Abstract
The effects of prednisolone treatment on the cellularity and cytokine (gamma interferon, interleukin-12, and inducible nitric oxide synthase) profiles of leprosy skin type 1 (reversal) reactions were studied using immunohistochemistry. Skin biopsies were taken from 15 patients with leprosy type 1 (reversal) reactions at days 0, 7, 28, and 180 after the start of steroid treatment. Prednisolone treatment had little effect at day 7, but by day 28 significant decreases were found in cytokine levels. Some patients maintained cytokine production at days 28 and 180. These results illustrate the strong Th1 profile of type 1 reactional lesions, the slow response to steroid therapy, and continuing activity at 180 days.
Leprosy has two polar forms, lepromatous and tuberculoid leprosy, with contrasting cytokine profiles. The Th1-type cytokines gamma interferon (IFN-γ), interleukin-2 (IL-2), and IL-12 dominate the tuberculoid pole, but a Th2-like cytokine profile occurs at the lepromatous pole (17). Between the two polar forms are the clinically and immunologically unstable forms borderline tuberculoid (BT) and borderline lepromatous (BL) leprosy. At least 15% of patients with borderline leprosy develop type 1 or reversal reactions (T1R) (9). This is due to the development of augmented cell-mediated immunity towards Mycobacterium leprae antigens, and patients present clinically with acute skin inflammation and neuritis. There is an influx of CD4+ cells into lesions (10), and elevated levels of cytokine mRNA and protein have been detected in situ, including IFN-γ, IL-1β, IL-2, IL-12, and tumor necrosis factor α (4, 7, 14–16). Furthermore, increased levels of the enzyme-inducible nitric oxide synthase (iNOS) are found in reactional lesions (8), indicating an enhanced intracellular killing capacity of macrophages. Reactions are painful and frequently result in significant nerve damage. Immediate treatment with corticosteroids is instituted to prevent nerve damage.
Steroids have multiple effects on cytokine production, principally through inhibition of NF-κB-induced transcription of cytokine mRNAs (13). Several proinflammatory cytokines, tumor necrosis factor α (6), IL-2 (11), IL-12 (5), IFN-γ (1), and also iNOS are downregulated in this way by glucocorticoids.
This clinical study assessed the in vivo effects of steroids on the cellular phenotypes within reactional lesions and on the expression of iNOS, IFN-γ, and IL-12.
Patients.
Fifteen patients (n of BT patients = 6, n of BL patients = 9) with a skin T1R were studied at the Dhoolpet Leprosy Research Centre (DLRC), Hyderabad, India. A T1R was defined as the appearance of erythema in either existing or new leprosy skin lesions within the previous 2 weeks, confirmed histologically. Patient consent was obtained. Skin biopsies (6-mm-diameter punch, stored in liquid nitrogen) were taken from the reactional site on presentation (day 0) (before prednisolone therapy was begun) and from the same area at days 7, 28, and 180 during therapy. Prednisolone (30 mg orally, daily) was used initially and reduced by 5 mg every month. The study design of taking only reactional patients allowed the comparison of levels of cytokine production both within individual patients and groupwise between reactional BT and BL patients, with the patients being their own controls.
Immunostaining.
Cryosections (thicknesses, 5 to 6 μm) were adhered to silane (Sigma, Poole, United Kingdom)-coated slides and acetone fixed. Sections were incubated with the following monoclonal antibodies: anti-IFN-γ (33 g/ml; Boehringer Mannhein), anti-IL-12 P40 subunit (25 g/ml; R&D Systems), and anti-iNOS (2 g/ml; Transduction Laboratories). Cell phenotypes were examined using monoclonal antibodies against CD3 (dilution, 1:20), CD4 (1:200), and CD8 (1:20) (Becton Dickinson). Antibody binding was revealed with peroxidase–anti-peroxidase antibody or avidin-biotin complex–peroxidase using rabbit-anti-mouse immunoglobulin or biotin-conjugated rabbit anti-mouse immunoglobulins, respectively. The enzymatic reaction was developed using 3,3′diaminobenzidine chromogen (Sigma) with hematoxylin counterstaining. Controls for the specificity of staining included the use of appropriate isotype antibodies and normal serum and omission of the primary antibody. Cell and cytokine staining was evaluated independently by two observers using agreed-upon scales (Table 1). Differences between all four groups were determined statistically using the Kruskall-Wallis test and between two groups using the Mann-Whitney test.
