Abstract
Background:
The tibial fixation site is considered the weak link in anterior cruciate ligament (ACL) reconstruction, and conflicting results regarding the biomechanical properties of various fixation methods have been reported.
Purpose:
To examine knots tied over a bone bridge and its biomechanical properties as a suitable tibial fixation method in ACL reconstruction.
Study Design:
Controlled laboratory study.
Methods:
We divided 40 fresh-frozen porcine tibiae into 4 equal groups to evaluate flexor tendon grafts set with standard tibial fixation techniques: (1) bone bridge (BB group), (2) suspension button (SB group), (3) combined interference screw and bone bridge (IFS/BB group), and (4) combined interference screw and suspension button (IFS/SB group). Each construct was subjected to cyclic loading (1500 cycles, 50-250 N, 1 Hz) with a servohydraulic materials testing machine to measure elongation; load-to-failure testing (displacement rate: 25 mm/s) was then performed. Load to failure, stiffness, and yield load were compared between constructs using 1-way analysis of variance.
Results:
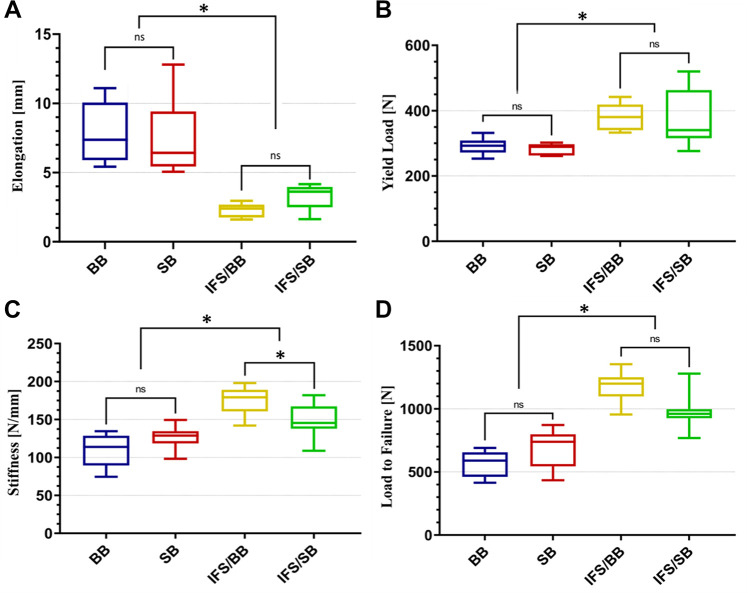
The hybrid fixation constructs (IFS/BB and IFS/SB groups) showed significantly better biomechanical properties than the isolated extracortical fixation constructs (BB and SB groups) (P < .05 for all). There were no differences between the isolated extracortical fixation constructs or between the hybrid fixation constructs in elongation or load to failure; however, stiffness of the IFS/BB group was significantly higher than that of the IFS/SB group (175.3 ± 16.6 vs 144.9 ± 20.1 N/mm, respectively; P < .05). Stiffness between the SB and BB groups was not significantly different.
Conclusion:
Hybrid fixation had superior biomechanical performance compared with isolated extracortical fixation. However, tibial graft fixation using a bone bridge either as isolated extracortical fixation or combined with an interference screw for hybrid fixation showed equivalent biomechanical properties compared with suspension button–based graft fixation.
Clinical Relevance:
The clinical use of a bone bridge for tibial graft fixation could reduce the cost for ACL reconstruction and lower the rate of implant-associated issues.
Keywords: anterior cruciate ligament, ACL reconstruction, bone bridge, graft fixation, hybrid fixation, biomechanics
Anterior cruciate ligament (ACL) reconstruction is one of the most frequently performed procedures in orthopaedic surgery, resulting in good to excellent clinical outcomes. ‡ However, reported rates of graft failure after ACL reconstruction remain highly variable, ranging from 4% to 17% of cases. 20,24,35,37,51,52 Because of the low bone density in the area of the proximal tibial metaphysis 7,23 and the long osseous integration period of soft tissue grafts of 3 to 6 months, 9,12,40,44 tibial fixation of ACL grafts and higher levels of activity during early rehabilitation have been considered the weak link in ACL reconstruction. 6,7,19,20,27,35,48 Therefore, biomechanically stable graft fixation, which has to withstand forces up to 500 N in the early postoperative rehabilitation period, 4,5,50 is essential to avoid early loosening and guarantee osseous integration. 6
As clinical studies show a high variability of surgical techniques and reported outcome parameters, the gold standard of tibial fixation remains yet unknown. In several studies, various tibial fixation devices demonstrated no differences in patient-reported outcomes. 16,18,25,30 Biomechanical studies, however, have shown superior results when using an interference screw, washer, or suspension button, 8,22,26,28 with better tendon-to-bone integration for the interference screw in animal studies. 49 Augmenting an interference screw with extracortical fixation yielded the highest primary stability but was associated with higher implant costs and soft tissue irritation. 3,6,53 These problems may be avoided when knotting sutures over a bone bridge onto the tibia, as first described by Pässler and Thermann. 36
This backup fixation method of using a bone bridge with an interference screw is part of our clinical routine for ACL reconstruction. The aim of the present study was to determine the biomechanical properties of this implant-free ACL–tibial bone bridge construct. We hypothesized that elongation would be greater while load to failure, yield load, and stiffness would be lower compared with standard suspension button fixation.
