Abstract
Cell-to-cell interactions in the intricate microenvironment of tissue have a significant impact on the progression of cancer at every stage. Both cancer cells and stromal cells are responsible for the secretion of soluble chemical compounds as well as membrane-encased components, which both influence and govern the cell-to-cell interactions within the micro-environment of tumor cells. These membrane structures are identified as extracellular vesicles (EVs), which include exosomes and microvesicles. These nanosized vesicles are made up of bilayered proteolipids and have dimensions ranging from 50 to 1000 nm. It has been speculated that extracellular vesicles that originate from cancer cells perform a variety of functions in the development and progression of cancer which may involve the transport of regulatory materials, such as oncogenic proteins between nearby cells and to distant biological locations. In addition, their level in the serum of cancer patients is noticeably higher than those of healthy controls. The release of extracellular vesicles into the extracellular space is a continual process in both healthy and diseased cells. These extracellular vesicles hold molecular signatures that are defining features of health as well as disease. And hence, the EVs present in biological fluids provide unparalleled and noninvasive access to the necessary molecular details about the health status of the cells. Recent discoveries about these complex extracellular organelles have accelerated the discovery of cancer-specific biological markers as well as the development of unique diagnostic tools based on extracellular vesicles. In this mini-review, we aim to highlight the hopes and hypes associated with the applications of extracellular vesicles as biomarkers for cancer diagnosis.
Keywords: cancer, extracellular vesicles, diagnosis, biomarkers, EVs
Introduction
Over the course of more than 200 years of cancer research, an enormous quantity of knowledge concerning tumor biology has been amassed and is now available to researchers worldwide. Although there is a wealth of information accessible and much effort is being made to discover curative medications, cancer remains a fatal disease.1,2 Innovative pharmaceuticals and ground-breaking cancer treatments have made it possible to successfully treat a variety of cancers, including prostate, testicular, melanoma, and thyroid cancer, with a high probability of curing the disease. Other types of tumors, such as ovarian, breast, and lung cancers, are also among those that can now be cured.3‐5 These advancements, in conjunction with the use of preventive screenings for cancer, resulted in a steady decline in the cancer fatality rates of nations that have well-developed healthcare systems.2 Compared to highly curable cancers, patients with other common tumor types arising from lung, breast, colorectal, or pancreatic tissue have an extremely low chance of survival if detected at an advanced stage. And hence it seems early detection and diagnosis is the only viable method for initiating therapy regimens in a timely manner and thereby improving survival rates in such patients.2,6‐9
It is common for tumorigenesis to develop over years or even decades. Early stages are characterized by asymptomatic progression, and it is practically impossible to detect tumors developing internally that are not directly visible to palpable using conventional imaging techniques like computed tomography, magnetic resonance imaging, or positron emission tomography.10,11 Hence, only some of the diagnostic screening methods for specific tumor types have been established as effective.6,7 These methods have a number of significant drawbacks, including a high level of discomfort for the individual being examined, the detection of only a single type of tumor, increased healthcare costs as a result of x-ray exposure, an increased risk of injury, and a tendency to over-diagnose and perform additional tests that are not necessary.6,7,12
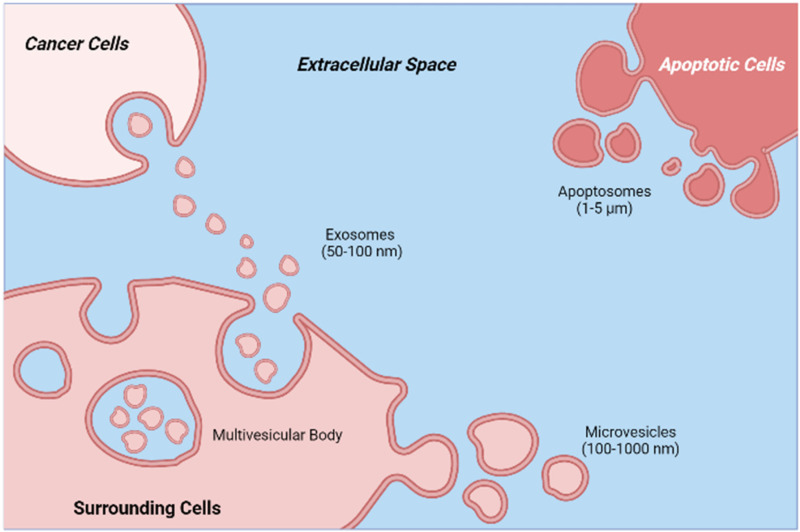
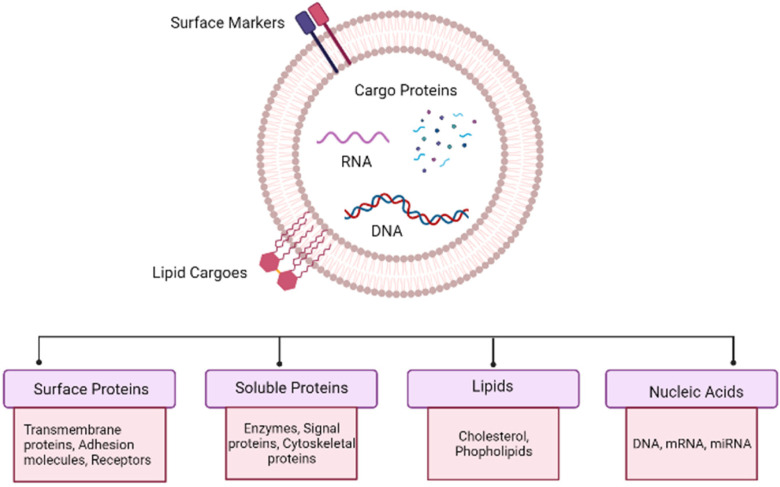