TABLE 1.
Scoring of cytokine and iNOS expression in sequential biopsies
| Patient | Classificationb
|
Scorea on indicated day of biopsy
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell infiltrate
|
IFN-γ
|
IL-12
|
iNOS
|
||||||||||||||||
| Class | MDT | Clinical improvement (28 Days) | 0 | 7 | 28 | 180 | 0 | 7 | 28 | 180 | 0 | 7 | 28 | 180 | 0 | 7 | 28 | 180 | |
| 131 | BL-RR | Previously | Complete | 2 | 3 | 3 | 3 | 2 | 2 | 2 | 1 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | |
| 141 | BL-RR | Yes | Partial | 3 | 2 | 1 | 2 | 2 | 2 | 1 | 3 | 2 | 2 | 3 | 3 | 2 | 2 | 3 | |
| 146 | BL-RR | No | Partial | 3 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 3 | 2 | 1 | ||||
| 186 | BL-RR | No | Complete | 2 | 1 | 1 | 2 | 2 | 0 | 2 | 2 | 1 | 2 | 2 | 1 | ||||
| 198 | BL-RR | No | Partial | 3 | 3 | 2 | 0 | 3 | 3 | 3 | 0 | 3 | 3 | 2 | 0 | 3 | 3 | 2 | 0 |
| 207 | BL-RR | No | Partial | 3 | 3 | 0 | 1 | 3 | 3 | 1 | 2 | 3 | 3 | 0 | 0 | 3 | 3 | 0 | 1 |
| 260 | BL-RR | Yes | Partial | 3 | 3 | 1 | 1 | 3 | 3 | 2 | 0 | 3 | 2 | 1 | 0 | 2 | 2 | 1 | 0 |
| 304 | BL-RR | No | Partial | 2 | 2 | 0 | 3 | 3 | 1 | 2 | 3 | 0 | 2 | 3 | 0 | ||||
| 343 | BL-RR | No | Partial | 1 | 2 | 0 | 3 | 3 | 0 | 3 | 3 | 0 | 3 | 3 | 0 | ||||
| 143 | BT-RR | No | Partial | 2 | 1 | 1 | 2 | 2 | 1 | 3 | 3 | 2 | 3 | 2 | 0 | ||||
| 162 | BT-RR | No | Partial | 3 | 2 | 2 | 3 | 3 | 2 | 3 | 3 | 2 | 4 | 3 | 2 | ||||
| 179 | BT-RR | Previously | Partial | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 4 | 3 | 2 | ||||
| 303 | BT-RR | No | None | 3 | 3 | 1 | 4 | 3 | 1 | 2 | 3 | 3 | 1 | 3 | 3 | 2 | |||
| 342 | BT-RR | No | Complete | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | ||||
| 380 | BT-RR | No | Complete | 3 | 3 | 1 | 3 | 3 | 2 | 3 | 3 | 2 | 3 | 2 | 1 | ||||
Scoring for cytokine staining was as follows: 0, negative; 1, <10% of cells staining positive; 2, 10 to 30% of cells staining positive; 3, 50 to 80% of cells staining positive; and 4, 80 to 100% of cells staining positive. Scoring for cellular infiltrate was as follows: 0, no cellular infiltrate; 1, small granulomas; 2, medium-sized granulomas; and 3, large granulomas.
BL-RR, borderline lepromatous leprosy in reversal reaction; BT-RR, borderline tuberculoid leprosy in reversal reaction; MDT, multidrug therapy.
After 28 days of prednisolone treatment, 4 patients had a complete resolution, 10 had a partial resolution, and 1 had no clinical improvement of their skin lesions.
Cellular infiltrate.