Methods
A total of 40 fresh-frozen porcine tibiae with a mean age of 11 ± 7 months were obtained from a local butcher. The tibiae were stored at –20°C and thawed for 24 hours at room temperature before the dissection of all soft tissue. The 40 tibiae were divided into 4 groups of 10 each based on the tibial fixation technique. The following fixation techniques were tested: (1) bone bridge (BB group), (2) suspension button (SB group), (3) combined interference screw and bone bridge (IFS/BB group), and (4) combined interference screw and suspension button (IFS/SB group). Half of the tibiae underwent ACL reconstruction with extracortical fixation using a bone bridge (Figure 1A) or suspension button (Endotack; Karl Storz) (Figure 1B). The other half of the tibiae underwent ACL reconstruction with hybrid fixation utilizing a 9 × 28–mm interference screw (Mega Fix CP; Karl Storz) combined with either a bone bridge or a suspension button. No ethical approval was needed for this study.
Figure 1.
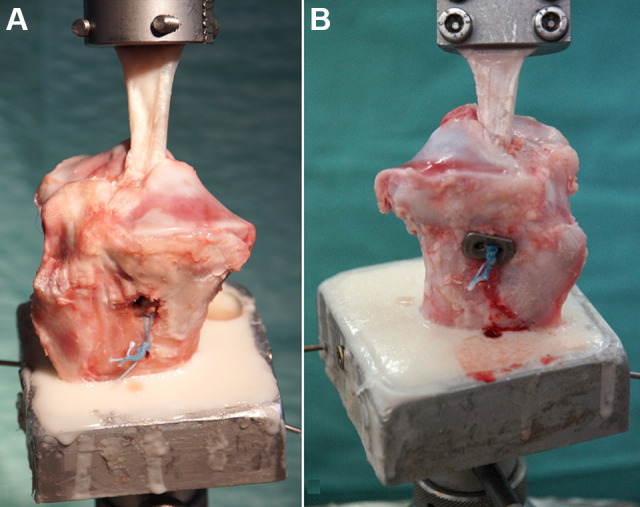
Fixation of the porcine tendon graft in the proximal tibia. The specimens were fixed in a custom-designed vise allowing axial testing of the anterior cruciate ligament graft using a (A) bone bridge or (B) suspension button.
Graft Preparation and Fixation
Porcine flexor digitorum tendons were used as a graft for ACL reconstruction. The tendon grafts were stored at –20°C and thawed at room temperature at least 1 hour before preparation. For all fixation techniques, the flexor digitorum tendons were prepared to a diameter of 9 mm and a total length of 80 mm with 30 mm of tibial tunnel length and 50 mm of extra-articular length. Consistent with previous studies, the Krackow locking stitch with doubled No. 2 braided composite suture (FiberWire; Arthrex) was used to whipstitch the distal free ends of the tendon grafts, imitating a standard soft tissue quadriceps tendon graft (Figure 2). 29,42
Figure 2.
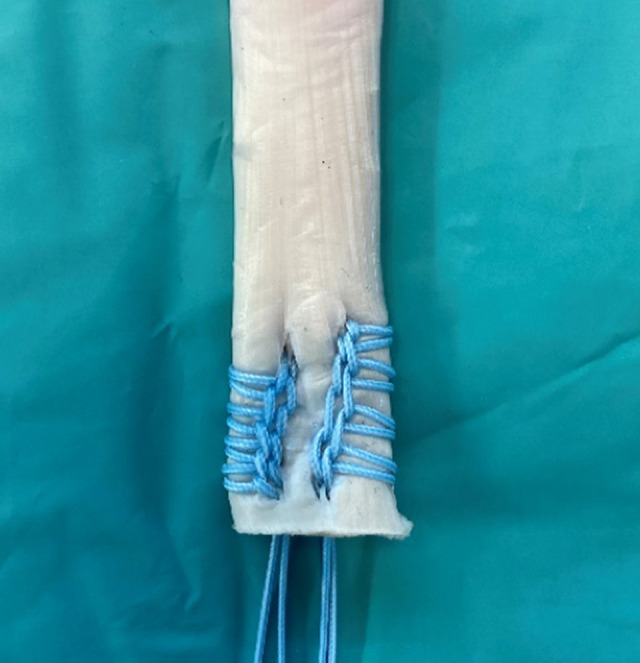
Prepared allograft using the Krackow locking stitch with doubled No. 2 braided composite suture (FiberWire; Arthrex) to whipstitch the distal free end of the porcine flexor digitorum tendon.