As a result, the above-mentioned preventive examinations are only suggested for people who are statistically at risk for the condition. Beginning at age 25 years (for cervical cancer), and continuing until age 45 to 50 years for other cancers, it is recommended that one undergo routine examinations.13,14 To address the apparent deficiencies of current cancer screening methods, considerable efforts have been devoted to identifying the cancer-specific biomarkers in easily accessible biological fluids that can be identified with high precision and are suited for low-threshold preventive screening. The most effective biomarkers must be indicative of an undiagnosed (asymptomatic) condition and provide biological information regarding the tumor, its stage of progression, and response to the therapeutic interventions, among other characteristics. In order to be effective, such biomarkers must be indistinguishable from nontumor cells in healthy individuals and detectable in significantly higher concentrations when tumor cells are present in the body. In other instances, such as with extracellular vesicles (EVs), specific markers may be lost in the diseased state, and their absence may also serve as an indication of the presence of a tumor.15,16 Recent research indicates that both healthy and malignant cells produce vesicles in the extracellular environment. These EVs contain the same genetic and proteomic material as the secreting cell. Mammalian cells release 2 distinct forms of EVs, exosomes, and microvesicles, with distinct biogenesis principles. Exosomes, with a diameter of 50 to 100 nm, are intraluminal vesicles that are released from multivesicular bodies by fusion with the plasma membrane. Microvesicles (sometimes referred to as ectosomes) of 100 to 1000 nm in diameter, are shed straight from the plasma membrane. During the blebbing of the plasma membrane, programmed cell death-afflicted cells generate apoptotic bodies. Prior to the discovery that they transport crucial information and substances from dying cells, these cells were considered to be garbage cells.17 The biogenesis and molecular composition of a typical EV can be seen in Figures 1 and 2, respectively.
Figure 1.
Schematic illustration of the biogenesis of apoptotic bodies (apoptosomes), microvesicles, and exosomes, along with their reported typical size ranges.
Figure 2.
Molecular snapshot of an extracellular vesicle (EV), depicting typical molecules that can be found in a typical EV
The most common way to find cancer is to take a sample of tissue, which is called a tissue biopsy. This is an invasive procedure, and the information it gives is limited to a single site, which makes it hard to get a full picture of cancer. Moreover, a tissue biopsy is not always feasible or reproducible. However, the revolutionary concept of liquid biopsy has completely transformed the field of oncology, as it allows the comprehensive and noninvasive molecular profiling of any tumor-derived substances that have been shed into the blood or other bodily fluids. In the past decades, numerous liquid biopsy biomarkers have been the subject of extensive research and evaluations. In addition to proteins, mRNAs, and miRNAs, circulating tumor cells, circulating tumor DNA, tumor-educated platelets, and EVs have been the focus of cancer diagnostic research.18‐23
Particularly, EVs are garnering a great deal of interest in the field of cancer diagnosis because of their special characteristics which are listed in Table 1.
Table 1.
Unique Hallmarks of Extracellular Vesicles (EVs).
| No. | Remarks | Reference |
|---|---|---|
| 1 | All the body cells studied to date produce EVs and are significantly more prevalent in bodily fluids than circulating tumor cells and circulating tumor DNA. | Allard et al,24 Fernando et al,25 Johnsen et al26 |
| 2 | Tumor-derived EVs contain a range of compounds, including potentially tumor-specific biomarkers, showcasing their molecular fingerprint. | Hurwitz et al,27 Kucharzewska et al,28 Clos-Garcia et al29 |
| 3 | EVs are quite stable under a variety of physiological conditions, preserving their contents (biomarkers) and facilitating the subsequent analysis. | Boukouris and Mathivanan30 |
Despite the fact that the precise mechanism behind how EVs are able to cross biological barriers is not completely understood, there is a tremendous amount of diagnostic potential offered by the ability of EVs to do so. This is especially true for cancers that are forming deep within tissues.31
Clinical Applications of EVs in Cancer Diagnosis
Several promising cancer biomarker proteins have been identified through proteomic research (Table 2). In spite of the many advances that have been made in medical science in recent decades, cancer remains the leading cause of death across the globe, accounting for one death in every 6 that occur. The failure to diagnose cancer at an early stage, which is associated with a better prognosis than diagnosing cancer at a later stage, is one of the primary factors that contribute to cancer deaths.32
Table 2.
