.4 | 1.48 | 73.2 | 0.02 | 11.16 | 0.44 | 281.9 | 0.14 | |
| Male | Female | 1.42 | 0.53 | 3.79 | 0.48 | 1.07 | 0.24 | 4.73 | 0.9 | 0.06 | 0.01 | 0.66 | 0.02 |
| Age | 1.01 | 0.98 | 1.04 | 0.7 | 0.97 | 0.93 | 1.02 | 0.22 | 1.17 | 1.1 | 1.28 | 0.002 | |
| PCI < 90 min | PCI > 90 min | 1.07 | 0.37 | 3.1 | 0.9 | 1.13 | 0.26 | 4.93 | 0.87 | 7.47 | 0.08 | 18.37 | 0.1 |
| Heart-broken index* | 0.16 | 0.27 | 0.43 | ||||||||||
| Heart-broken index (1) | 43.3 | 0.56 | 182 | 0.99 | 9.74 | 0.06 | 16.4 | 0.99 | 6.9 | 0.02 | 19.83 | 0.99 | |
| Heart-broken index (2) | 98.8 | 0.76 | 328 | 0.99 | 74.6 | 0.34 | 254 | 0.99 | 5.61 | 0.05 | 24.44 | 0.99 | |
| Heart-broken index (3) | 222 | 0.62 | 431 | 0.99 | 19 | 0.15 | 65.8 | 0.99 | 6.61 | 0.04 | 29.22 | 0.99 | |
| Heart-broken index (4) | 352 | 0.44 | 511 | 0.99 | 20.4 | 0.55 | 825 | 0.99 | 8.38 | 0.05 | 36.08 | 0.99 | |
| Killip score# | 0.01 | 0.01 | 0.99 | ||||||||||
| Killip score (1) | 17.5 | 6.71 | 45.5 | 0.01 | 41.7 | 8.4 | 201 | 0.01 | 1.28 | 0.1 | 15.93 | 0.99 | |
| Killip score (2) | 4.65 | 1.73 | 12.5 | 0.01 | 6.02 | 1.35 | 27 | 0.02 | 1.22 | 0.07 | 22.23 | 0.85 | |
| Killip score (3) | 9.52 | 2.36 | 38.5 | 0.01 | 11.4 | 0.97 | 124 | 0.05 | 0.992 | 0.04 | 25.46 | 0.9 |
CABG, coronary artery bypass graft; CAD, coronary artery disease; CI, confidence interval; DM, diabetes mellitus; ECG, electrocardiogram; ECMO, extracorporeal membrane oxygenation; ESRD, end-stage renal disease; HTN, hypertension; NSTE-ACS, non-ST-segment elevation myocardial infarction and unstable angina; OR, odds ratio; PCI, percutaneous coronary intervention; STEMI, ST-segment-elevation myocardial infarction.
* The reference group of heart-broken index is score 0. # The reference group of Killip score is class I.
DISCUSSION
The American Heart Association guidelines for STEMI and NSTE-ACS recommend that an ECG should be obtained in all patients with suspected STEMI/NSTE-ACS and ongoing chest pain within 10 min of presentation.5,8 Delayed ECG acquisition has been associated with delayed fibrinolytic treatment in patients with STEMI.9 However, it is unclear whether ECG acquisition time would influence the treatment of patients with NSTE-ACS. Therefore, this study examined the current practice with regards to ECG acquisition, identified factors that influenced adherence, and explored their relationships with adverse clinical outcomes in patients with STEMI/NSTE-ACS who presented to the ED.
The results of our study suggested that the current ED practice of obtaining an ECG adhered to the recommended guidelines in patients with chest pain. The median time-to-ECG was 5 min (IQR: 4-11 min) in all patients. However, for those who had a door-to-ECG time > 10 minutes, the median time-to-ECG was 31 min in the STEMI patients and 38 min in the NSTE-ACS patients. The delayed acquisition time may be due to the initial atypical presentations. Prior studies have also evaluated potential predictors of a delay in the acquisition of an ECG. Lambrew et al.10 reported that sex and mode of transportation were factors affecting the time-to-ECG in patients with STEMI. Phelan et al.11 identified two main causes of a delayed ECG acquisition time > 10 min: priority delay (e.g., completing triage and registration data entry tasks before the ECG), and failure to recognize patients with non-chest pain STEMI symptoms.