For all fixation techniques, the tibial tunnel was prepared using a tibial aiming guide (Karl Storz), which was positioned at the footprint of the native porcine ACL. After placing the entry site midway between the tibial tubercle and posteromedial cortex, a tunnel was reamed in an antegrade direction, matching the graft diameter of 9 mm. For half of the tibiae, which used a bone bridge for tibial fixation, a second hole with a diameter of 4 mm was drilled distal to the tibial tunnel, providing a cortical socket with a length of 15 mm as a post for the ACL sutures. This second hole was connected to the tibial tunnel of the ACL by compressing the cancellous bone of the metaphysis beneath the created cortical socket using an Overholt clamp (Figure 3).
Figure 3.
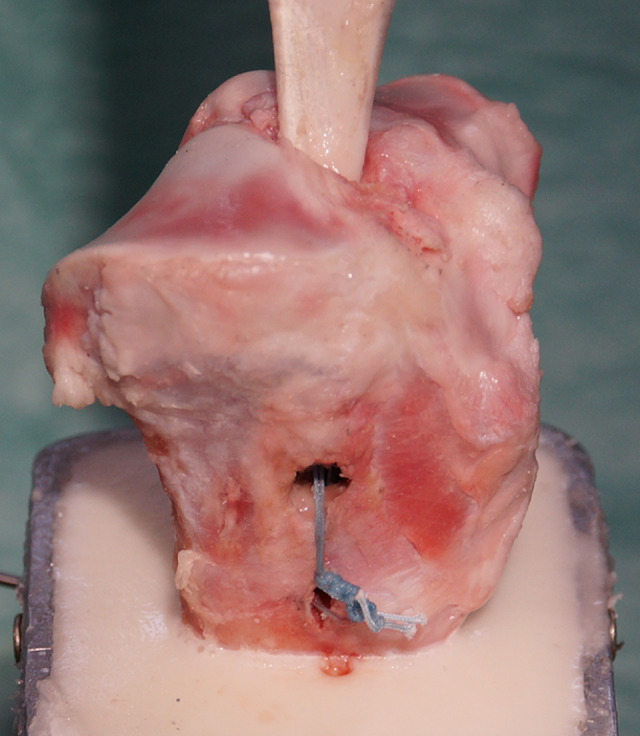
Extracortical fixation using a bone bridge. A second hole with a diameter of 4 mm was drilled distal to the tibial tunnel, providing a cortical socket with a length of 15 mm as a post for the bone bridge.
Then, the prepared tendon graft was shuttled through the tibial tunnel. For tibial fixation with a bone bridge, half of the free ends of the braided composite suture were shuttled around the cortical post and knotted 10 times. For the SB group, a suspension button was placed at the tibial entry site, and the braided composite suture was knotted 10 times onto the button. In addition, for the hybrid fixation groups, an interference screw of the same diameter as the graft was inserted before performing additional extracortical fixation.
Biomechanical Testing
Biomechanical testing was performed using a servohydraulic materials testing machine (Model 8874; Instron). Each tibia was fixed in a custom-designed vise using polymethyl methacrylate for unconstrained positioning of the specimens before testing. The specimens were then positioned in a way that the tibial tunnel ran parallel to the actuator axis, which allowed axial testing. The proximal end of the graft was fixed in a clamp, which was connected directly to the loading cell.
To minimize tissue hysteresis, a preload of 50 N was applied for 10 seconds to each specimen. 43 Subsequently, cyclic loading of 1500 cycles was conducted using between 50 and 250 N at a rate of 1 cycle per second (1 Hz). Finally, load-to-failure testing was performed at a displacement rate of 25 mm/s. Standard force displacement curves were then generated.
Statistical Analysis
Elongation, load to failure, yield load, and stiffness were quantified from the force displacement curves. Elongation was determined by subtracting the preload displacement from the displacement at 1500 cycles. Stiffness was defined as the steepest slope of the load-to-failure deformation curve, while yield load was determined at the point where the slope of the load displacement curve initially decreased. Maximum load to failure was defined as the highest point of the load displacement curve. The failure mode was recorded for each fixation technique after load-to-failure testing.
One-way analysis of variance was used to determine any statistically significant differences in elongation, load to failure, yield load, and stiffness between the 4 tibial fixation techniques. Significance was set at P < .05 divided by the number of tests (Bonferroni correction). Statistical analysis was performed using MATLAB (R2020a; MathWorks) and Prism (Version 9; GraphPad Software).