Pioneering Clinical Trials of Extracellular Vesicles (EVs).
| Cancer type | Phase | Result | Reference |
|---|---|---|---|
| Lung cancer | Phase II | In order to establish that dendritic cell-derived exosomes (Dex) can be used as maintenance immunotherapy after first-line chemotherapy in Non-Small Cell Lung Cancer (NSCLC), a phase II trial was conducted, which confirmed the capacity of Dex to boost the natural killer cell arm of antitumor immunity in patients with NSCLC. | Besse et al47 |
| Advanced nonsmall cell lung cancer | Phase I | A study was conducted to determine the safety, feasibility, and
efficacy of autologous Dex loaded with tumor antigens in
patients with NSCLC. Therapy was well tolerated and was completed by 9 of 13 patients. The formulations tested, were very well tolerated with just grade 1 and 2 side effects. Some patients experienced long-term stability of disease and activation of immune effectors. |
Morse et al48 |
| Colon cancer | Phase I | Ongoing | 49 |
| Malignant pleural effusion | Phase II | Ongoing | 50 |
| Lung cancer | Phase II | Ongoing | 51 |
| Ovarian, gastric, or colorectal cancer | Phase II | Ongoing | 52 |
The vast majority of tumors are identified in patients who already have symptoms that point to the presence of potentially malignant disease or in people who have evidence of the disease identified during preventative examinations that are performed on a routine basis. In order to obtain a definitive diagnosis, which is the foundation for further treatment, it may be necessary to use imaging (sonography, CT, or MRI), tumor markers (if available, which are typically detectable in blood tests), and a biopsy sample for histological evaluation. This is because acquiring a definitive diagnosis is the prerequisite for moving forward with treatment. Only by utilizing a combination of these various tests is it possible to determine the type of cancer, its grade as well as biomarkers. Possessing this information is essential for choosing the most appropriate course of treatment.
The creation of innovative diagnostic techniques that are reliable, easily accessible, and minimally invasive could allow for the early identification of cancers, which could result in a reduction in the overall mortality rate caused by cancer. Although cancerous cells and DNA that are found circulating in the blood provide significant information regarding the diagnosis and prognosis of cancer, their application in the context of clinical practice is limited because of low detection rates and high false-positive rates, both of which affect the precision and efficiency of the process.33,34
EV diagnostics has the great promise to acquire comprehensive molecular details about a suspected tumor if coupled with minimally or noninvasively obtained liquid biopsy samples. This information could reveal the suspected tumor's position, gene mutations, and progression state. It is feasible that tumor cells will release up to 10 times more EVs than normal healthy tissues (and that these EVs will have a higher amounts of protein and varying size profiles than normal healthy cells), which will provide an added benefit for the separation and assessment of cancer EVs. The ubiquitous nature of EVs in bodily fluids has led to their consideration as a potential noninvasive diagnostic aid for cancer.35‐37
The lipid bilayer safeguards the tumor-specific genetic content of EVs from the highly active nucleases located in biological fluids, enabling EVs to provide an exact reflection of the status of an originating cell as well as facilitate the early detection of just about any pathological occurrence at the genetic level.38‐41
It is necessary to identify and characterize the biomarkers that are existing in EVs before they can be utilized for the diagnosis and monitoring of cancer. When it comes to the detection of biomarkers, EVs derived from cancer cell lines and the bodily fluids of cancer patients are both excellent sources of EVs. Purification of EVs can be accomplished through a number of different techniques, such as centrifugation or ultracentrifugation.42
The application of quantitative proteomics makes it possible to locate particular vesicular proteins that are associated with cancer. There are 2 methods that can be used, “label-free methods and isotope-labelling methods.” These methods deliver quantitative information on particular vesicle proteins which are regarded to be regulated in a different manner in cancer. In addition to microarrays and sequencing of the next generation, another method for locating RNA biomarkers is next-generation sequencing. In melanoma-derived EVs, for instance, mRNA and miRNA arrays have been used to find out the progression status of melanoma.43 Validation of the identified RNA biomarker candidates is accomplished through the use of “quantitative real-time reverse transcription PCR.” EVs have an important role in the immune suppressive tumor microenvironment of squamous cell carcinomas of the head and neck (HNSCC). EVs isolated from HNSCC cell culture or patient plasma can be studied for their molecular cargo and biological significance in immunosuppression and tumor growth.44
The study of proteomics has found a wide range of proteins that have the potential to act as tumor markers in the near future. When especially in comparison to EVs obtained from the blood sample of normal controls and subjects with benign prostatic hyperplasia, EVs separated from the blood of patients with prostate cancer showed a remarkable increase in survival expression. This was found in contrast to EVs separated from the blood of subjects with benign prostatic hyperplasia.45,46 EV clinical trials are summarized in Table 2.
It's possible that EVs could be used as new diagnostic and monitoring templates for RNA-based cancer treatments based on RNA. Urine EVs which have been isolated from prostate cancer patients could be analyzed to determine how well the patients are doing with their disease. These EVs contain high levels of prostate cancer antigen-3 as well as the transmembrane protease serine-2.53
Examining EVs for the presence of microRNAs (miRNAs) that are unique to cancer is another method for diagnosing the disease. Significantly higher levels of miR-21 were detected in the EVs of patients with ovarian or esophageal cancer compared to healthy controls. Also seen in ovarian cancer patients’ circulating EVs are elevated amounts of miRNAs54,55 (Table 3).
