Abstract
Gastrointestinal cancer is one of the most malignant tumors with high morbidity and mortality, especially colorectal cancer, which has become the second leading cause of cancer-related deaths worldwide. Targeted drug treatment and precise endoscopic resection can significantly improve the overall survival rate and greatly extend the life span. Promising biomedical applications of hydrogels would represent hopeful therapeutic alternatives for patients with different kinds of diseases, particularly providing precise therapy for cancer patients. Although the intersection field of material science and biomedical science has made tremendous advances, major challenges remain. In this review, the application of hydrogel-based technology in cancer precision medicine is the focus of attention, which is the development trend of multidisciplinary cooperation in the future. First, we provide the current clinical landscape of hydrogel applications, and then we highlight precision oncology, including personalized drug treatment and accurate endoscopic intervention. Finally, we discuss major challenges for their clinical translation that have not yet been overcome and future perspectives on cancer precision medicine.
Keywords: hydrogel, biomedical application, cancer treatment, precision oncology, gastrointestinal cancer, clinical translation
Introduction
Hydrogels are a type of soft and crosslinked hydrophilic polymer network and can be adapted to meet the requirements of different settings by altering material components and chemical modifying approaches.1 Due to favorable physicochemical characteristics and high biocompatibility, various hydrogels have been designed and developed for biomedical applications, such as regenerative medicine,2,3 tissue engineering scaffolds,4,5 drug delivery system,6,7 and cancer precision medicine.8–10 In particular, there is an increasing utilization of hydrogels in the diagnosis and treatment of cancers. For example, gastrointestinal cancers are life-threatening malignant diseases originating from the gastrointestinal tract, consisting of esophageal cancer, gastric cancer, colorectal cancer, and others. Gastrointestinal cancer accounts for approximately 20% of all cancer diagnoses and 22.5% of cancer deaths worldwide. The 5-year survival rate of early-stage cancer is more than 90%, while that of advanced-stage cancer is less than 20%.11 Early endoscopic curative resection by using hydrogels can significantly improve the survival rate and quality of life of gastrointestinal cancer patients. Although a variety of hydrogel products have been investigated in preclinical research, the translation from material sciences to clinical application remains a challenge.1,12 Herein, recent advances in hydrogel-based biomedical applications are generally reviewed. This review also summarizes the current state of hydrogels associated with precision treatment in gastrointestinal cancer, including patient-specific drug screening, therapeutic delivery, and endoscopic precise removal of early-stage malignant cancer.
A fundamental classification of hydrogels is made, such as natural, synthetic, and semisynthetic hydrogels based on the material origin.13 Among these 3 types, the most common hydrogels are natural hydrogels. Owing to their good biocompatibility, controllable biodegradability, and flexible adaptability, natural hydrogels derived from polypeptides or polysaccharides are highly efficient in many biomedical applications. Through chemical modification and crosslinking during synthesis, synthetic hydrogels are obtained and can afford high tunability and versatile physical properties. To overcome the limitations of natural and synthetic hydrogels, such as stability and batch-to-batch variation, investigators have developed chemically modified natural hydrogels, which are defined as semisynthetic hydrogels.14 Physicochemical properties of hydrogels, such as mechanical strength, and biological characteristics, such as degradation behavior, can be regulated by varied compositions, different chemical crosslinking methods and density.14
Naturally, derived hydrogels include hyaluronic acid (HA), alginate, fibrin, collagen, gelatin, and chitosan. Of all natural hydrogels, HA and alginate are 2 notable types, and they are never reactive with the proteins in our body. HA is composed of repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine. Due to outstanding properties such as biocompatibility, biodegradability, and nonimmunogenicity, there is exponential growth in its biomedical applications.15 Owing to its high biological relevance and precise chemical tunability, alginate is often utilized to create tailored mechanical scaffolds and biomedical implants. Fibrin has also been explored in extensive biomedical investigations due to its biocompatibility and easy fabrication process; however, uncontrolled degradation is a major limitation in its clinical translation because it is easily affected by tissue factors and enzymes in-vivo.14 Additionally, gelatin is denatured from collagen and can be derived from diverse sources. Due to their relatively low antigenicity, short degradation period and similar structure to the extracellular matrix, gelatin-combined hydrogels have been explored widely.13 Moreover, chitosan is a natural, nontoxic, biodegradable polysaccharide, and ionic cross-linking is the most common method for preparing chitosan nanoparticles, which are often used as an antitumor therapy carrier for research due to their inherent antitumor activity. Since their highly hydrophilic and mechanical properties are similar to those of many soft tissues in-vivo, natural hydrogels are popularly utilized in a wide range of clinical applications. Here, we highlight promising biomedical applications of natural hydrogels.