Cellular infiltrates were assessed at days 0, 7, 28, and 180 (Table 1). The cellularity of the lesions decreased during prednisolone therapy (Fig. 1 and 2). All patients had cellular infiltration in the initial biopsy, with most having large granulomas and 10 patients having a score of 3. No significant difference was found between the median scores for day 0 and 7 biopsies (median score, 3), and eight patients still had a score of 3. The cellularity of lesions in biopsies declined significantly from days 0 to 28 (median, 1.5) (P < 0.02). However, two patients (patients 179 and 342) had no appreciable decrease in their cellular infiltrates over 28 days, and one patient (patient 131) maintained the same level of infiltration after 6 months of steroid treatment. No significant differences were found in the median CD4/CD8 ratios between day 0 (median, 1.8), day 7 (median, 1.7), and day 28 (median, 1.9) (P = 0.5), suggesting that prednisolone therapy does not alter the cell phenotypes attracted into the lesions. BL and BT reactional patients had similar CD4/CD8 ratios.
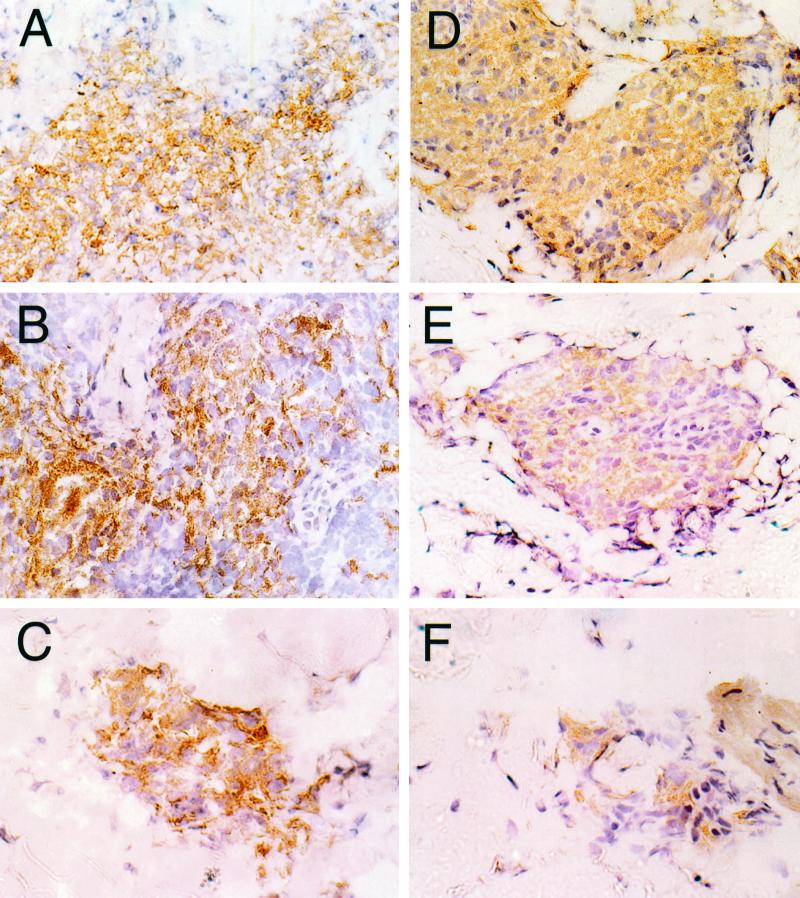
FIG. 1.
Photomicrographs of tissue sections stained with immunoperoxidase for IFN-γ and IL-12 expression in a BT patient (patient 380; BT-RR, 40× objective). (A) IFN-γ staining in a reactional granuloma in a biopsy taken at day 0. (B) A high level of IFN-γ expression is seen and appears unchanged at day 7. (C) Fewer cells are present in a smaller granuloma at day 28, and the level of IFN-γ expression has decreased compared with expression in the day 0 and 7 biopsies. IL-12 is strongly expressed in granulomas in biopsies taken at day 0 (D), and a similar level of positive staining is seen at day 7 (E). (F) The granuloma size and level of positive staining have decreased by day 28.