Table 3.
Promising Cancer Biomarkers in Extracellular Vesicles (EVs).
| Location of cancer | Sample type | Biomarkers | Reference |
|---|---|---|---|
| Breast | Plasma | miR-200a, miR-200c, miR-205 | Zhang et al56 |
| Lung | Plasma | miR-151a, miR-301a-3p, miR-200b-5p, miR-629, miR-154-3p, miR-223, miR-301 | Rodríguez et al57 and Cazzoli et al58 |
| Pancreas | Serum | miR-17-5p, miR-1246, miR-4644, miR-4306, miR-3976, miR-21 | Madhavan et al59 and Que et al60 |
| ovary | Serum | miR-21, miR-141, miR-200a, miR-200b, miR-200c, miR-203, miR-205, miR-214 | Taylor et al61 |
| Prostate | Serum, urine | miR-107, miR-375, PCA-3 | Nilsson et al53 and Bryant et al62 |
| Brain | Serum, CSF | miR-320, miR-574-3p, miR-21 | Manterola et al63 and Akers et al64 |
| Cervix | Cervicovaginal lavage | miR-146a, miR-21 | Liu et al65 |
| Colon or rectum | Serum | Let-7a,miR-1229, miR-1246 | Ogata-Kawata et al66 |
Forecasting the Progression of the Disease
Markers of prognosis are predictors that can be used to anticipate the normal course of the progression of cancer without the need for any therapeutic interventions. These markers are essential in assisting clinicians in determining the most appropriate course of treatment for a particular tumor, taking into account the molecular portfolio of the tumor as well as the aggressiveness of cancer. In the end, the aim is to maximize the therapeutic outcomes while significantly reducing the number of patients who receive overtreatment.15
During the course of the disease, it has been demonstrated that cancer cells are capable of releasing EVs that promote their own survival by inhibiting immune cell activation and inducing apoptosis in T-cells. This is accomplished by the transfer of galectin-9 from cancer cells to healthy T-cells. Galectin-9 was shown to be present in substantial proportions in the extracellular fluids released from pancreas and lung adenocarcinoma cells, which makes it an intriguing possible marker for determining the (harmful) growth stage of these and other types of tumors.16,67,68
Tissue factor (TF), which is directly correlated with a significant physiological function in the beginning stages of blood coagulation, is another putative biomarker that is linked to a bad prognosis. TF has been shown to be connected with a higher risk of mortality. It has been demonstrated that patients with cancer, as well as those suffering from a wide variety of other disorders, have higher levels of EVs in their blood that contain significant amounts of TF as compared to healthy individuals.69‐71 It is recognized that adenocarcinomas enhance the chance of developing venous thromboembolism. In this procedure, TF acts as a coagulation initiator. It was discovered that TF and other possible indications, epithelial mucin-1 (MUC1), which is expressed on EVs, are associated with extensive breast and pancreatic cancer, as well as a significant risk of venous thromboembolism in pancreatic cancer patients. The analysis of MUC1 levels on the EV revealed a substantial reduction postoperatively or, in one case, after the chemotherapy.70
During therapy for colorectal, small-cell lung, and pancreatic cancer, scientists discovered that higher concentrations of EV-bound TF were associated with a worse prognosis in pancreatic cancer, but not in colorectal or small-cell lung cancer. Higher levels of EV-bound TF were not related to a worse prognosis in colorectal or small-cell lung cancer, which surprised the researchers.69,70,72 MUC1 was found to be abundant in the extracellular spaces of subjects with “Non-small cell lung cancer,” and other adenocarcinomas; nevertheless, it was not found to be enriched in the total plasma or cellular membrane proteome, highlighting the promise of EV diagnostics.73
Individuals with head and neck squamous cell carcinomas had greater levels of EV-bound programmed death ligand-1 (PD-L1) in their blood than those who didn’t, suggesting that liquid biopsy might be used to test EV-bound PD-L1 in the suffering patients.74
Considering the involvement of tumor EVs in organotropism at the onset of a tumor's metastasizing phase is a relatively recent topic. This happens when the so-called pre-metastatic niche (PMN) emerges, resulting in the formation of a milieu that seems to favor the survival and growth of disseminated tumor cells.75
EVs from patients with pancreatic ductal adenocarcinoma (PDAC) have been analyzed, and a variety of proteins, notably glypican-1 and macrophage immigration inhibitory factor (MIF), have been detected there. These proteins have been connected to the development of PMNs in the liver. Although glypican-1 bound to EVs has been suggested as a possible indicator for the early diagnosis of pancreatic ductal adenocarcinoma (PDAC), it has also been observed to be elevated on EVs in subjects with mammary and colorectal cancer.76 However, blood tests relying on glypican-1 expression that included circulating tumor cells and EVs were able to effectively identify surgically excised PDAC tumors with a specificity of 80%. Other investigations have shown, the expression of glypican-1 on EVs solely wasn’t linked with PDAC tumor burden.77,78 The newly discovered marker class known as microRNAs (miRNAs) is currently the subject of intense research and also has the potential to complement protein biomarkers that are already in use. It has been shown that the prognosis of ovarian and breast cancer patients can be predicted not by a single miRNA, but rather by a collection of miRNAs that together make up a tumor-specific signature with a particular molecular pattern.79,80