Currently, natural hydrogels are commonly studied and applied in clinical settings such as regeneration medicine and precision oncology. Tissue regeneration and augmentation are common biomedical applications of hydrogels. For example, specific hydrogel patches are currently applied to facilitate the healing process in diabetic ulcers, burn wounds, and skin conditions such as eczema because a combination of preventing bacterial overgrowth and delivering therapeutic agents can be achieved by hydrogel patches.1 Tissue augmentation can provide mechanical support for compromised tissue, such as myocardial infarction and refractory heart failure. Yadid et al proposed that an engineered myocardial pump would represent a therapeutic alternative for millions of patients with end-stage heart disease, addressing their urgent need for heart donation.16 Additionally, with the increasing understanding of tissue engineering, hydrogel scaffolds have attracted increasing attention, such as intra-articular injectable hydrogels designed for the treatment of knee osteoarthritis. Cancer precision medicine is another important application of hydrogels. For example, carbopol-based hydrogels loaded with lipophilic bismuth nanoparticles can effectively inhibit the proliferation of cervical cancer, prostate cancer, and colorectal cancer cell lines without adversely affecting the control of nontumor cells.17 For another example, Vikas et al developed dual receptor-targeted chitosan nanoparticles and confirmed that they had good cytotoxicity and enhanced anticancer activity against lung cancer cell lines.18 Furthermore, nanoparticles and nanotechnology are also used in many other gastrointestinal treatments, such as phototriggered therapy (including photothermal therapy, photoimmunotherapy, and photodynamic therapy), nanopowders, nanoscaffolds, nanogels, and so on.19,20 Nanomedicine has the potential to improve diagnostic tools for gastrointestinal cancers and increase treatment options.21 However, there is very little available clinical data on nanomedicine applications compared with preclinical data.22 In addition to the development of more novel hydrogels for antitumor therapy, a series of cancer products have been developed and approved. Endo's Vantas® has received regulatory approval by the FDA as a subcutaneous hormonal therapy for the prevention of testosterone-dependent prostate cancer.1 TraceIT® of hydrogel systems is approved by the FDA for imaging in cancer diagnosis and treatment in clinical trials.1 Gelfoam matrix histoculture, first developed by Leighton Joseph, permits the determination of the cell cycle position of invading and noninvading cancer cells.23 SpaceOAR® hydrogel is primarily designed to protect normal tissues from radiation injury during radiation treatment of cancerous tissues.1 Furthermore, HA-based hydrogels are related to cancer behavior and could be prognostic agents for tumors. In cancer cases, the degradation of HA is highly associated with tumor malignancy, angiogenesis, and distal metastasis.14 Patient-specific drug screening, targeted drug delivery, and accurate endoscopic removal of tumors are also included in precise cancer therapy.