An a priori power analysis indicated that a sample size of 10 per group would lead to 90% power to detect a difference of 50 N between means at the β ≥ 0.8 level, based on the standard deviations found during cyclic loading of fixation constructs in porcine knee models. 15
Results
All specimens underwent cyclic loading. After load-to-failure testing, all specimens of the BB group and IFS/BB group failed by shearing through the bone bridge and pulling the graft out of the tunnel (Figure 4A). All specimens of the SB and IFS/SB groups failed by disruption of the braided composite suture (Figure 4B).
Figure 4.
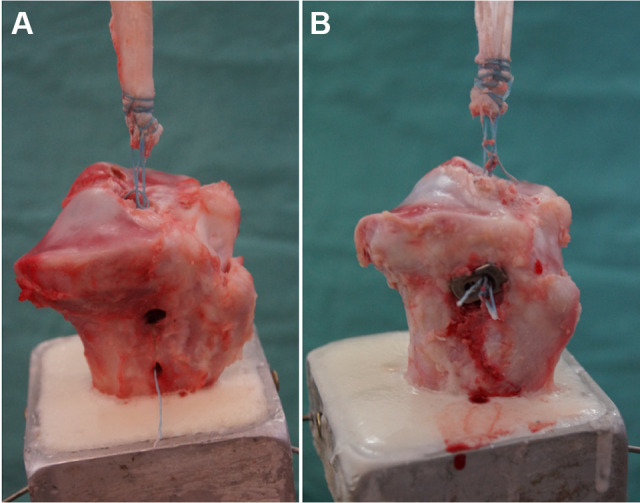
Failure mode for each graft fixation technique. (A) The braided composite suture sheared through the bone bridge. (B) Disruption of the braided composite suture during fixation with a suspension button.
The summarized results after biomechanical testing of the different fixation techniques are shown in Table 1 and Figure 5. The hybrid fixation groups (IFS/BB and IFS/SB) performed significantly better than the isolated extracortical fixation groups (BB and SB), with lower elongation values and higher load-to-failure, yield load, and stiffness values (P < .05 for all) (Figure 5). However, nearly 50% of elongation was observed after 50 of 1500 cycles.
Table 1.
Biomechanical Testing Results a
| Group | Elongation, mm | Load to Failure, N | Yield Load, N | Stiffness, N/mm |
|---|---|---|---|---|
| BB | 7.1 ± 1.5 | 557.3 ± 98.7 | 290.5 ± 22.8 | 112.8 ± 38.7 |
| SB | 5.7 ± 1.9 | 684.3 ± 139.3 | 283.4 ± 15.1 | 126.6 ± 13.1 |
| IFS/BB | 1.5 ± 0.3 | 1166.9 ± 99.1 | 381.8 ± 36.6 | 175.3 ± 16.6 |
| IFS/SB | 2.3 ± 0.7 | 962.7 ± 119.8 | 376.8 ± 82.2 | 144.9 ± 20.1 |
a Data are reported as mean ± SD. BB, bone bridge; IFS, interference screw; SB, suspension button.
Figure 5.
Box plots of the structural properties for the bone bridge (BB), suspension button (SB), interference screw/bone bridge (IFS/BB), and interference screw/suspension button (IFS/SB) groups: (A) elongation, (B) yield load, (C) stiffness, and (D) load to failure. The top and bottom of each box represents the first and third quartiles of the data set, while the median divides the box. In addition, the error bars at the beginning and end of the box plot represent the minimum and maximum of the data set. ns, not significant. *Statistically significant difference (P < .05).
Extracortical Fixation
There were no significant differences between the BB group and SB group with regard to elongation, load to failure, yield load, or stiffness.
Hybrid Fixation
There were no significant differences between the IFS/BB and IFS/SB groups in elongation, load to failure, or yield load. Stiffness was the only parameter in which a significant difference was found, with a higher value for the IFS/BB group compared with the IFS/SB group (175.3 ± 16.6 vs 144.9 ± 20.1 N/mm, respectively; P < .05).