It is entirely plausible that in the near future, EV cargo analysis will employ a large number of biomarkers with the aid of artificial intelligence-enhanced algorithms in order to achieve the increased levels of specificity and sensitivity needed for disease diagnosis and, eventually, preventative screenings.81
EVs in Diagnostic and Monitoring Tools Development
Before EVs can be purified and processed, they must first be exposed to biochemical and molecular examinations. Several of these processes, including ELISA, Northern blotting, Western blotting, and quantitative real-time PCR, take a significant amount of time to carry out. On the other hand, EVs are currently being utilized in the creation of a number of different procedures for the purposes of purification, diagnostics, and monitoring. In the recent past, microfluidic devices have been invented, and these devices provide a novel venue for the isolation and identification of EVs derived from biofluids, in particular blood.82,83 EV diagnostics have opened an opportunity for the isolation of EVs from biofluids. This platform makes use of a size-exclusion filter as well as immunoaffinity columns. Extracellular vesicle-associated RNAs are going to be utilized by the corporation in the hopes of producing more precise malignancy diagnostics. It was developed by Exosome Sciences, a division of Aethlon Medical, with the intention of detecting and quantifying EVs in biofluids. The presence of these vesicles in biological fluids can be an indication of the existence of a wide variety of cancers. An enzyme-linked lectin-specific test is a name given to this particular method. Recent advances in technology have made it possible to filter EVs or detect their presence utilizing methods that are uncomplicated, speedy, and dependable.84,85 However, further research needs to be done before circulating EVs can be used in clinical and commercial settings for cancer diagnosis and monitoring.
Pitfalls in EV-Based Diagnosis and Possible Solutions
Even though there is evidence from experiments to suggest that EVs could be helpful in the detection and monitoring of cancer, it is necessary to first get past a number of obstacles and restrictions before moving forward. These include pre-analytical, technical, temporal, as well as economic implications. During the preparation of a patient's blood, EVs can be depleted or synthesized, either of which can occur prior to their removal from the blood and subsequent purification. It is possible that considerable volumes of EVs will be lost in the process of removing blood cells or platelets using a centrifugation method that is inefficient. In addition, factors such as storage duration, temperatures, and tensile forces during blood preparation could be responsible for the destruction of EVs.86 These results suggest that a wide variety of environmental factors have an effect on the discharge of EVs; hence, all preparation phases need to be standardized for the sake of diagnostic purposes.
When it comes to isolating an adequate quantity (and quality) of EVs in a reliable and reproducible manner, as well as the cargo molecules that are generated as a result of this isolation, the first methodological challenges present themselves. Therefore, it is of the utmost importance to keep in mind that factors and confounding variables that can have an influence on the properties, amount, and cargo of EVs arise during the so-called pre-analytic stage, which takes place during the process of collecting bio-samples. This phase takes place at the time when bio-samples are being collected. These aspects include the specifics of the sample matrix, the characteristics of the patient (such as postprandial condition, medication, and age), the sample-taking equipment, the processing timelines for the sample, and the sample preservation. Therefore, variability in pre-analytical procedures should be reduced by developing robust operating procedures.87‐89
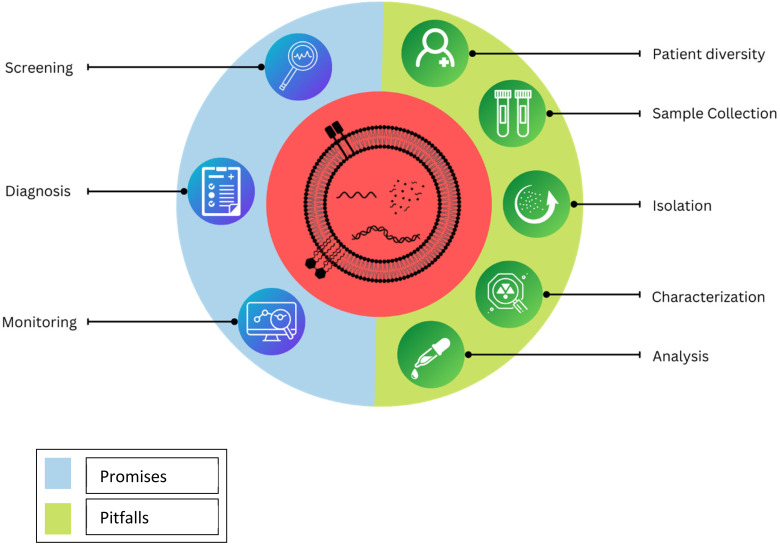
Plasma is suggested over the serum as serum contains a high percentage of platelet-derived EVs, which are formed mostly during clotting. Ethylenediamine tetraacetic acid (EDTA), out of regularly used anticoagulants in clinical settings, like heparin and citrate, is preferred as it inhibits the formation of EV-cell complexes and the release of platelet EV, whereas heparin inhibits the polymerase chain reactions (PCRs) exceptionally well. The time between blood collection and preliminary centrifugation has to be kept minimum (ideally about 30 min). Plasma and isolated EVs should be kept at −80 °C and freeze-thaw cycles should be avoided. Regarding blood collection, EV-specific advice involves overnight fasting for patients, rejecting the first several milliliters of blood, utilizing specified syringe types, and limiting the use of a tourniquet if feasible, as this induces uncontrollable EV release from the blood cells. Unlike traditional liquid biopsy approaches that are designed to analyze the circulating tumor cells, circulating tumor DNA, and other readily circulating tumor-informative analytes, preservatives are not needed for the liquid biopsy-derived EVs, as they are very much stable and capable to protect their cargo from the enzymatic activities which take place in the biofluids.90 Possible pitfalls and promises of EVs can be seen in Figure 3.
