Abstract
Splenic lymphangioma is a benign cystic tumor that develops as a result of lymphatic vessels’ congenital abnormalities. It is a rare condition that mostly occurs in children and young adults. Due to the lack of typical symptoms and signs, splenic lymphangioma is difficult to diagnose and often incidentally revealed during radiological examinations. We report a case of a 55-year-old Asian female, who presented with left upper quadrant abdominal pain in the past 3 days. She had mild upper abdominal tenderness, with no other specific findings. Abdominal contrast material–enhanced computed tomography revealed three hypodense lesions arising from a normal-sized spleen. The histologic findings after laparoscopic splenectomy demonstrated a 3-cm-diameter yellowish-white tumor made up of multiple cystic structures. Primary benign splenic tumors are exceedingly rare, especially in adults over 20. While small lesions are mostly asymptomatic, bigger lesions can cause organ compression or even rupture. Therefore, even in adults with pain in the left upper quadrant abdomen or enlarged spleen, splenic lymphangioma should be taken into account in the differential diagnosis. The case serves as an example of a rare congenital splenic tumor. Treatment of this benign splenic abnormality with laparoscopic splenectomy is a good, safe approach.
Keywords: Lymphangioma, splenic lymphangioma, splenic tumor, laparoscopic splenectomy
Introduction
Lymphangiomas are considered to be benign congenital malformations of lymphatic vessels, with more than 90% of cases occurring in the neck and axillary region.1,2 The less commonly cases, associated with lymphangiomatosis syndrome, are found in the mediastinum, lung, liver and retroperitoneum.2,3 Meanwhile, isolated splenic lymphangiomas are considered a much rarer form. Since first described by Frink in 1885, splenic lymphangioma was reported in less than 0.007% of all tumors at autopsy and surgical intervention.4,5 It is characterized by the increase in the size and number of thin-walled lymphatic vessels which are forming a single or multiple benign cystic tumor in the spleen.2,6 Splenic lymphangioma typically occurrs in children between 80% and 90% of cases, so it is extremely rare in adults.7
The clinical manifestations of splenic lymphangioma are related to lesion size. Large lesions may cause nonspecific symptoms, including left upper quadrant pain, nausea, vomiting, a palpable mass and the loss of appetite.2–4 Splenic lymphangioma is usually detected incidentally on imaging findings.2 Splenectomy is the main choice of treatment to avoid serious complications.7 In this article, we report a case of a 55-year-old female who was hospitalized with the increasing upper left abdominal pain. The abdominal ultrasound showed a heterogeneous echogenic structure in the spleen. Splenic lymphangioma was diagnosed by laparoscopic splenectomy, histopathology and immunohistochemistry.
Case presentation
A 55-year-old female patient was admitted to the hospital after 3 days of increasing upper left abdominal pain. The patient had no history of fever, weight loss, hematemesis or melena. Vital signs revealed a body temperature of 37°C, pulse rate of 88°beats per minute, respiratory rate of 18 breaths per minute and blood pressure of 150/80 mm°Hg. Physical examination showed mild upper abdominal tenderness. The results of hematological, biochemical and coagulation tests were within normal limits. The patient had no history of previous abdominal surgery or trauma. Her past medical and family histories showed no known comorbidities. The abdominal ultrasound showed an anechoic cystic structure, 3.4 × 3.4 cm2, in the spleen. Spleen border was normal, normal size and splenic vein not dilated.
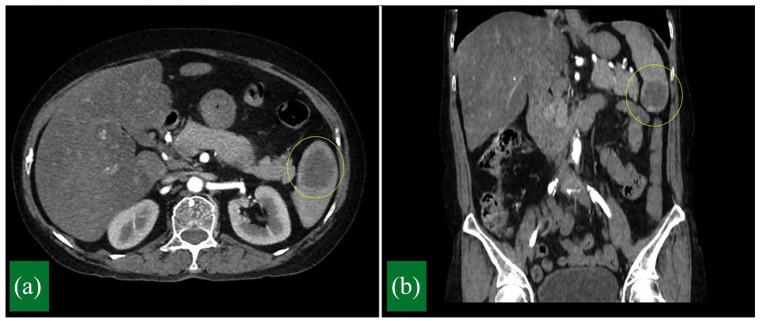
The abdominal computed tomography scan with intravenous contrast showed normal size spleen with three hypodense lesions. The largest lesion was in the lower pole of the spleen and measured 3.4 × 2.4 × 2.6 cm3 (Figure 1). This lesion presented peripheral nodular enhancement in the arterial phase and progressive peripheral enhancement in the later phases. No other abnormalities were detected in the abdomen. The initial differential diagnosis included hamartoma, hemangioma, lymphangioma and lymphoma. Therefore, we indicated laparoscopic splenectomy to confirm diagnosis and relieve symptoms.