Precise Cancer Treatment
Drug Screen
To our knowledge, major disadvantages of regular cancer therapy are that a large number of patients have to go through multiple rounds of drug treatment to eradicate tumors and afford tremendous cost spending on cancer therapy.14 Therefore, there is a pressing need to develop precise and efficient oncology models that highly recapitulate the genetic and morphological composition and mimic the arrangement pattern of cancer cells in the original tumor. Over the past few years, a variety of drug screening tools have been investigated, such as tumor cell lines,24,25 tumor organic,26 organ-on-a-chip, reprogramming technology, and hydrogel-based tumor models. In terms of tumor cell lines, the low culture success rate and limited proliferative capacity of ex-vivo culture of tumor cells from patients are major roadblocks in their utilization to evaluate therapeutic effectiveness.23 For organ-on-a-chip technology, making a tumor or physiologically relevant disease on a chip has been a logical step in the field of cancer research. For instance, Huh et al developed a lung-on-a-chip model that can mimic the physiological environment of the lung.14,27
Hydrogels are one of the most versatile technologies for personalized drug screen.1,28 Recently, Suzuka et al reported an innovative hydrogel, defined as a double-network hydrogel, that can rapidly reprogram tumor cells into cancer stem cells with advanced reprogramming technology. It is important to develop novel cancer therapies and screen personalized therapeutic agents targeting cancer stem cells with available double-network hydrogels.29 Additionally, using hydrogels to create engineered tumor models is an emerging trend in cancer precision medicine, and researchers have demonstrated that the tumor microenvironment plays an important role in tumor development and metastasis.14 Hydrogel-based tumor models, accurately recapitulating the tumor microenvironment, serve as in-vitro platforms for better screening of novel precise cancer therapeutics, as well as further study of mechanisms underlying cancer development and metastasis.14,30,31 There is promising translatability and wide-scale use of hydrogel-based tumor models for less expensive and more controllable therapeutic evaluation than in-vivo.
Drug Delivery
Injectable hydrogels are regarded as favorable carriers, loading and delivering therapeutic agents to the surrounding environment and targeted sites.10,32 While hydrogels encapsulate therapeutics and circulate in the bloodstream, initial efforts should focus on their biological properties to reduce phagocytic uptake and clearance.15 It is expected that delivering therapeutic agents to the targeted sites can significantly enhance therapeutic efficacy while decreasing adverse effects. The effectiveness of drug delivery is also determined by the size of agents, mesh size of gels, and interaction affinity of the agent-hydrogel.1
Initially, Szoka et al used HA liposomes loaded with anticancer drugs to facilitate targeted drug delivery by upregulating CD44 receptors on murine tumor cells. Since then, a variety of CD44-induced HA-based hydrogels for targeted therapy have been developed.15 Recently, pH-sensitive hydrogels have been proposed as an important method of drug-targeted delivery. Due to their high sensitivity to detect minute pH changes of as small as 10−5 pH units, they are capable of targeting cancer and prolonging drug release within the blood circulation.33 The pH of the tumor microenvironment is directly or indirectly influenced by O2, angiogenesis, cytokines, and interactions with each other. By monitoring pH changes in tumor sites and blood in individual precision cancer treatment, pH-sensitive hydrogels can provide tailored release doses of chemotherapy drugs, the best timing for drug release, and records of the response of cancer cells to anticancer drugs.33 For instance, Dai et al used a pH-sensitive hydrogel to deliver anticancer chemotherapy drugs to tumor sites where the pH was different from the physiological range.34 Fully taking advantage of hydrogel biomaterials in the field of cancer research has made it possible to successfully transition from drug discovery to personalized medicine.