Figure 3.
Plausible promises and pitfalls of extracellular vesicles (EVs).
Conclusion and Future Prospects
EV research has come a long way since the days when it was just seen as a way to collect biowaste. In the past few years, a lot of knowledge has been gathered about the role of EVs in the development and spread of cancer, and so far, the majority of research on EVs has focused on their functions which have shown the tremendous potential that EVs hold. All the research done to the date had suggested EVs to be excellent biomarkers as they contain information about the actual tissue they came from and a snapshot of the cells’ physiological state. Despite the fact that we have learned a lot about EVs and made great strides in the technology for isolating EVs and figuring out what do they carry, there are still some hurdles that make it hard to employ EVs in clinical practice. However, oncology will undoubtedly undergo a sea change as a result of the forthcoming research into this field; which certainly seems a reason to have a sense of hope that the current shortcomings associated with the use of EVs will be conquered.
Acknowledgments
Not applicable.
Footnotes
Ethical Statement: Not applicable, because this article includes the committee approval number—for animal and human studies.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article
ORCID iD: Vaishali Yogesh Londhe https://orcid.org/0000-0003-3718-9858
References
- 1.Cancer Research UK. Worldwide cancer statistics | Cancer Research UK [Internet]. Cancer Res. UK. 2016 [cited 2022 Apr 22]. p. 1–5. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer.
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7‐34. [DOI] [PubMed] [Google Scholar]
- 3.Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019:175‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuth JC, Jones TS, Hanje J, Motl Moroney SE. Monoclonal antibodies in cancer. Pharm Biotechnol Fundam Appl Third Ed. Antibodies (Basel). 2016:339‐363. [Google Scholar]
- 5.Hong M, Clubb JD, Chen YY. Engineering CAR-T cells for next-generation. Cancer Therapy. 2020:473‐488. [DOI] [PubMed] [Google Scholar]
- 6.Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. 2016;11:967‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le-Petross HT, Shetty MK. Magnetic resonance imaging and breast ultrasonography as an adjunct to mammographic screening in high-risk patients. Semin Ultrasound CT MR. 2011;32:266‐272. [DOI] [PubMed] [Google Scholar]
- 8.Screening for Pancreatic Cancer: US Preventive Services Task Force Reaffirmation Recommendation Statement. The Center for Health and Community. [cited 2022 Apr 22]. Available from: https://chc.ucsf.edu/publications/screening-pancreatic-cancer-us-preventive-services-task-force-reaffirmation.
- 9.https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/pancreatic-cancer Pancreatic cancer statistics | Cancer Research UK. [cited 2022 Apr 22]. Available from:
- 10.Bashir U, Mallia A, Stirling J, et al. PET/MRI in oncological imaging: state of the art. Diagnostics. 2015;5:333‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraczek M, Kamecki H, Kamecka A, et al. Evaluation of lymph node status in patients with urothelial carcinoma-still in search of the perfect imaging modality: a systematic review. Transl. Androl. 2018:783‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilic D, Djulbegovic M, Jung JH, et al. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ. 2018:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponti A, Anttila A, Ronco G CS, et al. Cancer screening in the European Union. Report on the implementation of the Council Recommendation on cancer screening (second report). 2016;160. [Google Scholar]
- 14.Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American cancer society. CA Cancer J Clin. 2020;70:321‐346. [DOI] [PubMed] [Google Scholar]
- 15.Mordente A, Meucci E, Martorana GE, Silvestrini A. Cancer biomarkers discovery and validation: State of the art, problems and future perspectives. Adv Exp Med Biol. 2015:9‐26. [DOI] [PubMed] [Google Scholar]
- 16.Hoshino A, Kim HS, Bojmar L, et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182:1044‐1061.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581‐593. [DOI] [PubMed] [Google Scholar]
- 18.Arantes LMRB, De Carvalho AC, Melendez ME, Carvalho AL. Serum, plasma and saliva biomarkers for head and neck cancer. Expert Rev Mol Diagn. 2017;18:85‐112. [DOI] [PubMed] [Google Scholar]
- 19.Kahraman M, Röske A, Laufer T, et al. MicroRNA in diagnosis and therapy monitoring of early-stage triple-negative breast cancer. Sci Rep. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balázs K, Antal L, Sáfrány G, Lumniczky K. Blood-derived biomarkers of diagnosis, prognosis and therapy response in prostate cancer patients. J Pers Med. 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dovizio M, Ballerini P, Fullone R, Tacconelli S, Contursi A, Patrignani P. Multifaceted functions of platelets in cancer: from tumorigenesis to liquid biopsy tool and drug delivery system. Int J Mol Sci. 2020;2020:9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6:479‐491. [DOI] [PubMed] [Google Scholar]
- 23.Poulet G, Massias J, Taly V. Liquid biopsy: general concepts. Acta Cytol. 2019;63:449‐455. [DOI] [PubMed] [Google Scholar]
- 24.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897‐6904. [DOI] [PubMed] [Google Scholar]