Discussion
The most important finding of the present study was that, contrary to our hypothesis, tibial graft fixation using a bone bridge, either as isolated extracortical fixation or combined with an interference screw for hybrid fixation, showed equivalent biomechanical properties compared with isolated and hybrid suspension button–based graft fixation in ACL reconstruction, while the hybrid construct using the bone bridge provided higher stiffness. Additionally, we found that hybrid fixation had superior biomechanical performance compared to isolated extracortical fixation. Load to failure in all 4 tested groups exceeded the expected loads that occur during rehabilitation. 4,5,50
It is widely accepted that tibial fixation is the weak point in ACL reconstruction, especially during early rehabilitation, which is necessary to restore range of motion, strength, and function. § In a previous study, Scannell et al 43 compared the biomechanical properties of a WasherLoc and 3 different designs of interference screws and demonstrated that the WasherLoc was the strongest on load-to-failure testing (630 ± 129 N) and less stiff than interference screw fixation (97 ± 12 N), which was similar to the values of the BB group in the present study. Similarly, Robert et al 39 compared a TightRope, a WasherLoc, a Delta screw, and a Tape Locking Screw and determined that the Tape Locking Screw exhibited the highest load to failure (1015 ± 129 N) and less slippage (1.23 ± 0.36 N/mm). Their WasherLoc fixation, representing extracortical fixation, yielded a mean load to failure of 511 ± 95 N and a mean stiffness of 131.8 ± 56 N/mm, which were lower than for their Tape Locking Screw fixation but comparable with our BB group.
Conversely, using a porcine model, Fogel and colleagues 13 demonstrated that suspension button fixation featured a higher load to failure (880.1 ± 176.4 N) and stiffness (112.8 ± 24.1 N/mm) compared with several other fixation devices. The present study showed lower but comparable results for both bone bridge and suspension button fixation. This may be because of the different suspension button devices (GraftLink [Arthrex] vs Endotack [Karl Storz]) used in their experiment and shorter cyclic loading of 500 cycles compared with 1500 cycles in the present study.
In contrast to the consistency of the above data, our study’s results showed slightly higher elongation for isolated extracortical fixation (BB group: 7.1 ± 1.5 mm; SB group: 5.7 ± 1.9 mm) compared with the aforementioned (2.5-5.6 mm) studies. 13,39,43 One potential explanation for this may be possible lengthening of the used tendon construct during biomechanical testing. In a biomechanical analysis examining the biomechanical properties of various stitching methods of human quadriceps tendons, Michel et al 29 observed a greater mean elongation of 10.6 ± 2.6 mm after whipstitching the tendons with doubled No. 2 braided composite suture using the Krackow locking stitch, which was similar to our study’s findings. Furthermore, when looking at the present study’s cyclic loading results, nearly 50% of elongation was observed after 50 of 1500 cycles. Another possible explanation may be the differences in testing setups and the exact calculation of the elongation values. Compared with the studies of Scannell et al, 43 Robert et al, 39 and Fogel et al, 13 the present study used a more aggressive testing protocol and determined elongation by subtracting the preload displacement from the displacement at 1500 cycles. Fogel et al, however, determined elongation as the net change in displacement after completing 500 cycles under loads of 250 N, which resulted in lower elongation values. During cyclic loading of isolated extracortical fixation constructs (BB and SB groups), elongation exceeded the clinical threshold of >3 mm38 and might hold the risk of clinical failure during early rehabilitation, while hybrid fixation constructs (IFS/BB group: 1.5 ± 0.3 mm; IFS/SB group: 2.3 ± 0.7 mm) achieved similar or lower values compared with the aforementioned studies (2.5-5.6 mm). 13,39,43
Intratunnel fixation devices such as the interference screw are positioned on the cancellous bone within the tunnel and enable fixation directly at the joint level, which shortens the mechanically loaded graft length and increases the stiffness of the construct. 16,22,44 Conversely, extracortical fixation devices such as the suspension button or bone bridge are positioned on the cortical bone at the tunnel entrance and are characterized by higher primary stability with a pullout strength of 600 to 800 N. 6,13,16,39,43 Combining the advantages of both methods, hybrid fixation techniques have shown better biomechanical properties with the highest pullout strength and stiffness of the graft combined with a reduced length. 3,6,53 According to the present study, the IFS/BB and IFS/SB groups yielded the highest primary stability, with a mean load to failure of 1166.9 ± 99.1 and 962.7 ± 119.8 N, respectively. The stiffness of the hybrid fixation constructs was nearly double that of the isolated extracortical fixation constructs, with superior values for the IFS/BB group (175.3 ± 16.6 N/mm; P < .05). These results for hybrid fixation were similar to those found by Yoo and colleagues 53 in a comparable test setup. According to their findings, hybrid fixation combining an interference screw with a cortical screw and spiked washer yielded a load to failure of up to 800 N and stiffness of 120 N/mm. However, the results of our study do not show whether backup fixation (with either a bone bridge or suspension button) is necessary when using an interference screw.
Graft fixation in both the BB and IFS/BB groups failed by shearing through the bone bridge, which requires a high density of the cortical bone of the proximal tibia to ensure biomechanical stability of the implant-free ACL–tibial bone bridge construct. Thus, the tested bone bridge construct may not be ideal in the cases of middle-aged female patients with early osteoporosis, and an implant-based fixation strategy should be considered instead. Nevertheless, implant-free fixation strategies seem to be increasingly coming into focus for knee surgeons, especially in these times of steadily increasing socioeconomic pressure on hospitals. 10,47 From a biomechanical point of view, hybrid fixation of ACL grafts is superior to isolated extracortical or intratunnel fixation 53 ; however, hybrid fixation is associated with significantly higher material expenditure and costs. Therefore, hybrid fixation consisting of an interference screw and a bone bridge could reduce the costs of ACL reconstruction without compromising biomechanical stability.