Endoscopic Removal
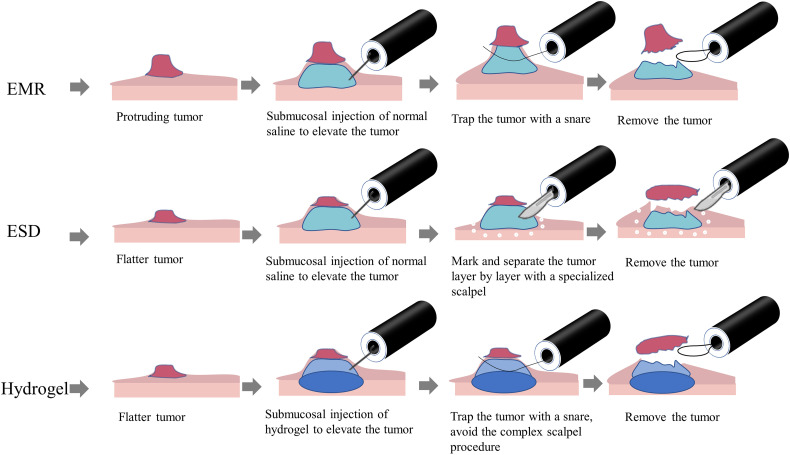
Gastrointestinal cancer is one of the most malignant tumors with high morbidity and mortality worldwide.35,36 Endoscopic removal of early cancer and premalignant neoplasia, including endoscopic mucosal resection (EMR)37 and endoscopic submucosal dissection (ESD),38,39 is the most effective approach to prevent tumor development and progression. A key consideration factor for successful endoscopic therapy is an ideal injectable submucosal solution. Normal saline is commonly used as a submucosal liquid cushion; however, the accuracy of endoscopic treatment is greatly affected because normal saline can be maintained for only a very short time due to its high permeability and fast diffusion. To address these limitations, submucosal injectable hydrogels (3-5 mL) offer a competitive strategy for endoscopic precise treatment (Figure 1), and there has been exponential growth in preclinical investigations of injectable submucosal hydrogels.40 However, very few studies have actually entered clinical trials (Table 1). Ideal submucosal hydrogels must have low viscosity and sufficient elasticity in local sites to maintain their volume and sustained submucosal elevation height. In addition, repeated submucosal injections are avoided compared to normal saline. There are 2 approaches for submucosal injection. One approach is directly injecting synthetic gels into the submucosal layer in sites of interest; shear-thinning polymer hydrogels are extensively explored to overcome the paradox between viscosity and elasticity.40 Another approach is to inject material precursors that form gels via in-situ chemical crosslinking within the physiological environment.
Figure 1.
Illustration of endoscopic treatment of gastrointestinal cancer.
Table 1.
Clinical Trials of Hydrogels in Gastrointestinal Cancer.
| Number | ClinicalTrials.gov identifier | Condition/Disease | Intervention/Treatment | Phase |
|---|---|---|---|---|
| 1 | NCT04595266 | Colorectal cancer metastatic | Drug: FOLFOX regimen Biological: Anti-EGFR or Bevacizumab Drug: LIVERPEARLS-Irinotecan |
Phase 2 |
| 2 | NCT03258541 | Rectal cancer | Device: TraceIT® Radiation: Volumetric arc therapy (VMAT) Procedure: Surgery |
Not applicable |
| 3 | NCT03321396 | Submucosal tumor of gastrointestinal tract | Device: sodium alginate mixed with calcium lactate | Not applicable |
| 4 | NCT04124588 | Nonvariceal upper gastrointestinal bleeding | Device: Nexpowder (hemostatic powder) Device: Conventional technique |
Not applicable |
| 5 | NCT04062721 | Unresectable colorectal liver metastases | Drug: Chemotherapy Procedure: Radiofrequency ablation (RFA) Drug: In-situ immunotherapy |
Phase 1 |
Diverse natural hydrogels have been explored for use as therapeutic submucosal injections for precision treatment of gastrointestinal early cancer and premalignant lesions. Significant efforts have been devoted to optimizing the physicochemical properties of natural hydrogels by altering various components and crosslinking technologies. Of all natural hydrogels studied in preclinical investigations, a nature-derived hydrogel of gelatin-oxidized alginate (G-OALG) was first reported by Fan et al, showing higher performance in controllable gelation, higher viscosity, and more stable properties. Due to good biocompatibility, excellent endoscopic injectability, and prolonged submucosal elevation, G-OALG could be a promising submucosal injection agent for ESD.41 Similarly, a study conducted by Massachusetts Institute of Technology developed endoscopically injectable shear-thinning hydrogels (EISHs), which can serve as safe and easily injectable agents to provide durable and ideally elevated submucosal cushions. It has been validated in large live animal models for accurate removal at the early stage of colorectal tumors.40 Fibrin glue (FG) has been explored in extensive biomedical applications due to its biocompatibility and easy fabrication process. Comparing the capability of maintaining submucosal elevation among FG, HA, and normal saline, Takao et al demonstrated that the FG had the best submucosal lifting.42 However, uncontrolled degradation is a major limitation in its clinical translation because it is easily affected by tissue factors and enzymes in-vivo. There are still many other natural hydrogels available, but they are often not easily transferable to clinical applications and wide-scale industrial use.43–46