- 25.Fernando MR, Jiang C, Krzyzanowski GD, Ryan WL. New evidence that a large proportion of human blood plasma cell-free DNA is localized in exosomes. PLoS One. 2017;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnsen KB, Gudbergsson JM, Andresen TL, Simonsen JB. What is the blood concentration of extracellular vesicles? Implications for the use of extracellular vesicles as blood-borne biomarkers of cancer. Biochim Biophys Acta - Rev Cancer. 2019;1871:109‐116. [DOI] [PubMed] [Google Scholar]
- 27.Hurwitz SN, Rider MA, Bundy JL, Liu X, Singh RK, Meckes DG. Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget. 2016;7:86999‐87015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kucharzewska P, Christianson HC, Welch JE, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110:7312‐7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clos-Garcia M, Loizaga-Iriarte A, Zuñiga-Garcia P, et al. Metabolic alterations in urine extracellular vesicles are associated to prostate cancer pathogenesis and progression. J Extracell Vesicles. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin Appl. 2015;9:358‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morad G, Carman CV, Hagedorn EJ, et al. Tumor-derived extracellular vesicles breach the intact blood-brain barrier via transcytosis. ACS Nano. 2019;13:13853‐13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cancer [Internet]. [cited 2022 Apr 25]. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer.
- 33.Merker JD, Oxnard GR, Compton C, et al. Circulating tumor DNA analysis in patients with cancer: American society of clinical oncology and college of American pathologists joint review. Arch Pathol Lab Med. 2018;142:1242‐1253. [DOI] [PubMed] [Google Scholar]
- 34.Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14:623‐631. [DOI] [PubMed] [Google Scholar]
- 35.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vlassov A V, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940‐948. [DOI] [PubMed] [Google Scholar]
- 37.Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83:1484‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654‐659. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442‐17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54:1237‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.György B, Szabó TG, Pásztói M, et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667‐2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao D, Ohlendorf J, Chen Y, et al. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One. 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofmann L, Ludwig S, Vahl JM, Brunner C, Hoffmann TK, Theodoraki MN. The emerging role of exosomes in diagnosis, prognosis, and therapy in head and neck cancer. Int J Mol Sci. 2020;21:1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell PJ, Welton J, Staffurth J, et al. Can urinary exosomes act as treatment response markers in prostate cancer? J Transl Med. 2009;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Zhang Y, Qiu F, Qiu Z. Proteomic identification of exosomal LRG1: a potential urinary biomarker for detecting NSCLC. Electrophoresis. 2011;32:1976‐1983. [DOI] [PubMed] [Google Scholar]
- 47.Besse B, Charrier M, Lapierre V, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morse MA, Garst J, Osada T, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.https://clinicaltrials.gov/ct2/show/NCT01294072 Study Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Colon Cancer Tissue - Full Text View – ClinicalTrials.gov [Internet]. [cited 2022 Oct 28]. Available from:
- 50.https://clinicaltrials.gov/ct2/show/NCT01854866 Safety and Effectiveness Study of Tumor Cell-derived Microparticles to Treat Malignant Ascites and Pleural Effusion – Full Text View - ClinicalTrials.gov [Internet]. [cited 2022 Oct 28]. Available from:
- 51.https://clinicaltrials.gov/ct2/show/NCT02657460 Clinical Trial of Tumor Cell-derived Microparticles Packaging Chemotherapeutic Drugs to Treat Malignant Pleural Effusion – Full Text View – ClinicalTrials.gov [Internet]. [cited 2022 Oct 28]. Available from:
- 52.https://clinicaltrials.gov/ct2/show/NCT03230708 Clinical Study of Autologous Erythrocytes Derived MPs Packaging MTX Peritoneal Perfusion to Treat Malignant Ascites – Full Text View – ClinicalTrials.gov.pdf [Internet]. [cited 2022 Oct 28]. Available from:
- 53.Nilsson J, Skog J, Nordstrand A, et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100:1603‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baran J, Baj-Krzyworzeka M, Weglarczyk K, et al. Circulating tumour-derived microvesicles in plasma of gastric cancer patients. Cancer Immunol Immunother. 2010;59:841‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang G, Zhang W, Li B, et al. MicroRNA-200c and microRNA-141 are regulated by a FOXP3-KAT2B axis and associated with tumor metastasis in breast cancer. Breast Cancer Res. 2017;19:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodríguez M, Silva J, López-Alfonso A, et al. Different exosome cargo from plasma/bronchoalveolar lavage in non-small-cell lung cancer. Genes, Chromosom Cancer. 2014;53:713‐724. [DOI] [PubMed] [Google Scholar]
- 58.Cazzoli R, Buttitta F, Di Nicola M, et al. MicroRNAs derived from circulating exosomes as noninvasive biomarkers for screening and diagnosing lung cancer. J Thorac Oncol. 2013;8:1156‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madhavan B, Yue S, Galli U, et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int J Cancer. 2015;136:2616‐2627. [DOI] [PubMed] [Google Scholar]
