Abstract
Traditional Chinese medicine (TCM) has been frequently used as skin lightning agents. However, the mechanism of action of their effect is unclear. The present study aims to evaluate anti-tyrosinase activity of 10 commonly used TCM on mushroom (ab), human (hs) and mouse melanoma B16F0 (mm) tyrosinase (TYR) respectively. The results showed that at 1.0 mg/mL, extracts from Rosa rugosa Thumb, Morus alba L. and Paeonia lactiflora Pall were active against both abTYR and hsTYR (>50% inhibition), extracts from Bletilla striata (Thunb.) Rchb. F., Centella asiatica (L.) Urb, Cynanchum atratum L., Rosa canina L., Rhus chinensis Mill. and Glycyrrhiza urolensis Fisch. Ex DC. inhibited either abTYR or hsTYR (>50%), while extract from Tribulus terrestris L. had no/minimal activity (<10% inhibition). When treated with melanoma B16F0 cells, M. alba also significantly reduced mmTYR activity (70% at 250 μg/mL) and melanin content (50% at 250 μg/mL). These findings demonstrated inhibitory effects of 9 TCM against TYR and hence support their application as skin lightning agents. Our results also showed discrepancies in TYR activity from different sources, suggesting a testing regime of combining abTYR, hsTYR and mmTYR when developing depigmentation agents for human application.
Keywords: Traditional Chinese medicine (TCM), Tyrosinase, Tyrosinase inhibitor, Skin lightning
Traditional Chinese medicine (TCM); Tyrosinase, Tyrosinase inhibitor, Skin lightning.
1. Introduction
Melanin, the main pigment in human skin, plays a crucial role in preventing skin damage caused by UV light and reactive oxygen species (ROS) (Gragnani et al., 2014). Excessive production or uneven distribution of melanin can cause various dermatological disorders, such as hyperpigmentation, lentigines, age spot, freckles, melasma, post inflammatory melanoderma as well as DNA damage, gene mutation, photoaging, and immune system impairment (Meredith and Sarna, 2006; Ortonne and Bisset, 2008; Nicolaidou and Katsambas, 2014). Tyrosinase (TYR) is a copper-containing metalloprotein and controls the rate of the first two steps in melanin biosynthesis, a process also known as melanogenesis. TYR catalyses the hydroxylation of tyrosine to 3,4-dihydroxyl-L-phenylalanine (L-DOPA) and the subsequent oxidation of L-DOPA to dopaquinone (Del Marmiol and Beermann, 1996; Ortonne and Bisset, 2008; D’mello et al., 2016), leading to the synthesis of eumelanin and pheomelanin. Mammalian TYR is found in highly specialized cells known as melanocytes, and it is responsible for the pigmentation of skin, eye, and hair (Hearing and Jimenez, 1989; Jimenez-Cervantes et al., 1993; Choi et al., 2018). Inhibition of TYR reduces or breaks down the production of melanin, posing as the most prominent and validated target for skin depigmentation (Chang, 2012). Several agents, such as hydroquinone, arbutin, azelaic acid, kojic acid and ascorbic acid, are commonly used in skin depigmentation formulations; however, the compounds have limitations in terms of poor-skin penetration, low stability, high toxicity, and insufficient activity (Callendar et al., 2011; Choi et al., 2018).
For several decades, mushroom (Agaricus bisporus) tyrosinase (abTYR) has been a gold standard in developing tyrosinase inhibitors. It is commercially available, economic and rich in natural source. However, recent research on human tyrosinase (hsTYR) revealed differences in the binding sites between abTYR and hsTYR (Xu et al., 1997; Dolinska et al., 2019), and many molecules identified through abTYR gave unsatisfactory activity against hsTYR (McEvilly et al., 1992). Therefore, there has been a paradigm shift in searching for effective TYR inhibitors, with both abTYR and hsTYR recommended in the testing regime, in order to identify true TYR inhibitors for human applications (Kim and Lee, 1998). In addition, it has been recommended that TYR inhibitors should also be tested in a cellular system, such as the pigmented mouse (mmTYR) or human melanoma cells or primary human melanocytes for their potential in vivo anti-melanogenic effects (Lee et al., 2010; Chan et al., 2011).
