Take-home points
• According to the 2022 Korean Liver Cancer Association–National Cancer Center Korea practice guidelines for hepatocellular carcinoma (HCC), nodules that do not meet the noninvasive diagnostic criteria for “definite” HCC can be categorized as either “probable” HCC or “indeterminate” nodules on the basis of the ancillary findings of the first-line imaging study.
• Diagnostic examinations recommended for “probable” HCC and “indeterminate” nodules have been elaborated.
• Contrast-enhanced ultrasound with a Kupffer cell-specific contrast agent has been adopted as a second-line imaging study for HCC diagnosis.
• The imaging criteria for “probable” HCC have been modified to differ in their application depending on whether arterial phase hyperenhancement is present.
An updated version (v2022) of the Korean Liver Cancer Association (KLCA)-National Cancer Center (NCC) Korea practice guidelines for hepatocellular carcinoma (HCC) management has recently been released [1]. Several important changes have been made since the previous version (v2018) [2] pertaining to the imaging diagnosis of HCC, after considering published research and practical issues.
In addition, the KLCA-NCC v2022 guidelines define imaging features using the standardized Liver Imaging Reporting and Data System (LI-RADS) lexicon (https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS), which was last updated in 2021. However, the definition of “washout” on gadoxetic acid-enhanced magnetic resonance imaging (MRI) in the KLCA-NCC v2022 guidelines (that is, it can be assessed in the portal venous, transitional, or hepatobiliary phase) differs from that in the LI-RADS lexicon (that is, it should be assessed only in the portal venous phase); moreover, it is the same as that in the KLCA-NCC v2018 guidelines. Furthermore, the LI-RADS lexicon is only available for contrast-enhanced ultrasound (CEUS) with a blood-pool contrast agent; it is not available for CEUS with a Kupffer cell-specific contrast agent.
Diagnostic Algorithm
The KLCA-NCC guidelines v2022 allow for HCC diagnosis using both first- and second-line imaging studies. First-line imaging studies include multiphasic computed tomography (CT) and multiphasic MRI (with an extracellular contrast agent or a hepatocyte-specific contrast agent). Conversely, second-line imaging studies include multiphasic CT and MRI and CEUS (with a blood-pool or Kupffer cell-specific contrast agent).
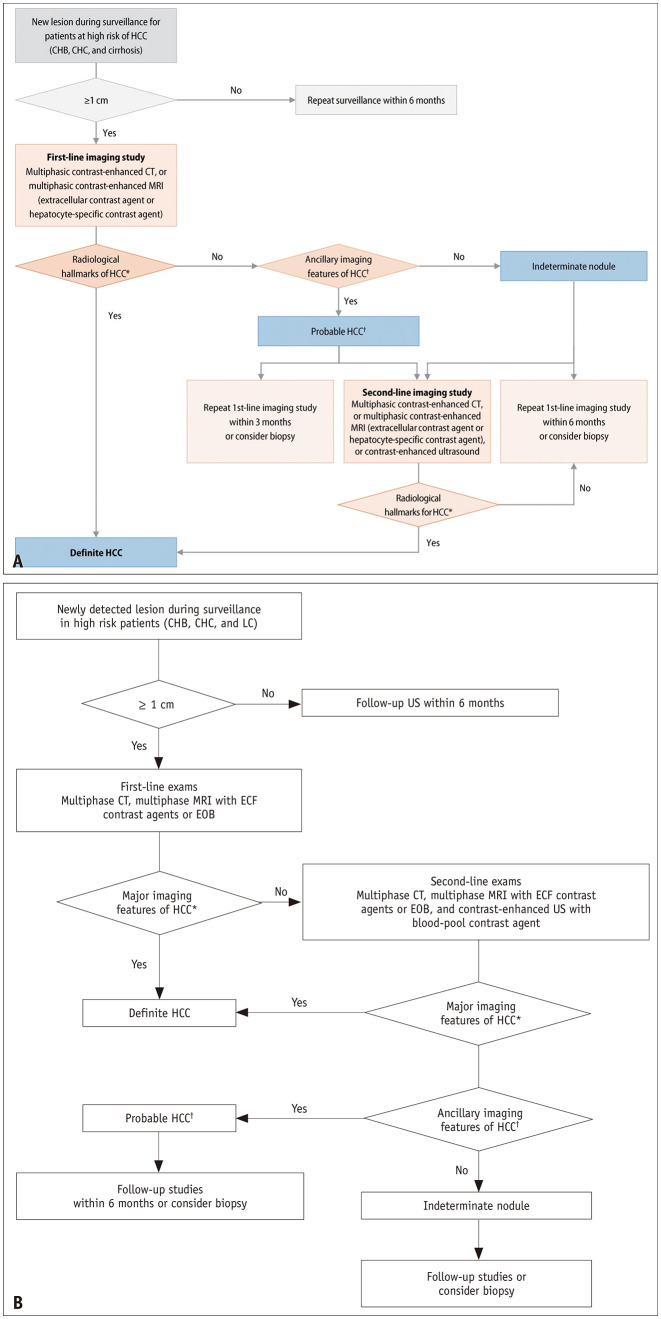
In the diagnostic algorithm of the KLCA-NCC v2018 guidelines, the only recommendation in cases where “definite” HCC cannot be diagnosed on the basis of the first-line imaging findings is a second-line imaging study [2]. However, in real-world practice, it may be difficult to conduct an additional study with an alternative imaging modality owing to constraints related to patient characteristics or the medical environment. Moreover, core-needle biopsy is sometimes performed instead of an additional imaging study to confirm HCC. To provide a more practical guide for nodules that are not definitively diagnosed on the basis of the first-line imaging findings, the algorithm has been changed in the KLCA-NCC v2022 guidelines to include multiple options such as a second-line imaging study, follow-up imaging using the same modality as that of the first-line study, and biopsy.