Limitations
The present study had some limitations inherent to biomechanical testing. First, a porcine model was used to simulate tibial fixation of ACL grafts. 8,13,22,39,43 Although it has been previously reported that porcine tibiae are a nonideal substitute for human cadaveric tibiae because of the higher volumetric density of the porcine bone, 32,33 other authors found a fairly similar bone density between porcine and human tibiae. 6,31 Second, biomechanical testing simulated forces acting at time zero when biological integration processes and graft healing were not taken into account. This unidirectional testing simulated a worst-case scenario with forces acting directly on the graft and may, therefore, not have mimicked loads that occur clinically. Third, the present study only tested 1 device as a representation for each fixation method, even though several other products are available for extracortical or intratunnel fixation and may feature different ultimate strength and stiffness values. Finally, the present study’s setup only allowed the measurement of biomechanical properties of each construct, which do not represent primary endpoints in clinical studies.
Conclusion
In the current study, hybrid fixation had superior biomechanical performance compared with isolated extracortical fixation. Tibial graft fixation using a bone bridge either as isolated extracortical fixation or combined with an interference screw for hybrid fixation showed equivalent biomechanical properties compared with isolated and hybrid suspension button–based graft fixation in ACL reconstruction, while the hybrid construct using the bone bridge provided higher stiffness.
Footnotes
Final revision submitted September 3, 2022; accepted September 13, 2022.
One or more of the authors has declared the following potential conflict of interest or source of funding: Support was received from the Open Access Publication Fund of the University of Münster. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval was not sought for the present study.
References
- 1. Ahldén M, Samuelsson K, Sernert N, et al. The Swedish National Anterior Cruciate Ligament Register: a report on baseline variables and outcomes of surgery for almost 18,000 patients. Am J Sports Med. 2012;40(10):2230–2235. [DOI] [PubMed] [Google Scholar]
- 2. Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011;45(7):596–606. [DOI] [PubMed] [Google Scholar]
- 3. Balazs GC, Brelin AM, Grimm PD, et al. Hybrid tibia fixation of soft tissue grafts in anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2016;44(10):2724–2732. [DOI] [PubMed] [Google Scholar]
- 4. Beynnon BD, Fleming BC. Anterior cruciate ligament strain in-vivo: a review of previous work. J Biomech. 1998;31(6):519–525. [DOI] [PubMed] [Google Scholar]
- 5. Boileau P, Remi M, Lemaire M, et al. Plea for accelerated rehabilitation after ligament plasty of the knee by a bone-patellar tendon-bone graft. Article in French. Rev Chir Orthop Reparatrice Appar Mot. 1999;85(5):475–490. [PubMed] [Google Scholar]
- 6. Brand J, Jr, Weiler A, Caborn DN, Brown CH, Jr, Johnson DL. Graft fixation in cruciate ligament reconstruction. Am J Sports Med. 2000;28(5):761–774. [DOI] [PubMed] [Google Scholar]
- 7. Brand JC, Jr, Pienkowski D, Steenlage E, et al. Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med. 2000;28(5):705–710. [DOI] [PubMed] [Google Scholar]
- 8. Coleridge SD, Amis AA. A comparison of five tibial-fixation systems in hamstring-graft anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(5):391–397. [DOI] [PubMed] [Google Scholar]
- 9. Colombet P, Graveleau N, Jambou S. Incorporation of hamstring grafts within the tibial tunnel after anterior cruciate ligament reconstruction: magnetic resonance imaging of suspensory fixation versus interference screws. Am J Sports Med. 2016;44(11):2838–2845. [DOI] [PubMed] [Google Scholar]
- 10. Deviandri R, van der Veen HC, Lubis AMT, van den Akker-Scheek I, Postma MJ. Cost-effectiveness of ACL treatment is dependent on age and activity level: a systematic review. Knee Surg Sports Traumatol Arthrosc. Published online August 23, 2022. doi:10.1007/s00167-022-07087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ejerhed L, Kartus J, Sernert N, Kohler K, Karlsson J. Patellar tendon or semitendinosus tendon autografts for anterior cruciate ligament reconstruction? A prospective randomized study with a two-year follow-up. Am J Sports Med. 2003;31(1):19–25. [DOI] [PubMed] [Google Scholar]