Traditional Chinese Medicine (TCM) has been documented and practically used for its skin lightning properties for thousands of years (Duraisamy et al., 2011). It is regarded as a safer alternative due to its low cost, less side effects and abundance of resources (Lee et al., 1997). A comprehensive literature review on Chinese Pharmacopoeia, compendium of Materia Medica and German Plant Drug Analysis revealed more than 120 TCM as active ingredients in cosmetic products. Further research on published scientific papers (Duraisamy et al., 2011; Chang 2012; Hsieh et al., 2015) revealed that the following 10 TCM are commonly used as single herb or in formulations, including Morus alba L., Glycyrrhiza uralensis Fisch. Ex DC., Rosa rugosa Thumb, Rosa canina L., Rhus chinensis Mill, Paeonia lactiflora Pall, Tribulus terrestris L., Cynanchum atratum L., Bletilla striata (Thunb.) Rchb. F., and Centella asiatica (L.) Urb. Studies have shown that several TCM extracts exhibited promising activity against abTYR, while some have also displayed inhibitory activity in mouse melanoma cell assays (Xue and Li, 1999). However, there have been no studies available investigating the activities of the TCM against hsTYR (Xue and Li, 1999).
With our ongoing interest in skin lightning TCM, enzymatic assays against ab- and hsTYR and cell assay using mouse melanoma B16F0 cells were established. The assays were used to evaluate the activities of the 10 commonly used depigmentation TCM. The antioxidant activities of the TCM extracts were also measured as previous research showed a direct link between oxidation and TYR activity (Lee and Kim, 1995; Bernard and Berthon, 2000; Wong et al., 2006). Considering the traditional water decoction and wine tincture herbal preparation methods, extractions using different ratios of water and ethanol were carried out. Extraction efficiency and the efficacy of the extracts against TYR were investigated.
2. Materials and methods
2.1. General experimental procedure
Ethanol, tyrosine (99.4%), abTYR (8503 units/mg), dimethyl sulfoxide (DMSO), potassium dihydrogen phosphate, sodium phosphate dibasic, L-DOPA, kojic acid, and arbutin were purchased from Sigma Aldrich, a subsidiary company of Merck Australia. hsTYR (Bioresearch, >85%, Mwt 52Kda) was purchase from Bioresearch USA. Tyrosine, abTYR and hsTYR solutions were prepared freshly 30 mins before each use in phosphate buffer solution, yielding a concentration of 0.625 mM, 217 units/mL and 20 mg/mL, respectively. 0.1 M sodium phosphate buffer solution (pH = 6.8) and 50 mM potassium phosphate (pH = 7.0) were used for the preparation of samples. A Branson B2500S-DTH sonicator was used as an aid to improve the extraction potency. Shen Sheng rotary evaporator and Fevik freeze dryer were used for the evaporation of solvent. A Schimadzu HPLC LC-20AD equipped with DGU-20A5R degassing unit, SIL-20AC autosampler, SPD-M40 PDA detector, CTO-20AC column oven, CBM-20A communication bus and FRC-10A fraction collection unit, together with LB Solution DB version was used for HPLC chemical analysis. A Bio-Strategy SpectraMax ABS Plus multi-plate reader was used for the measurement of the UV absorption of the samples and controls for TYR assay.