If an imaging diagnosis of “definite” HCC cannot be established, the nodule can be categorized as “probable” HCC or “indeterminate” nodule on the basis of the ancillary imaging features of HCC. We have described the imaging diagnoses of “probable” HCC and “indeterminate” nodule in detail subsequently. Unlike the KLCA-NCC v2018 guidelines, which recommend classification into “probable” HCC and “indeterminate” nodule based on second-line imaging findings, the KLCA-NCC v2022 guidelines recommend categorization according to the ancillary imaging features in the first-line study. This change was driven by the idea that the classification of lesions according to the relative probability of HCC or relative risk of future progression would help determine the appropriate next step after the first-line imaging study. In the KLCA-NCC v2022 guidelines, a follow-up imaging study using one of the first-line imaging modalities is an option after the first-line study, and follow-up imaging is recommended within 3 months for “probable” HCC and within 6 months for “indeterminate” nodule. The two versions (v2022 and v2018) of the diagnostic algorithm are compared in Figure 1.
Fig. 1. Diagnostic algorithms of the 2022 version (A) and 2018 version (B) of the KLCA-NCC guidelines.
Adapted from KLCA and NCC. Korean J Radiol 2022;23:1126-1240 [1] and Korean J Radiol 2019;20:1042-1113 [2]. KLCA = Korean Liver Cancer Association, NCC = National Cancer Center
CEUS with a Kupffer Cell-Specific Contrast Agent
The updated KLCA-NCC guidelines (v2022) are the first major guidelines to adopt CEUS with a blood-pool contrast agent (e.g., Sonovue®/Lumason® and Luminity®/Definity®) and Kupffer cell-specific contrast agent (e.g., Sonazoid®) as diagnostic modalities for HCC. The previous version (v2018) of the KLCA-NCC guidelines only included CEUS with a blood-pool contrast agent as a secondary imaging tool; this was also the case in the 2018 version of the European Association for the Study of the Liver guidelines [3]. CEUS with a Kupffer cell-specific contrast agent has been adopted in the 2017 version of the Asian Pacific Association for the Study of the Liver guidelines, although CEUS with a blood-pool contrast agent has not been adopted [4]. The decision to add CEUS with a Kupffer cell-specific contrast agent to the diagnostic imaging modalities of KLCA-NCC v2022 is based on recent studies demonstrating that its diagnostic performance is good and comparable with that of CEUS with a blood-pool contrast agent and multiphasic CT and MRI [5,6,7]. However, as in the KLCA-NCC v2018 guidelines, CEUS is only recommended as a second-line imaging study because of its limitations in determining tumor extent and staging.