Our findings demonstrated that age and heart-broken index were predictors of a timely ECG acquisition in the patients with NSTE-ACS, but that only heart-broken index was a predictor of an ECG being performed within 10 min of presentation in the patients with STEMI. Multiple studies have shown that a protocol-driven evaluation of patients with STEMI improves time-to-ECG and treatment.12-14 Glickman et al.14 reported that the predictors of patients requiring an immediate ECG in the ED were: age ≥ 30 years with chest pain; age ≥ 50 years with shortness of breath, altered mental status, upper extremity pain, syncope, or generalized weakness; and age ≥ 80 years with abdominal pain or nausea/vomiting. They had a sensitivity of 91.9% for STEMI and a negative predictive value of 99.98%. A simple ECG prioritization rule based on age and presenting symptoms in the ED can identify patients during triage who are at high risk of STEMI so that they receive an immediate 12-lead ECG.
Other triage protocols are also applied for ACS patients in the hospitals in Taiwan. For example, the ASAP Score developed by China Medical University Hospital is composed of: male age > 50 years, female age > 60 years, symptoms of altered consciousness/general weakness/epigastric pain/vomiting and past history of diabetes mellitus/hypertension/previous ACS event. The ECG is performed within 10 min in patients with more than 3 of these characteristics. The score has been shown to improve the recognition of an atypical presentation of ACS and lower the ECG acquisition time. In addition, with the application of artificial intelligence systems to assist in the interpretation of ECG, the specificity of diagnosing ACS was 86% and positive predictive value was 100%.17 Although the actual sensitivity and specificity were not obtained in this study, the heart-broken index in our hospital is composed of only 4 symptoms, and it demonstrated good results with regards to timely ECG acquisition. Future triage protocols and assisted interpretation of ECG by artificial intelligence systems may significantly lower the door-to-balloon time and improve the outcomes of ACS patients. This triage system may also be promoted in more hospitals.
In-hospital mortality was higher in those who had ECG performed after more than 10 min. However, in the multivariable logistic regression analysis, ECG acquisition time was not significantly associated with in-hospital mortality, and the results remained the same in both the patients with STEMI and in the patients with NSTE-ACS. We supposed that those who had ECG performed after more than 10 min presented with atypical symptoms and also had more comorbidities. Therefore, the in-hospital mortality rate was higher but not associated with ECG acquisition time in the multivariable logistic regression analysis. Although we found that delayed ECG acquisition was not predictive of adverse events in our study, Diercks et al.15 found an increase in the risk of recurrent myocardial infarction or death in or out of the hospital in STEMI patients with an ECG acquisition time > 10 min. However, ECG acquisition time was much longer in their study with a median time of 20 min. We propose that the timely ECG acquisition owing to the triage protocol at our institution led to an early PCI. Therefore, ECG acquisition time was not significantly associated with adverse events.
The overall in-hospital mortality rate of the ACS patients in our study was 7%, which is lower than that (16%) reported in 2015 from the population in Taiwan.16 In subgroup analysis, the in-hospital mortality rates of both STEMI and NSTE-ACS patients in our study were also lower (STEMI: 5.26% vs. 7.62%; NSTEMI: 8.88% vs. 13.45%). We propose that the timely ECG acquisition owing to the triage protocol at our institution led to an early PCI and thus better outcomes for the ACS patients in our study.
Limitations
This study has several limitations. First, it is a retrospective study from chart review in a single institution. The study size may be underpowered. Hence, the results cannot be generalized to other institutions or in other countries. A prospective, multicenter, cohort study is still necessary to verify our results. Second, because we used ED discharge diagnosis to group patients, classification error is possible in the patients initially identified as having noncardiac chest pain who were later diagnosed with myocardial infarction based on subsequent laboratory findings. Third, the multivariable logistic regression analysis may have involved overfitting and collinearity. The results should therefore be interpreted with caution.
CONCLUSION
We found that patients with an ECG acquisition time < 10 min usually presented with a younger age and typical symptoms. Timely ECG acquisition owing to the triage protocol with the heart-broken index at our institution led to an early PCI and thus better outcomes for the ACS patients in our study. The implementation of a protocol-driven timely evaluation of patients with ACS and prompt PCI are important.