2.2. TCM materials
M. alba, G. uralensis, R. rugosa, R. canina, R. chinensis, P. lactiflora, T. terrestris, C. atratum, B. striata, and C. asiatica were sourced from Ferngrove Pharmaceutical and authenticated by Australian Therapeutic Goods Administration (TGA) licenced analytical research laboratory in Southern Cross University (TGA licence number:MI-01122004-LI-000264-1). Authentication reference numbers are ARL2205667 for M. alba, ARL2205668 for G. uralensis, ARL2205669 for R. rugosa, ARL2205670 for R. canina, ARL2205671 for R. chinensis, ARL2205662 for P. lactiflora, ARL2205663 for T. terrestris, ARL2205664 for C. atratum, ARL2205665 for B. striata, and ARL2205666 for C. asiatica.
2.3. Extraction of TCM material
TCM herbal material was first washed with water, then air dried and ground into fine powder. Dried plant powder (600 mg) was macerated in respective extraction solvent (15 mL), namely 95% ethanol, 70% ethanol, 30% ethanol or H2O, at room temperature overnight, followed by sonication in each solvent (15 mL) 4 times (10 min each). The supernatant from each extraction was combined and dried by a rotary evaporator under vacuum and freeze-dried to obtain crude extract.
2.4. HPLC analysis
TCM crude extract (5 mg) was dissolved in MeOH (1 mL) and the solution (200 μL) was injected into a Schimadzu HPLC system with a Diode Array detector for chemical analysis. Waters Xtterra C18 reverse phase column (250 × 4.6mm, 5μm) was used with gradient elution of 0.1% formic acid water solution and methanol. The gradient elution was from 0% methanol to 100% methanol in 70 min at the flow rate of 1 mL/min.
2.5. abTYR assay
abTYR assay was set up based on a reported method with modifications (Baurin et al., 2002). The extract (10 mg) was dissolved in DMSO (10–50 μL depending on solubility) and the solution was diluted by sodium phosphate buffer solution (0.1 M, pH 6.8) to yield a concentration of 1.0 mg/mL. In a 96-well plate, the extract (1.0 mg/mL, 100 μL), abTYR (217 U/mL in sodium phosphate buffer, 20μL) and freshly prepared L-tyrosine (0.625mM, 80μL) in sodium phosphate buffer solution were added and the assay mixture was incubated at 37 °C for 30 min. After the incubation, the absorbance was read at 475 nm to determine the formation of L-DOPA using a Bio-Strategy SpectraMax ABS Plus multi-plate reader (Fu et al., 2003; Chen et al., 2008). A blank, two negative controls and two positive controls were prepared. Kojic acid and arbutin (1.0 mg/mL) were used in place of the extract as positive controls. L-tyrosine, abTYR and TCM extracts were the test reactions (A1). L-tyrosine and the extract without abTYR were used as negative control 1 (A2). L-tyrosine and abTYR without the extract were used as negative control 2 (A3). L-tyrosine and sodium phosphate buffer were used as a blank (A4). The inhibition percentage of the extracts was calculated using the following equation:
| Inhibition% = [1-(A1-A2/A3-A4)] x 100% |
2.6. hsTYR assay
The hsTYR assay was based on a reported method with (Pomeranz, 1963). The assay was conducted similarly to that of abTYR except that potassium phosphate buffer (50 mM, pH 7.0) was used to dilute the extract. The reaction mixture containing the extract (1.0 mg/mL, 1.0 mL) and hsTYR (50 mM in potassium phosphate buffer, 0.5 mL) was incubated at 37 °C for 5 min before L-DOPA (3 mM, 0.5 mL) in potassium phosphate buffer solution was added. The assay mixture was then incubated at 37 °C for 10 min before the absorbance was read at 484 nm (Pomeranz, 1963) to determine the formation of dopachrome. The blank, negative controls, positive controls and treated group were the same as those for abTYR. The inhibition percentage of the extracts was calculated using the following equation:
| Inhibition% = [1-(A1-A2/A3-A4)] x 100% |
2.7. Total antioxidant capacity assay