In the KLCA-NCC v2022 guidelines, the diagnostic criteria for “definite” HCC, based on the findings of CEUS with a Kupffer cell-specific contrast agent, are arterial phase hyperenhancement (APHE) with late (≥ 60 seconds) and mild washout or washout appearance in the Kupffer phase. They differ from the criteria for CEUS with a blood-pool contrast agent in that the washout appearance in the Kupffer phase is included. Because the Kupffer phase is a post-vascular phase and “washout” reflects hemodynamic properties, “washout in the Kupffer phase” may be a misnomer. However, the KLCA-NCC v2022 guidelines include extended criteria for washout and use “washout in the Kupffer phase” to indicate that the Kupffer phase defect (relative hypo-enhancement compared with the liver parenchyma) can be considered as an alternative for washout. Recent studies have shown that the extended criteria for washout that include the Kupffer phase show better sensitivity for HCC diagnosis without significant loss of specificity than do those that do not include the Kupffer phase [8,9]. These results are similar to those obtained for extended criteria for washout that include the hepatobiliary phase of MRI with a hepatocyte-specific contrast agent [10,11]. To exclude non-HCC malignancies or hemangiomas, the diagnostic criteria for CEUS with either a blood-pool or Kupffer cell-specific contrast agent should not be applied to lesions with rim or peripheral globular enhancement in the arterial phase, early washout within 60 seconds, or punched-out pattern of washout within 120 seconds.
Imaging Diagnosis of “Probable” HCC
The imaging criteria for “probable” HCC were modified in KLCA-NCC v2022 guidelines to make their application dependent on the presence of APHE (Fig. 2). Specifically, in nodules ≥ 1 cm that do not meet the noninvasive diagnostic criteria for “definite” HCC, a diagnosis of “probable” HCC can be established on the basis of the ancillary imaging features. Nodules without APHE can be diagnosed as “probable” HCC only when the lesion fulfills at least one item from each of the following: 1) ancillary imaging features favoring malignancy in general (mild-to-moderate T2 hyperintensity, restricted diffusion, and threshold growth) and 2) ancillary imaging features favoring HCC in particular (enhancing or non-enhancing capsule, mosaic architecture, nodule-in-nodule appearance, and fat or blood products in the mass). However, nodules with APHE but without washout can be diagnosed as “probable” HCC when at least one of the ancillary imaging features in either of the aforementioned two lists is present.
Fig. 2. Diagnostic algorithm of the 2022 version of the KLCA-NCC guidelines for classifying lesions as “probable” HCC or “indeterminate” nodule on the basis of multiphasic contrast-enhanced CT or MRI.
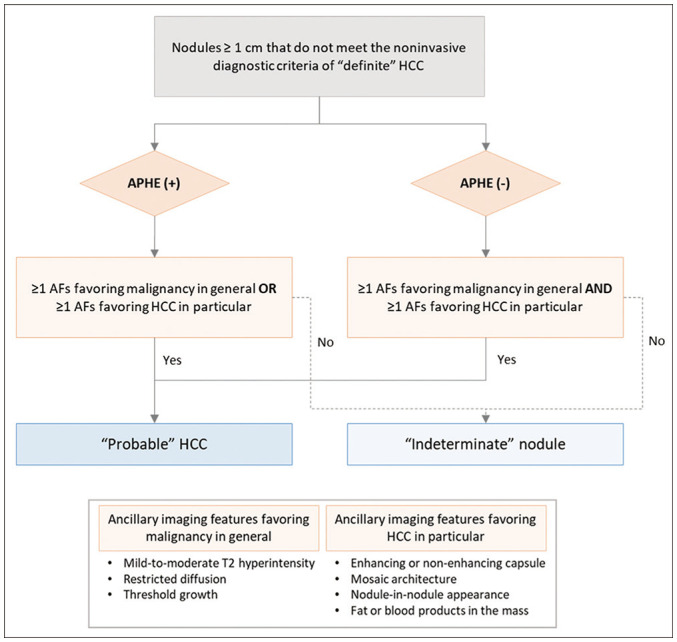
AF = ancillary feature, APHE = arterial phase hyperenhancement, CT = computed tomography, KLCA = Korean Liver Cancer Association, MRI = magnetic resonance imaging, NCC = National Cancer Center
According to the KLCA-NCC v2018 guidelines, nodules can be diagnosed as “probable” HCC when at least one item from each of the two lists of ancillary imaging features is present, regardless of the presence of APHE. However, in the updated guidelines (v2022), “probable” HCC can be diagnosed more easily in nodules with APHE than in those without APHE. This change was based on studies demonstrating that lesions with APHE had a higher probability of HCC and chance of progression to HCC than did those without APHE [12,13,14]. As discussed in the “Diagnostic algorithm” section, classifying lesions as “probable” HCC or “indeterminate” nodule is important because it affects the selection of the next diagnostic step. The diagnostic ability of these new criteria for “probable” HCC should be evaluated in future studies.