Gastrointestinal Hemorrhage
The most common complication of gastrointestinal tumors, or any other type of cancer that attacks the digestive tract, is a malignant ulcer accompanied by uncontrolled bleeding, which can even threaten the lives of patients.47 Accumulating studies have demonstrated that the older population has a significantly higher frequency of developing different cancers. However, a large proportion of older patients cannot tolerate invasive surgical resection and prefer to choose noninvasive drug treatment when acute gastrointestinal bleeding occurs. There are several agents used to treat gastrointestinal bleeding, such as hemostatic spray powders, oral thrombin, and adrenaline solution. However, the hemostatic effect of these agents usually lasts for a short time and needs repeated administration due to low adhesion to the ulcer and fast dissolution in the digestive environment.48 Thus, developing efficient biomaterials to treat gastrointestinal bleeding is highly desired in clinical practice.
There is a rapidly increasing investigation of hemostatic hydrogels. To form therapeutic hydrogels at target sites, especially in fluidically and mechanically dynamic gastrointestinal environments, appropriate gelation time and bioadhesion to the target site are 2 major consideration factors during the preparation of hydrogels. Endoscopic injectable pH-responsive hydrogels were subsequently developed, which are suitable for biological use in monitoring the pH of ulcer sites, stopping bleeding, and accelerating the self-healing process.48 For example, He et al presented endoscopic injectable pH-responsive adhesive and self-healing hydrogels for the treatment of gastrointestinal bleeding. It has been validated through animal models that this multifunctional hydrogel shows a suitable gelation time and efficient and good hemostatic properties.48 Contrary to pH-responsive hydrogels, Xu et al explored hydrogels that exhibited ultrafast gelation and sufficient adhesion independent of pH, providing a protective barrier and accelerating the healing process of ulcers.49 Compared with previous hemostatic powders, these therapeutic hydrogels can reduce the potential risk of biliary orifice obstruction, poor pancreatic drainage, and even choking. Rapid in-situ formation of stable and adhesive hydrogels achieves precision therapy of gastrointestinal ulcer.
Discussion and Future Perspectives
At present, the following problems are commonly encountered in clinical translation. First, despite the development of hydrogel-based drug screening and delivery systems, key technological challenges and practical adaptability remain major hurdles in their successful clinical translation. Second, immunological adverse events of hydrogels, such as inflammation, local pain, fibrosis, and indefinite long-term impact, remain a worrying limitation for wide-scale clinical translation. Third, although engineered tumor models have potential translatability in the clinic, great efforts are still needed to recapitulate the heterogeneity of tumors and enhance their ability to keep tumor samples viable outside the body. Last, as we have already described, most early precancerous lesions of the digestive tract require ESD treatment. However, ESD requires clinicians with more than 5 years of experience to perform the operation and has many disadvantages, such as general anesthesia, high bleeding risk, high perforation probability, and unaffordable hospitalization costs. Through endoscopic submucosal injection of individually tailored hydrogels, the complex and high-risk ESD procedure can be transformed into a simple and low-risk EMR procedure, which will save patients and the country huge medical expenses. Therefore, we believe that the hydrogel will have great potential in the application of endoscopic early-stage precancerous lesion resection in the future.