Figure 1.
Contrast-enhanced computed tomography. (a) Axial view and (b) coronal view showing a hypodense lesion in the lower pole of the spleen.
Two weeks before the surgery, the patient received pneumococcal vaccination. After administering general anesthesia, the nasogastric tube and Foley catheter were inserted. The patient was placed in a supine position with a right rotation of 30°. A 10 mm trocar was inserted below the umbilicus and CO2 pneumoperitoneum at 12 mm°Hg. The remaining trocars were established consisting of a 5 mm trocar in the the right upper quadrant, another 5 mm trocar in the epigastrium and a 12 mm trocar in the left subcostal area. During operation, the tumor was found in the lower pole of the spleen. The procedure was started by dissecting the splenocolic and phrenocolic ligaments for splenic flexure mobilization. Subsequently, the gastrosplenic ligament was separated till the upper pole of spleen to expose the pancreatic tail and the splenic hilum. The short gastric vessels were controlled with LigaSure. The splenophrenic ligament and the posterior of splenic pedicle were freed. Endo GIA 60 mm Articulating Tri-Staple was used for control and transection of the splenic vessels, and the pancreatic tail was protected. An elastic drainage tube was placed in the splenic fossa. The intact spleen was removed through an incision approximately 4 cm in the umbilical trocar. The operative time was 125 min, and the estimated blood loss was 100°mL. The nasogastric tube was removed at the end of the operation.
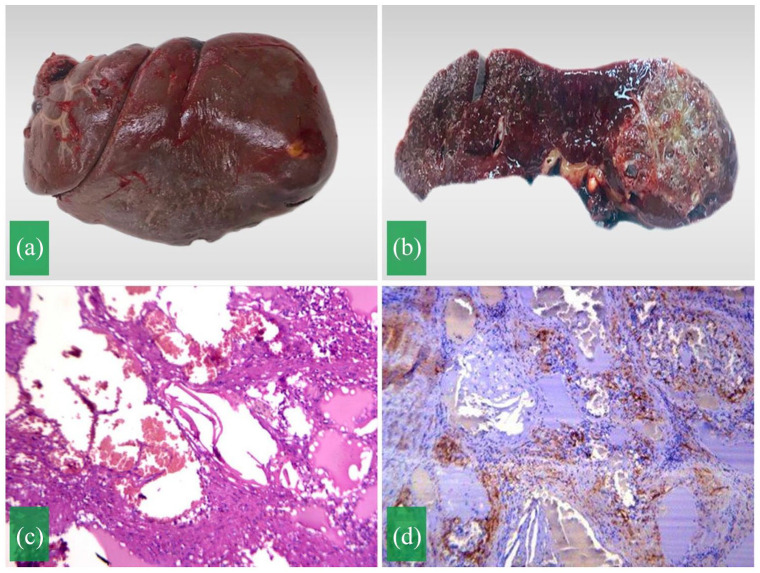
The spleen was sent for histopathological evaluation. On macroscopic examination, the spleen measured 10 × 6 × 4 cm3 (Figure 2(a)). The cut section showed brown-red tissue and a yellowish-white tumor with a diameter of 3 cm (Figure 2(b)). On microscopic examination, multiple cystic structures with variable sizes lined by flattened endothelial cells (Figure 2(c)). These structures are thin-walled and contained lymphocytes and red blood cells. Immunohistochemical stains showed the cells were positive for CD34 (Figure 2(d)), and negative for CK AE1/AE3, P63, desmin and Ki67. The histopathology results were consistent with the diagnosis of splenic lymphangioma. The patient had a stable recovery from the surgery and was discharged on the sixth postoperative day. She remained stable after 6 months of follow-up.
Figure 2.
(a) Gross appearance of the spleen with tumor. (b) The gross-section revealing a yellowish-white tumor in the lower pole of the spleen. (c) Microscopic image presenting multiple cysts with variable sizes lined by flattened endothelial cells. (d) Immunohistochemical stain showing positive for CD34.
Discussion
Splenic lymphangioma remains poorly reported in the literature with only fewer than 200 cases, since the first case was described by Frink in 1885.2 Most of cases occur in children before 2 years of age, accounting for 80%–90% and a very small number of cases are found in adults.7,8 In most cases, splenic lymphangiomas are associated with synchronous or metachronous cystic hygroma in the neck, or lymphangiomatosis syndrome or Klippel–Trenaunay syndrome.2,7,8 There were only nine cases of solitary splenic lymphangioma reported from 1990 to 2010.9 Isolated lymphangiomatosis of spleen, thus, is an extremely rare condition, especially in aldults.