- 60.Que R, Ding G, Chen J, Cao L. Analysis of serum exosomal microRNAs and clinicopathologic features of patients with pancreatic adenocarcinoma. World J Surg Oncol. 2013;11:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13‐21. [DOI] [PubMed] [Google Scholar]
- 62.Bryant RJ, Pawlowski T, Catto JWF, et al. Changes in circulating microRNA levels associated with prostate cancer. Br J Cancer. 2012;106:768‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manterola L, Guruceaga E, Pérez-Larraya JG, et al. A small noncoding RNA signature found in exosomes of GBM patient serum as a diagnostic tool. Neuro Oncol 2014;16:520‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akers JC, Ramakrishnan V, Kim R, et al. miR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development. PLoS One. 2013;8:e78115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu J, Sun H, Wang X, et al. Increased exosomal microRNA-21 and microRNA-146a levels in the cervicovaginal lavage specimens of patients with cervical cancer. Int J Mol Sci. 2014;15:758‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogata-Kawata H, Izumiya M, Kurioka D, et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS One. 2014;9:e92921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szajnik M, Derbis M, Lach M, et al. Exosomes in plasma of patients with ovarian carcinoma: Potential biomarkers of tumor progression and response to therapy. Gynecol Obstet. 2013(Suppl 4):3‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kasthuri RS, Hisada Y, Ilich A, Key NS, Mackman N. Effect of chemotherapy and longitudinal analysis of circulating extracellular vesicle tissue factor activity in patients with pancreatic and colorectal cancer. Res Pract Thromb Haemost. 2020;4:636‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tesselaar MET, Romijn FPHTM, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520‐527. [DOI] [PubMed] [Google Scholar]
- 71.Erez O, Romero R, Vaisbuch E, et al. Tissue factor activity in women with preeclampsia or SGA: a potential explanation for the excessive thrombin generation in these syndromes. J Matern Neonatal Med. 2018;31:1568‐1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gezelius E, Flou Kristensen A, Bendahl PO, et al. Coagulation biomarkers and prediction of venous thromboembolism and survival in small cell lung cancer: a sub-study of RASTEN – a randomized trial with low molecular weight heparin. PLoS One. 2018;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pan D, Chen J, Feng C, et al. Preferential localization of MUC1 glycoprotein in exosomes secreted by non-small cell lung carcinoma cells. Int J Mol Sci. 2019;20:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1 + exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24:896‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lai X, Wang M, McElyea SD, Sherman S, House M, Korc M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017;393:86‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buscail E, Alix-Panabières C, Quincy P, et al. High clinical value of liquid biopsy to detect circulating tumor cells and tumor exosomes in pancreatic ductal adenocarcinoma patients eligible for up-front surgery. Cancers (Basel). 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X, Kong D, Wang C, et al. Circulating microRNAs as novel potential diagnostic biomarkers for ovarian cancer: a systematic review and updated meta-analysis. J Ovarian Res. 2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pan C, Stevic I, Müller V, et al. Exosomal microRNAs as tumor markers in epithelial ovarian cancer. Mol Oncol. 2018;12:1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iyer V, Yang Z, Ko J, Weissleder R, Issadore D. Advancing microfluidic diagnostic chips into clinical use: a review of current challenges and opportunities. Lab Chip. 2022;22:3110‐3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davies RT, Kim J, Jang SC, Choi EJ, Gho YS, Park J. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip. 2012;12:5202‐5210. [DOI] [PubMed] [Google Scholar]
- 83.Shao H, Chung J, Balaj L, et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med. 2012;18:1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aethlon Medical, Inc. (AEMD) [Internet]. [cited 2022 Apr 29]. Available from: https://www.aethlonmedical.com/.
- 85.ExosomeDx. ExosomeDx: Exosome Based Liquid Biopsies for Prostate Cancer [Internet]. ExosomeDx. 2021 [cited 2022 Apr 29]. Available from: https://www.exosomedx.com/.
- 86.Rubin O, Crettaz D, Tissot JD, Lion N. Pre-analytical and methodological challenges in red blood cell microparticle proteomics. Talanta. 2010;82:1‐8. [DOI] [PubMed] [Google Scholar]
- 87.Gandham S, Su X, Wood J, et al. Technologies and standardization in research on extracellular vesicles. Trends Biotechnol. 2020;38:1066‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maass KK, Schad PS, Finster AME, et al. From sampling to sequencing: a liquid biopsy pre-analytic workflow to maximize multi-layer genomic information from a single tube. Cancers (Basel). 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coumans FAW, Brisson AR, Buzas EI, et al. Methodological guidelines to study extracellular vesicles. Circ Res. 2017;120:1632‐1648. [DOI] [PubMed] [Google Scholar]
- 90.Geeurickx E, Hendrix A. Targets, pitfalls and reference materials for liquid biopsy tests in cancer diagnostics. Mol Aspects Med. 2020;72:100828. [DOI] [PubMed] [Google Scholar]