- 12. Ekdahl M, Wang JH, Ronga M, Fu FH. Graft healing in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):935–947. [DOI] [PubMed] [Google Scholar]
- 13. Fogel H, Golz A, Burleson A, et al. A biomechanical analysis of tibial fixation methods in hamstring-graft anterior cruciate ligament reconstruction. Iowa Orthop J. 2019;39(1):141–147. [PMC free article] [PubMed] [Google Scholar]
- 14. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622–627. [DOI] [PubMed] [Google Scholar]
- 15. Glasbrenner J, Domnick C, Raschke MJ, et al. Adjustable buttons for ACL graft cortical fixation partially fail with cyclic loading and unloading. Knee Surg Sports Traumatol Arthrosc. 2019;27(8):2530–2536. [DOI] [PubMed] [Google Scholar]
- 16. Han DL, Nyland J, Kendzior M, Nawab A, Caborn DN. Intratunnel versus extratunnel fixation of hamstring autograft for anterior cruciate ligament reconstruction. Arthroscopy. 2012;28(10):1555–1566. [DOI] [PubMed] [Google Scholar]
- 17. Harris JD, Abrams GD, Bach BR, et al. Return to sport after ACL reconstruction. Orthopedics. 2014;37(2):e103–e108. [DOI] [PubMed] [Google Scholar]
- 18. Ilahi OA, Nolla JM, Ho DM. Intra-tunnel fixation versus extra-tunnel fixation of hamstring anterior cruciate ligament reconstruction: a meta-analysis. J Knee Surg. 2009;22(2):120–129. [DOI] [PubMed] [Google Scholar]
- 19. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaeding CC, Pedroza AD, Reinke EK, et al. Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: prospective analysis of 2488 primary ACL reconstructions from the MOON cohort. Am J Sports Med. 2015;43(7):1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kostogiannis I, Ageberg E, Neuman P, et al. Activity level and subjective knee function 15 years after anterior cruciate ligament injury: a prospective, longitudinal study of nonreconstructed patients. Am J Sports Med. 2007;35(7):1135–1143. [DOI] [PubMed] [Google Scholar]
- 22. Kousa P, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction, part II: tibial site. Am J Sports Med. 2003;31(2):182–188. [DOI] [PubMed] [Google Scholar]
- 23. Kurosaka M, Yoshiya S, Andrish JT. A biomechanical comparison of different surgical techniques of graft fixation in anterior cruciate ligament reconstruction. Am J Sports Med. 1987;15(3):225–229. [DOI] [PubMed] [Google Scholar]
- 24. Leys T, Salmon L, Waller A, Linklater J, Pinczewski L. Clinical results and risk factors for reinjury 15 years after anterior cruciate ligament reconstruction: a prospective study of hamstring and patellar tendon grafts. Am J Sports Med. 2012;40(3):595–605. [DOI] [PubMed] [Google Scholar]
- 25. Lubowitz JH, Ahmad CS, Anderson K. All-inside anterior cruciate ligament graft-link technique: second-generation, no-incision anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(5):717–727. [DOI] [PubMed] [Google Scholar]
- 26. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35–43. [DOI] [PubMed] [Google Scholar]
- 27. Maletis GB, Inacio MC, Funahashi TT. Risk factors associated with revision and contralateral anterior cruciate ligament reconstructions in the Kaiser Permanente ACLR registry. Am J Sports Med. 2015;43(3):641–647. [DOI] [PubMed] [Google Scholar]
- 28. Mayr R, Heinrichs CH, Eichinger M, et al. Biomechanical comparison of 2 anterior cruciate ligament graft preparation techniques for tibial fixation: adjustable-length loop cortical button or interference screw. Am J Sports Med. 2015;43(6):1380–1385. [DOI] [PubMed] [Google Scholar]
- 29. Michel PA, Domnick C, Raschke MJ, et al. Soft tissue fixation strategies of human quadriceps tendon grafts: a biomechanical study. Arthroscopy. 2019;35(11):3069–3076. [DOI] [PubMed] [Google Scholar]
- 30. Monaco E, Fabbri M, Redler A, et al. Anterior cruciate ligament reconstruction is associated with greater tibial tunnel widening when using a bioabsorbable screw compared to an all-inside technique with suspensory fixation. Knee Surg Sports Traumatol Arthrosc. 2019;27(8):2577–2584. [DOI] [PubMed] [Google Scholar]
- 31. Nagarkatti DG, McKeon BP, Donahue BS, Fulkerson JP. Mechanical evaluation of a soft tissue interference screw in free tendon anterior cruciate ligament graft fixation. Am J Sports Med. 2001;29(1):67–71. [DOI] [PubMed] [Google Scholar]
- 32. Nurmi JT, Jarvinen TL, Kannus P, et al. Compaction versus extraction drilling for fixation of the hamstring tendon graft in anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30(2):167–173. [DOI] [PubMed] [Google Scholar]