The clinical features are asymptomatic or not usually typical, including left upper quadrant pain, nausea, loss of appetite or abdominal distension. In some cases, patients were admitted to the hospital due to the splenomegaly with the compression of neighboring organs. In addition, patients can present with some complications such as bleeding, consumptive coagulopathy, hypersplenism or portal hypertension.8,10 Our patient only had the symptom of increasing upper left abdominal pain. Physical examination revealed no sign of splenomegaly, and tests of hematology, biochemistry and coagulation were normal. Hence, radiology examinations were indicated including abdominal ultrasound and computed tomography. The ultrasound result of our patient showed an anechoic cystic structure in the normal-sized spleen. The abdominal computed tomography with intravenous contrast confirmed three hypodense lesions in the spleen. These lesions were close together and peripheral nodular enhancement in the arterial phase. The preoperative differential diagnosis included hamartoma, hemangioma, lymphoma and lymphangioma. No other abnormalities of the abdomen and the absence of lymphadenopathy were detected. Imaging findings of splenic lymphangioma have been little described in the literature and they are not specific.5,11 Most are hypoechoic and anechoic cystic lesions with internal septations and peripheral calcification on ulatrasound.11 Findings of computed tomography include subcapsular thin-walled lesions, hypodense, peripheral rim calcification and no enhancement.3,5 The sign of significant contrast enhancement is not typically seen. Some lesions have septation enhancement, but others have contrast enhancement of solid component.2,3 In our patient, computed tomography showed hypodense lesions with peripheral nodular enhancement in the arterial phase and progressive peripheral enhancement in the later phases. This type of enhancement favored the diagnosis of hemangioma rather than lymphangioma. Nevertheless, characteristics of imaging are not typical. This leads to mistaken identification or difficulty in differentiating splenic lymphangioma from hemangioma or lymphoma according to some studies.8,12 In recent years, image-guided percutaneous biopsies have been widely used in the evaluation of splenic tumors. However, there was still a false negative rates and the hemorrhagic risk has been reported in the literature varies between 0% and 2%.13 For splenic lymphangiomas, percutaneous biopsies are rarely performed because of the hemorrhagic risk and the limited amount of tissue for an accurate diagnosis.14 Therefore, the diagnosis is usually based on the histopathologic findings after splenectomy. In our case, lesion in the spleen pointed to lymphangioma based on imaging studies. If biopsy confirms lymphangioma, surgery should also be indicated. In case of no surgery, the patient needs to be continuously monitored for clinical symptoms, re-examined and possibly performed a second biopsy to re-evaluate the lesion. Moreover, this lesion was no longer small and has caused symptoms. Therefore, we have chosen the method of splenectomy for both histopathological diagnosis after surgery and treament.
According to the literature as well as the previous studies, laparoscopic splenectomy is considered as more effective in treating lymphangioma than other therapeutic treatment such as drainage, irradiation and aspiration.15,16 An excessively large spleen may be a contraindication to laparoscopy. Partial splenectomy is also an option to avoid post-splenectomy infection.7 However, previous studies evaluated that the recurrence rate is more common in cases of incomplete resection.2,7 In addition, surgery should be indicated early to avoid possible complications such as rupture, infection and tumor enlargement.2 In our case, laparoscopic splenectomy was indicated immediately after the diagnosis of suspected lymphangioma. After surgery, the cross-section of the spleen showed a large subcapsular lesion with multiple thin-walled cysts of variable size separated by fibrous tissues. The macroscopic findings of lymphangioma include solitary nodules, multiple nodules or even diffuse lymphangiomatosis.2 On microscopic characteristics, the lesions of lymphangioma are dilated lymphatic vessels and usually situate in subcapsular region. The nodule is composed of a large thick fibrous walled cyst or multiple thin-walled cysts which are surrounded by flat endothelial cells between fat, fibrous and lymphatic structures.2,4,7 Lymphangioma is divided into capillary, cavernous and cystic types. Among them, cystic type is the most common.2,4 Regarding our patient, histopathology result favored the diagnosis of splenic lymphangioma with cystic type. Immunohistochemical exam was continously performed with the result of positive CD34. CK AE1/AE3, P63, desmin and Ki67 were negative. This was also suitable for the diagnosis of lymphangioma of the spleen. The marker CD34 stains the endothelial cells of both blood and lymphatic vessels.2,7 Several authors have evaluated the reactivity for podoplanin (D2-40), which is a selective marker for lymphatic endothelium. Although our facility did not evaluate D2-40, the combination of histopathology and immunohistochemistry exams confirmed splenic lymphangioma.