Footnotes
Conflicts of Interest: Ijin Joo and Jeong Min Lee who is on the editorial board of the Korean Journal of Radiology was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement: None
Availability of Data and Material
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
References
- 1.Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2022;23:1126–1240. doi: 10.3348/kjr.2022.0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2019;20:1042–1113. doi: 10.3348/kjr.2019.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Liu C, Yan J, Liu K. Perfluorobutane contrast-enhanced ultrasonography for the diagnosis of HCC: a systematic review and meta-analysis. Abdom Radiol (NY) 2021;46:4619–4628. doi: 10.1007/s00261-021-03141-5. [DOI] [PubMed] [Google Scholar]
- 6.Kang HJ, Lee JM, Yoon JH, Lee K, Kim H, Han JK. Contrast-enhanced US with sulfur hexafluoride and perfluorobutane for the diagnosis of hepatocellular carcinoma in individuals with high risk. Radiology. 2020;297:108–116. doi: 10.1148/radiol.2020200115. [DOI] [PubMed] [Google Scholar]
- 7.Hsiao CY, Chen PD, Huang KW. A prospective assessment of the diagnostic value of contrast-enhanced ultrasound, dynamic computed tomography and magnetic resonance imaging for patients with small liver tumors. J Clin Med. 2019;8:1353. doi: 10.3390/jcm8091353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang HJ, Kim JH, Yoo J, Han JK. Diagnostic criteria of perfluorobutane-enhanced ultrasonography for diagnosing hepatocellular carcinoma in high-risk individuals: how is late washout determined? Ultrasonography. 2022;41:530–542. doi: 10.14366/usg.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Zheng W, Wang J, Han J, Guo Z, Hu Y, et al. Contrast-enhanced ultrasound using perfluorobutane: impact of proposed modified LI-RADS criteria on hepatocellular carcinoma detection. AJR Am J Roentgenol. 2022;219:434–443. doi: 10.2214/AJR.22.27521. [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Choi SH, Kim SY, Kim MJ, Lee SS, Byun JH. Gadoxetic acid–enhanced MRI of hepatocellular carcinoma: value of washout in transitional and hepatobiliary phases. Radiology. 2019;291:651–657. doi: 10.1148/radiol.2019182587. [DOI] [PubMed] [Google Scholar]
- 11.Joo I, Lee JM, Lee DH, Jeon JH, Han JK. Retrospective validation of a new diagnostic criterion for hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout with the aid of ancillary features? Eur Radiol. 2019;29:1724–1732. doi: 10.1007/s00330-018-5727-1. [DOI] [PubMed] [Google Scholar]
- 12.Tang A, Bashir MR, Corwin MT, Cruite I, Dietrich CF, Do RKG, et al. Evidence supporting LI-RADS major features for CT- and MR imaging-based diagnosis of hepatocellular carcinoma: a systematic review. Radiology. 2018;286:29–48. doi: 10.1148/radiol.2017170554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim TK, Lee KH, Jang HJ, Haider MA, Jacks LM, Menezes RJ, et al. Analysis of gadobenate dimeglumine-enhanced MR findings for characterizing small (1-2-cm) hepatic nodules in patients at high risk for hepatocellular carcinoma. Radiology. 2011;259:730–738. doi: 10.1148/radiol.11101549. [DOI] [PubMed] [Google Scholar]
- 14.Sofue K, Burke LMB, Nilmini V, Alagiyawanna M, Muir AJ, Choudhury KR, et al. Liver imaging reporting and data system category 4 observations in MRI: risk factors predicting upgrade to category 5. J Magn Reson Imaging. 2017;46:783–792. doi: 10.1002/jmri.25627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.