- 33. Nurmi JT, Sievanen H, Kannus P, Jarvinen M, Jarvinen TL. Porcine tibia is a poor substitute for human cadaver tibia for evaluating interference screw fixation. Am J Sports Med. 2004;32(3):765–771. [DOI] [PubMed] [Google Scholar]
- 34. Nwachukwu BU, Voleti PB, Berkanish P, et al. Return to play and patient satisfaction after ACL reconstruction: study with minimum 2-year follow-up. J Bone Joint Surg Am. 2017;99(9):720–725. [DOI] [PubMed] [Google Scholar]
- 35. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242–1246. [DOI] [PubMed] [Google Scholar]
- 36. Pässler HH, Thermann H. Neue Techniken: Kniechirurgie. Steinkopff ; 2002. [Google Scholar]
- 37. Pinczewski LA, Lyman J, Salmon LJ, et al. A 10-year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft: a controlled, prospective trial. Am J Sports Med. 2007;35(4):564–574. [DOI] [PubMed] [Google Scholar]
- 38. Poehling-Monaghan KL, Salem H, Ross KE, et al. Long-term outcomes in anterior cruciate ligament reconstruction: a systematic review of patellar tendon versus hamstring autografts. Orthop J Sports Med. 2017;5(6):2325967117709735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Robert H, Bowen M, Odry G, et al. A comparison of four tibial-fixation systems in hamstring-graft anterior ligament reconstruction. Eur J Orthop Surg Traumatol. 2015;25(2):339–347. [DOI] [PubMed] [Google Scholar]
- 40. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel: a biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795–1803. [DOI] [PubMed] [Google Scholar]
- 41. Sajovic M, Stropnik D, Skaza K. Long-term comparison of semitendinosus and gracilis tendon versus patellar tendon autografts for anterior cruciate ligament reconstruction: a 17-year follow-up of a randomized controlled trial. Am J Sports Med. 2018;46(8):1800–1808. [DOI] [PubMed] [Google Scholar]
- 42. Sakaguchi K, Tachibana Y, Oda H. Biomechanical properties of porcine flexor tendon fixation with varying throws and stitch methods. Am J Sports Med. 2012;40(7):1641–1645. [DOI] [PubMed] [Google Scholar]
- 43. Scannell BP, Loeffler BJ, Hoenig M, et al. Biomechanical comparison of hamstring tendon fixation devices for anterior cruciate ligament reconstruction, part 2: four tibial devices. Am J Orthop (Belle Mead NJ). 2015;44(2):82–85. [PubMed] [Google Scholar]
- 44. Scheffler SU, Sudkamp NP, Gockenjan A, Hoffmann RF, Weiler A. Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques: the impact of fixation level and fixation method under cyclic loading. Arthroscopy. 2002;18(3):304–315. [DOI] [PubMed] [Google Scholar]
- 45. Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292–299. [DOI] [PubMed] [Google Scholar]
- 46. Shelbourne KD, Wilckens JH. Current concepts in anterior cruciate ligament rehabilitation. Orthop Rev. 1990;19(11):957–964. [PubMed] [Google Scholar]
- 47. Stewart BA, Momaya AM, Silverstein MD, Lintner D. The cost-effectiveness of anterior cruciate ligament reconstruction in competitive athletes. Am J Sports Med. 2017;45(1):23–33. [DOI] [PubMed] [Google Scholar]
- 48. Webster KE, Feller JA. Exploring the high reinjury rate in younger patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(11):2827–2832. [DOI] [PubMed] [Google Scholar]
- 49. Weiler A, Hoffmann RF, Bail HJ, Rehm O, Südkamp NP. Tendon healing in a bone tunnel, part II: histologic analysis after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy. 2002;18(2):124–135. [DOI] [PubMed] [Google Scholar]
- 50. West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197–207. [DOI] [PubMed] [Google Scholar]
- 51. Wright RW, Dunn WR, Amendola A, et al. Risk of tearing the intact anterior cruciate ligament in the contralateral knee and rupturing the anterior cruciate ligament graft during the first 2 years after anterior cruciate ligament reconstruction: a prospective MOON cohort study. Am J Sports Med. 2007;35(7):1131–1134. [DOI] [PubMed] [Google Scholar]
- 52. Wright RW, Magnussen RA, Dunn WR, Spindler KP. Ipsilateral graft and contralateral ACL rupture at five years or more following ACL reconstruction: a systematic review. J Bone Joint Surg Am. 2011;93(12):1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yoo JC, Ahn JH, Kim JH, et al. Biomechanical testing of hybrid hamstring graft tibial fixation in anterior cruciate ligament reconstruction. Knee. 2006;13(6):455–459. [DOI] [PubMed] [Google Scholar]