Conclusion
The referred case presents a rare case of splenic lymphangioma in adults. In spite of its rarity, it should be distinguished from other cystic lesions of the spleen. Laparoscopic splenectomy is performed not only to relieve symptoms but also to obtain histopathological findings for an accurate diagnosis.
Acknowledgments
We thank our colleagues from Department of Pathology, Hue Central Hospital, Hue city, Vietnam, who provided nice pictures of pathology.
Footnotes
Author contributions: Minh Duc Pham: Data collection, conception and design of the article, writing the article.
Minh Thao Nguyen, Minh Tri Thi Vo, Van Trung Hoang: Conception and design of the article, writing the article.
Ngoc Trinh Thi Pham: Writing the article, critical revision and final approval.
All authors approved the final article.
Data availability: All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
ORCID iDs: Van Trung Hoang  https://orcid.org/0000-0001-7857-4387
https://orcid.org/0000-0001-7857-4387
Ngoc Trinh Thi Pham  https://orcid.org/0000-0002-3543-0532
https://orcid.org/0000-0002-3543-0532
References
- 1. de Perrot M, Rostan O, Morel P, et al. Abdominal lymphangioma in adults and children. Br J Surg 1998; 85(3): 395–397. [DOI] [PubMed] [Google Scholar]
- 2. Ioannidis I, Kahn AG. Splenic lymphangioma. Arch Pathol Lab Med 2015; 139(2): 278–282. [DOI] [PubMed] [Google Scholar]
- 3. Yang F. Splenic lymphangioma that manifested as a solid-cystic mass: a case report. World J Gastroenterol 2013; 19(5): 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chung SH, Park YS, Jo YJ, et al. Asymptomatic lymphangioma involving the spleen and retroperitoneum in adults. World J Gastroenterol 2009; 15(44): 5620–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perez A, Perez MEC, Yuga AC, et al. Splenic lymphangioma in adulthood: a case report. Int J Surg Case Rep 2020; 67: 250–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duvvada S, Senapati D, Challa SR, et al. Cystic lymphangioma of spleen in adults. BMJ Case Rep. Epub ahead of print 25 January 2017. DOI: 10.1136/bcr-2016-216267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al-Shaikh SA, Mubarak AM, Harb ZF. Splenic lymphangioma in an adult. Saudi Med J 2017; 38(11): 1148–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Efared B, Atsame-Ebang G, Zabeirou A, et al. Isolated splenic lymphangioma presenting as a huge mass causing anemia and abdominal distension in an adult patient: a case report. J Med Case Rep 2018; 12(1): 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar P, Kumar S, Husain N, et al. Isolated cystic lymphangiomatosis of spleen in an adult: a diagnostic conundrum. BMJ Case Rep 2018. DOI: 10.1136/bcr-2017-223856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodríguez-Montes JA, Collantes-Bellido E, Marín-Serrano E, et al. Splenic lympangioma. A rare tumour. Presentation of 3 cases and a literature review. Cir Cir 2016; 84(2): 154–159. [DOI] [PubMed] [Google Scholar]
- 11. Giovagnoni A, Giorgi C, Goteri G. Tumours of the spleen. Cancer Imaging 2005; 5(1): 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eghtedari M, Sicklick J, Kono Y, et al. Unusual imaging profile of a solitary splenic lymphangioma. Acta Radiol Short Rep. Epub ahead of print 24 September 2012. DOI: 10.1258/arsr.2012.120033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caraway NP, Fanning CV. Use of fine-needle aspiration biopsy in the evaluation of splenic lesions in a cancer center. Diagn Cytopathol 1997; 16(4): 312–316. [DOI] [PubMed] [Google Scholar]
- 14. George Verghese B, Kalvehalli Kashinath S, Kanth RR. Lymphangioma of the spleen — a rare tumor rarely seen in an adult: a case report and a comprehensive literature review. Euroasian J Hepatogastroenterol 2013; 3(1): 64–69. [Google Scholar]
- 15. Yu X, Yu J, Liang P, et al. Real-time contrast-enhanced ultrasound in diagnosing of focal spleen lesions. Eur J Radiol 2012; 81(3): 430–436. [DOI] [PubMed] [Google Scholar]
- 16. Makrin V, Avital S, White I, et al. Laparoscopic splenectomy for solitary splenic tumors. Surg Endosc 2008; 22(9): 2009–2012. [DOI] [PubMed] [Google Scholar]