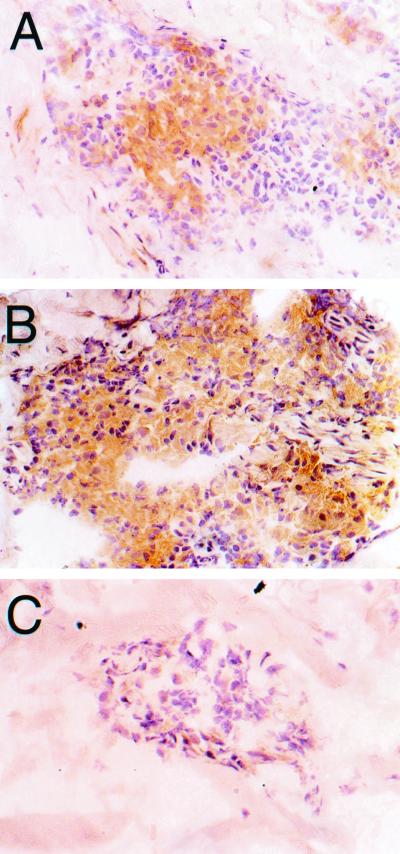
FIG. 2.
Immunoperoxidase staining of iNOS in biopsies taken from reactional lesions of a BL patient (patient 186). Strong positive staining is seen in the granulomas at day 0 (A) and day 7 (B). (C) At day 28, only a few scattered cells are found to stain positively.
Cytokines.
All patient biopsies had positive staining for IFN-γ and IL-12 on day 0 (Table 1), and most patient biopsies stained strongly for both cytokines, with median scores of 3, which corresponds to 30 to 50% of cells staining positive (Fig. 1). IFN-γ staining was unchanged between day 0 (median, 3) and day 7 (median, 3). A significant reduction was seen between days 0 and 28 (median, 1; P < 0.0005), by which time only two patients scored 3, five scored 2, and six scored 1. In two patients, IFN-γ was undetectable by day 28. Results for some individuals contradicted the group trend; three patients (patients 131, 198, and 342) had no reduction in IFN-γ levels by day 28. Two patients had increased IFN-γ levels between days 28 and 180, going from a score of 1 to 2 (patients 207 and 303). Staining for IL-12 revealed a pattern similar to that for IFN-γ (Fig. 1). IL-12 staining was unchanged between day 0 (median, 3) and day 7 (median, 3). A significant reduction occurred between days 0 and day 28 (median, 1; P < 0.002). Three patients were negative for IL-12 at day 28. Again, three patients (patients 131, 179, and 342) showed no reduction in IL-12 over the 28 days. One patient (patient 141) had increased IL-12 expression between days 28 and 180.
iNOS.
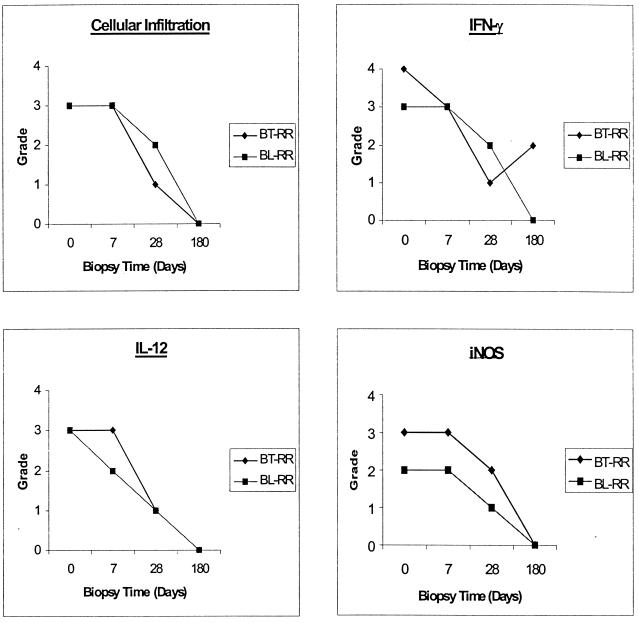
iNOS staining paralleled that for IFN-γ and IL-12 (Fig. 2). Positive staining in the initial biopsy was strong, with a median score of 3 (30 to 50% of cells were positive). Two patients showed very high levels of iNOS expression at day 0, with a score of 4, and both of these patients were classified as BT in reaction. Again there was no difference detected between the day 0 and 7 (median, 3) biopsies. Staining was significantly reduced from days 0 to 28 (median, 1; P < 0.0004). Two patients (patients 131 and 342) maintained constant levels of expression between days 0 and 28. Figure 3 shows time courses for the staining for cellularity and IFN-γ, IL-12, and iNOS staining in typical BT and BL patients. Cellularity and cytokine expression are maintained from days 0 to 7 and decline only by day 28. Of the patients who had continuing high levels of cellularity and cytokine expression, patients 131 and 342 stand out as having continued IFN-γ and IL-12 expression at day 28 and patient 303 showed even an increase in IFN-γ at 6 months.