Acknowledgments
We acknowledged the language editing and proofreading service provided by Enago, Crimson Interactive Incorporated.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 3.Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/ SCAI Guideline for coronary artery revascularization:a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79:e21–e129. doi: 10.1016/j.jacc.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Yin WH, Lu TH, Chen KC, et al. The temporal trends of incidence, treatment, and in-hospital mortality of acute myocardial infarction over 15 years in a Taiwanese population. Int J Cardiol. 2016;209:103–113. doi: 10.1016/j.ijcard.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Jean-Philippe C, Holger T, Emanuele B, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2021;42:2298. [Google Scholar]
- 6.Li YH, Wang YC, Wang YC, et al. 2018 Guidelines of the Taiwan Society of Cardiology, Taiwan Society of Emergency Medicine and Taiwan Society of Cardiovascular Interventions for the management of non ST-segment elevation acute coronary syndrome. J Formos Med Assoc. 2018;117:766–790. doi: 10.1016/j.jfma.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Li AH, Hsu JC, Chen KC, et al. Abstract 15022: using Heart Broken Index to improve the diagnostic accuracy of patient with ST elevation myocardial infarction and shorten door-to-balloon time on emergency room. Circulation. 2011;124:Suppl 21. [Google Scholar]
- 8.O'Gara PT, Kushner FG, Ascheim DD, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction:a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 9.Shi O, Khan AM, Rezai MR, et al. Factors associated with door-in to door-out delays among ST-segment elevation myocardial infarction (STEMI) patients transferred for primary percutaneous coronary intervention: a population-based cohort study in Ontario, Canada. BMC Cardiovasc Disord. 2018;18:204. doi: 10.1186/s12872-018-0940-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambrew CT, Bowlby LJ, Rogers WJ, et al. Factors influencing the time to thrombolysis in acute myocardial infarction. Arch Intern Med. 1997;157:2577–2582. [PubMed] [Google Scholar]
- 11.Phelan MP, Glauser J, Smith E, et al. Improving emergency department door-to-electrocardiogram time in ST segment elevation myocardial infarction. Crit Pathw Cardiol. 2009;8:119–121. doi: 10.1097/HPC.0b013e3181b5a6f3. [DOI] [PubMed] [Google Scholar]
- 12.Takakuwa KM, Burek GA, Estepa AT, et al. A method for improving arrival-to-electrocardiogram time in emergency department chest pain patients and the effect on door-to-balloon time for ST-segment elevation myocardial infarction. Acad Emerg Med. 2009;16:921–927. doi: 10.1111/j.1553-2712.2009.00493.x. [DOI] [PubMed] [Google Scholar]
- 13.Osborne AD, Ali K, Lowery-North D, et al. Ability of triage decision rules for rapid electrocardiogram to identify patients with suspected ST-elevation myocardial infarction. Crit Pathw Cardiol. 2012;11:211–213. doi: 10.1097/HPC.0b013e31826f4e8e. [DOI] [PubMed] [Google Scholar]
- 14.Glickman SW, Shofer FS, Wu MC, et al. Development and validation of a prioritization rule for obtaining an immediate 12-lead electrocardiogram in the emergency department to identify ST-elevation myocardial infarction. Am Heart J. 2012;163:372–382. doi: 10.1016/j.ahj.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Diercks DB, Kirk JD, Lindsell CJ, et al. Door-to-ECG time in patients with chest pain presenting to the ED. Am J Emerg Med. 2006;24:1–7. doi: 10.1016/j.ajem.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Lee CH, Fang CC, Tsai LM, et al. Patterns of acute myocardial infarction in Taiwan from 2009 to 2015. Am J Cardiol. 2018;122:1996–2004. doi: 10.1016/j.amjcard.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 17.Chang KC, Hsieh PH, et al. Usefulness of machine learning-based detection and classification of cardiac arrhythmias with 12-lead electrocardiograms. Can J Cardiol. 2021;37:94–104. doi: 10.1016/j.cjca.2020.02.096. [DOI] [PubMed] [Google Scholar]