In the future, as the avenues toward personalized precision medicine take a leap to explore more precise and more efficient hydrogel-based tumor models in-vitro for patient-tailored treatment. In particular, hydrogel-based tumor models currently represent innovative tools to address the research gap between basic development and clinical translation, moving complex tumor progression and novel drug development into the age of precision medicine.
Acknowledgments
We thank Shun Duan, PhD, Professor, and Ruo-nan Wu, PhD, for their encouragement and support in the field of biomaterials for our review writing.
Abbreviations
- HA
hyaluronic acid
- FDA
food and drug administration
- EMR
endoscopic mucosal resection
- ESD
endoscopic submucosal dissection
- G-OALG
gelatin-oxidized alginate
- EISHs
endoscopically injectable shear-thinning hydrogels
- FG
fibrin glue.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Statement: Our study did not require an ethical board approval because it did not contain human or animal trials.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Key Development Plan for Precision Medicine Research (grant number 2017YFC0910002).
ORCID iD: Qian-qian Wang https://orcid.org/0000-0002-7709-2121
References
- 1.Mandal A, Clegg JR, Anselmo AC, Mitragotri S. Hydrogels in the clinic. Bioeng Transl Med. 2020;5(2):e10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown TE, Anseth KS. Spatiotemporal hydrogel biomaterials for regenerative medicine. Chem Soc Rev. 2017;46(21):6532‐6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasani-Sadrabadi MM, Sarrion P, Pouraghaei S, et al. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci Transl Med. 2020;12(534):eaay6853. [DOI] [PubMed] [Google Scholar]
- 4.Sun Han Chang RA, Shanley JF, Kersh ME, Harley BAC. Tough and tunable scaffold-hydrogel composite biomaterial for soft-to-hard musculoskeletal tissue interfaces. Sci Adv. 2020;6(34):eabb6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji X, Yuan X, Ma L, et al. Mesenchymal stem cell-loaded thermosensitive hydroxypropyl chitin hydrogel combined with a three-dimensional-printed poly(ε-caprolactone) /nano-hydroxyapatite scaffold to repair bone defects via osteogenesis, angiogenesis and immunomodulation. Theranostics. 2020;10(2):725‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S, Ermann J, Succi MD, et al. An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci Transl Med. 2015;7(300):300ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amiri M, Khazaeli P, Salehabadi A, Salavati-Niasari M. Hydrogel beads-based nanocomposites in novel drug delivery platforms: recent trends and developments. Adv Colloid Interface Sci. 2021;288:102316. [DOI] [PubMed] [Google Scholar]
- 8.Qian Q, Wang D, Shi L, et al. A pure molecular drug hydrogel for post-surgical cancer treatment. Biomaterials. 2021;265:120403. [DOI] [PubMed] [Google Scholar]
- 9.Liu M, Cao Z, Zhang R, Chen Y, Yang X. Injectable supramolecular hydrogel for locoregional immune checkpoint blockade and enhanced cancer chemo-immunotherapy. ACS Appl Mater Interfaces. 2021;13(29):33874‐33884. [DOI] [PubMed] [Google Scholar]
- 10.Norouzi M, Nazari B, Miller DW. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov Today. 2016;21(11):1835‐1849. [DOI] [PubMed] [Google Scholar]
- 11.Kuntz S, Krieghoff-Henning E, Kather JN, et al. Gastrointestinal cancer classification and prognostication from histology using deep learning: systematic review. Eur J Cancer. 2021;155:200‐215. [DOI] [PubMed] [Google Scholar]
- 12.Correa S, Grosskopf AK, Lopez Hernandez H, et al. Translational applications of hydrogels. Chem Rev. 2021;121(18):11385‐11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma S, Tiwari S. A review on biomacromolecular hydrogel classification and its applications. Int J Biol Macromol. 2020;162:737‐747. [DOI] [PubMed] [Google Scholar]