FIG. 3.
Time course for cellular infiltration and IFN-γ, Il-12, and iNOS expression in two representative BT (patient 303) and BL (patient 260) patients.
This study showed a decline in cellularity and intralesional cytokine production on steroid treatment. In all the patients there was a strong Th1 response with a marked cellular infiltrate with high levels of IFN-γ, IL-12, and iNOS in reactional lesions. It was surprising that the cellular infiltration and cytokine expression were maintained at pretreatment levels for at least 7 days. We had expected a decrease in cytokine expression by day 7, as by then many patients show marked clinical improvement. Our data suggest that, even after steroid treatment has started, there is continued recruitment of cells and cytokine production within lesions. Maybe steroid therapy acts in two ways, by a rapid reduction of edema which manifests as clinical improvement and then by a slower switching off of the Th1 response. Asthma biopsies of the bronchial tree after 2 weeks of prednisolone treatment showed a significant decrease in IFN mRNA cells but no change in CD4 or CD8 cells (2). These results are similar to our findings. In asthma patients, corticosteroid therapy results in decreased IL-12 and IFN-γ production by T cells and monocytes (3). Unreleased cytokine protein may also be stored in cells for 7 days or more after the start of treatment. Furthermore, our scoring system may not be sensitive enough to detect subtle changes in cell numbers or protein expression. Although the sections were evaluated by two observers, it may be difficult to detect small changes in the percentages of positively stained cells.
These data show that BT and BL patients have similar pathologies when in T1R, with initial high levels of cellular infiltrate and cytokine and iNOS expression and similar CD4/CD8 ratios. As a group, our patients showed a consistent response to treatment, with a decrease in all parameters by day 28, regardless of classification. It is not surprising that some patients failed to follow the predicted trend, given the range of individual clinical and immune presentations in leprosy. The individual heterogeneity in immune responses despite the BT or BL classification emphasizes the importance of studying individual patients. A subgroup of leprosy patients have recurrent reactions; in one Ethiopian cohort, 27% of the patients who had reactions had more than one episode (12). Our observation of continued immune activity in reactional skin lesions at 6 months may explain this phenomenon, and persistent cytokine production may cause their chronic pathology.
Acknowledgments
This project and D.L. were funded by a grant from Glaxo Wellcome through LEPRA (Colchester, United Kingdom), and S.K.-Y. and A.C. are supported by LEPRA. The DLRC is supported by MRC (London, United Kingdom) through LEPRA (Hyderabad, India).
We thank the patients and staff at the DLRC, in particular, Raj Gopal Reddy, Muzaffir, Mohammed Ismail, and Jusuf, for recruiting patients, documenting clinical progress, and taking and maintaining skin biopsies.