- 14.Park KM, Lewis D, Gerecht S. Bioinspired hydrogels to engineer cancer microenvironments. Annu Rev Biomed Eng. 2017;19:109‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi KY, Han HS, Lee ES, et al. Hyaluronic acid-based activatable nanomaterials for stimuli-responsive imaging and therapeutics: beyond CD44-mediated drug delivery. Adv Mater. 2019;31(34):e1803549. [DOI] [PubMed] [Google Scholar]
- 16.Yadid M, Oved H, Silberman E, Dvir T. Bioengineering approaches to treat the failing heart: from cell biology to 3D printing. Nat Rev Cardiol 2021;19:83‐99. doi: 10.1038/s41569-021-00603-7. [DOI] [PubMed] [Google Scholar]
- 17.Cabral-Romero C, Solís-Soto JM, Sánchez-Pérez Y, et al. Antitumor activity of a hydrogel loaded with lipophilic bismuth nanoparticles on cervical, prostate, and colon human cancer cells. Anticancer Drugs. 2020;31(3):251‐259. [DOI] [PubMed] [Google Scholar]
- 18.Vikas, Viswanadh MK, Mehata AK, et al. Bioadhesive chitosan nanoparticles: dual targeting and pharmacokinetic aspects for advanced lung cancer treatment. Carbohydr Polym. 2021;274:118617. [DOI] [PubMed] [Google Scholar]
- 19.Nassani N, Alsheikh M, Carroll B, Nguyen D, Carroll RE. Theranostic gastrointestinal endoscopy: bringing healing light to the lumen. Clin Transl Gastroenterol. 2020;11(3):e00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen-Trinh QN, Trinh KXT, Trinh NT, et al. A silica-based antioxidant nanoparticle for oral delivery of Camptothecin which reduces intestinal side effects while improving drug efficacy for colon cancer treatment. Acta Biomater. 2022;143:459‐470. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Chen L, Luan S, et al. The development and progress of nanomedicine for esophageal cancer diagnosis and treatment. Semin Cancer Biol. 2022;86(Pt 2):873‐885. [DOI] [PubMed] [Google Scholar]
- 22.Jefremow A, Neurath MF. Nanoparticles in gastrooncology. Visc Med. 2020;36(2):88‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Z, Xu H, Zhao X. Designer self-assembling peptide hydrogels to engineer 3D cell microenvironments for cell constructs formation and precise oncology remodeling in ovarian cancer. Adv Sci (Weinh). 2020;7(9):1903718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murai Y, Jo U, Murai J, et al. SLFN11 Inactivation induces proteotoxic stress and sensitizes cancer cells to ubiquitin activating enzyme inhibitor TAK-243. Cancer Res. 2021;81(11):3067‐3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, Kim H, Lee JE, et al. Selective cytotoxicity of the NAMPT inhibitor FK866 toward gastric cancer cells with markers of the epithelial-mesenchymal transition, due to loss of NAPRT. Gastroenterology. 2018;155(3):799‐814.e13. [DOI] [PubMed] [Google Scholar]
- 26.Luo X, Fong ELS, Zhu C, et al. Hydrogel-based colorectal cancer organoid co-culture models. Acta Biomater. 2021;132:461‐472. [DOI] [PubMed] [Google Scholar]
- 27.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328(5986):1662‐1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliva N, Conde J, Wang K, Artzi N. Designing hydrogels for on-demand therapy. Acc Chem Res. 2017;50(4):669‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuka J, Tsuda M, Wang L, et al. Rapid reprogramming of tumour cells into cancer stem cells on double-network hydrogels. Nat Biomed Eng. 2021;5(8):914‐925. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Luo J, Alu A, Han X, Wei Y, Wei X. cGAS-STING pathway in cancer biotherapy. Mol Cancer. 2020;19(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Q, Zhang D, Qian H, et al. Superior antitumor efficacy of IFN-alpha2b-incorporated photo-cross-linked hydrogels combined with T cell transfer and low-dose irradiation against gastric cancer. Int J Nanomedicine. 2020;15:3669‐3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narayanaswamy R, Torchilin VP. Hydrogels and their applications in targeted drug delivery. Molecules. 2019;24(3):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hendi A, Umair Hassan M, Elsherif M, et al. Healthcare applications of pH-sensitive hydrogel-based devices: a review. Int J Nanomedicine. 2020;15:3887‐3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu RS, Lin J, Xing YM, Dai ZL, Wang LW, Zhang XP. pH-sensitive black phosphorous-incorporated hydrogel as novel implant for cancer treatment. J Pharm Sci. 2019;108(8):2542‐2551. [DOI] [PubMed] [Google Scholar]
- 35.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 36.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 37.Yang D, Othman M, Draganov PV. Endoscopic mucosal resection vs endoscopic submucosal dissection for Barrett’s esophagus and colorectal neoplasia. Clin Gastroenterol Hepatol. 2019;17(6):1019‐1028. [DOI] [PubMed] [Google Scholar]
- 38.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2015;47(9):829‐854. [DOI] [PubMed] [Google Scholar]
- 39.Bourke MJ, Neuhaus H, Bergman JJ. Endoscopic submucosal dissection: indications and application in western endoscopy practice. Gastroenterology. 2018;154(7):1887‐1900.e5. [DOI] [PubMed] [Google Scholar]
- 40.Pang Y, Liu J, Moussa ZL, et al. Endoscopically injectable shear-thinning hydrogels facilitating polyp removal. Adv Sci (Weinh). 2019;6(19):1901041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan C, Xu K, Huang Y, et al. Viscosity and degradation controlled injectable hydrogel for esophageal endoscopic submucosal dissection. Bioact Mater. 2021;6(4):1150‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takao M, Takegawa Y, Takao T, et al. Fibrin glue: novel submucosal injection agent for endoscopic submucosal dissection. Endosc Int Open. 2021;9(3):E319‐E323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Y, Xu JX, Cheng J, et al. A novel injectable thermo-sensitive binary hydrogels system for facilitating endoscopic submucosal dissection procedure. United Eur Gastroenterol J. 2019;7(6):782‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morisawa T, Okada A, Kataoka Y, Masaki S, Hayashi T. Cellulose nanofiber dispersion as a new submucosal injection material for endoscopic treatment: preliminary experimental study. Endosc Int Open. 2020;8(5):E623‐E627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujimoto A, Uraoka T, Nishizawa T, et al. Rebamipide solution: a novel submucosal injection material to promote healing speed and healing quality of ulcers induced by endoscopic submucosal dissection. Gastrointest Endosc. 2018;87(4):1114‐1120. [DOI] [PubMed] [Google Scholar]
- 46.Spadaccini M, Hassan C, Maselli R, et al. Efficacy and safety of SIC-8000 (Eleview(R)) for submucosal injection for endoscopic mucosal resection and endoscopic submucosal dissection in an in vivo porcine model. Dig Liver Dis. 2018;50(3):260‐266. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi D, Yoshida H, Ikeda K, et al. Colorectal endoscopic mucosal resection with submucosal injection of epinephrine versus hypertonic saline in patients taking antithrombotic agents: propensity-score-matching analysis. BMC Gastroenterol. 2019;19(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He J, Zhang Z, Yang Y, et al. Injectable self-healing adhesive pH-responsive hydrogels accelerate gastric hemostasis and wound healing. Nanomicro Lett. 2021;13(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu X, Xia X, Zhang K, et al. Bioadhesive hydrogels demonstrating pH-independent and ultrafast gelation promote gastric ulcer healing in pigs. Sci Transl Med. 2020;12(558):eaba8014. [DOI] [PubMed] [Google Scholar]