REFERENCES
- 1.Arya S K, Wong-Staal F, Gallo R C. Dexamethasone-mediated inhibition of human T cell growth factor and γ-interferon messenger RNA. J Immunol. 1984;133:273–276. [PubMed] [Google Scholar]
- 2.Bentley A M, Hamid Q, Robinson D S, Schotman E, Meng Q, Assoufi B, Kay A B, Durham S R. Reduction in the numbers if eosinophils, T cells, tryptase-only positive mast cells, and modulation of IL-4, IL-5, and interferon-gamma cytokine gene expression within the bronchial mucosa. Am J Respir Crit Care Med. 1996;153:551–556. doi: 10.1164/ajrccm.153.2.8564096. [DOI] [PubMed] [Google Scholar]
- 3.Blotta M H, DeKruyff R H, Umetsu D T. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J Immunol. 1997;158:5589–5595. [PubMed] [Google Scholar]
- 4.Cooper C L, Mueller C, Sinchaisri T A, Pirmez C, Chan J, Kaplan G, Young S M M, Weissman I L, Bloom B R, Rea T H, Modlin R L. Analysis of delayed type hypersensitivity reactions in leprosy by in situ hybridization. J Exp Med. 1989;169:1565–1581. doi: 10.1084/jem.169.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeKruyff R H, Fang Y, Umetsu D T. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol. 1998;160:2231–2237. [PubMed] [Google Scholar]
- 6.Han J, Thompson P, Beutler B. Dexamethasone and pentoxyfylline inhibit endotoxin-induced cachectin/tumour necrosis factor synthesis at separate points in the signaling pathway. J Exp Med. 1990;172:391–394. doi: 10.1084/jem.172.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanolkar-Young S, Rayment N, Brickell P M, Katz D R, Vinayakumar S, Colston M J, Lockwood D N J. Tumour necrosis factor alpha (TNF-α) synthesis is associated with the skin and peripheral nerve pathology of leprosy reversal reactions. Clin Exp Immunol. 1995;99:196–202. doi: 10.1111/j.1365-2249.1995.tb05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khanolkar-Young S K, Snowdon D, Lockwood D N J. Immunocytochemical localization of inducible nitric oxide synthase and transforming growth factor-beta (TGF-β) in leprosy lesions. Clin Exp Immunol. 1998;113:438–442. doi: 10.1046/j.1365-2249.1998.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lockwood D N J, Vinayakumar S, Stanley J N A, McAdam K P W J, Colston M J. Clinical features and outcome of reversal (type 1) reactions in Hyderabad. Int J Lepr Other Mycobact Dis. 1993;61:8–15. [PubMed] [Google Scholar]
- 10.Narayanan R B, Laal S, Sharma A K, Bhutani L K, Nath I. Differences in predominant T cell phenotypes and distribution pattern in reactional lesions of tuberculoid and lepromatous leprosy. Clin Exp Immunol. 1995;55:623–628. [PMC free article] [PubMed] [Google Scholar]
- 11.Paliogianni F, Raptis A, Ahuja S S, Najjar S M, Boumpas D T. Negative transcriptional regulation of human interleukin 2 (IL-2) gene by glucocorticoids through interference with nuclear transcription factors AP-1 and NF-AT. J Clin Investig. 1993;91:1481–1489. doi: 10.1172/JCI116353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunderson P, Gebre S, Desta K, Byass P, Lockwood D N J. The pattern of neuritis in the AMFES patients: definitions, incidence, risk factors and outcome. Lepr Rev. 2000;71:285–308. doi: 10.5935/0305-7518.20000033. [DOI] [PubMed] [Google Scholar]
- 13.Scheinman R I, Cogswell P C, Lofquist A K, Baldwin A S. Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 14.Sreenivasan P, Misra R S, Wilfred D, Nath I. Lepromatous leprosy patients show T helper 1-like cytokine profile with differential expression of interleukin-10 during type 1 and 2 reactions. Immunology. 1998;95:529–536. doi: 10.1046/j.1365-2567.1998.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verhagen C E, Wierenga E A, Buffing A A M, Chand M A, Faber W R, Das P K. Reversal reaction in borderline leprosy is associated with a polarized shift to type 1-like Mycobacterium leprae T cell reactivity in lesional skin. J Immunol. 1997;159:4474–4483. [PubMed] [Google Scholar]
- 16.Yamamura M, Wang X H, Ohmen J D, Uyemura K, Rea T H, Bloom B R, Modlin R L. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992;149:1470–1475. [PubMed] [Google Scholar]
- 17.Yamamura M, Uyemura K, Deans R J, Weinberg K, Rea T H, Bloom B R, Modlin R L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]