A total antioxidant capacity (TAC) assay kit purchased from Sigma (MA334, Sigma) was used to measure the total antioxidant activity of the extracts (Curto et al., 1999). The antioxidant activity testing method was adopted from Sigma Aldrich product instructions. The antioxidant kit measures total antioxidant capacity in which Cu2+ is reduced by an antioxidant to Cu+. The resulting Cu+ specifically forms a coloured complex with a dye reagent, the colour intensity at 570 nm is proportional to TAC in the sample (Rudeekulthamrong and Kaulpiboon, 2020; Nerya et al., 2003; Zheng and Wang, 2001). Trolox standard stock solution (1 mM) was used to prepare 0, 300, 600, 800, 1000 μM of standard solutions. Then 20 μL of each standard solution was transferred into a 96-well plate in triplicate to obtain a standard curve. To test the extracts, 20 μL of each standard solution and 20 μL extract MeOH solution (1.0 mg/mL) were transferred into a 96 well plate. Later, 100 μL of Cu2+ working solution was added to each well. The plate was mixed on a shaker and incubated at room temperature for 10 min. After the incubation, the absorbance was measured at 570nm on a SpectraMax ABS Plus multi-plate reader (Bio-Strategy USA). Standard curve was plotted using Trolox at concentrations ranging from zero to 1000 μM. The total antioxidant capacity is calculated using the formula:
| TAC(μM) = [(A570)sample - (A570)blank]/Slope(μM−1) x n |
where:
| (A570) sample = the absorbance of the sample |
| (A570) blank = the absorbance of the medium blank (water) |
n is the sample dilution factor for each preparation.
2.8. Cell culture
Mouse melanoma cell line B16F0 (ATCC catalogue number CRL-6322™) was cultured in RPMI 1640 media, containing 10% (v/v) foetal bovine serum (FBS) (Bovogen Biologicals, Melbourne, Australia), 200 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (Life Technologies, CA, USA). Cells were incubated in a T-75 flask at 37 °C, in a 5% CO2 incubator and were split every two days. The normal human dermal fibroblast (NHDF) cell line (PromoCell, Heidelberg, Germany) was cultured in fibroblast basal media 2 (PromoCell, Heidelberg, Germany), containing 2% (v/v) FBS, 1 ng/mL basic fibroblast growth factor and 5 μg/mL insulin (PromoCell, Heidelberg, Germany). Cells were incubated in a T-75 flask at 37 °C, in a 5% CO2 incubator, and split every two days.
2.9. Resazurin-based cytotoxicity assay
Cells were seeded at 3,300 (B16F0) or 10,000 (NHDF) cells per well in a 96-well plate and incubated at 37 °C/5% CO2 overnight. The following day, cells were treated with specific concentrations of the extract to make a total volume of 100 μL in triplicate. Growth media without cells was used as vehicle control (blank), and cells treated with 10% DMSO were used as a positive control. Then, the cells were incubated at 37 °C for 3 days. After 3 days, 5 μL of CellTiter-Blue® (Resazurin) Reagent (Promega) was added to each well, and the plate was mixed for 10 s using the plate reader. Cells were then incubated for 3 h at 37 °C/5% CO2. The total well fluorescence was measured at 560Ex/590Em using FLUOstar Omega multi-mode plate reader (BMG Labtech, Ortenberg, Germany).
2.10. mmTYR assay
TYR activity was measured as described previously with some modifications (Parvez et al., 2007). B16F0 cells (100,000) were seeded in a 6-well plate and incubated at 37 °C/5% CO2 overnight. On the following day, cells were treated with the extracts for 3 days in the presence of 0.1 μM α-MSH (Sigma-Aldrich, Sydney, Australia). After 3 days, cells were collected, washed, and resuspended with 100 μL of 1% Triton X-100 ice-cold lysis buffer (in 50mM sodium phosphate buffer, pH 6.8) followed by sonication at 20 kHz for 30 s. The cell lysates were then centrifuged at 7,500 × g for 30 min at 4 °C. Then, 20 μL of cell lysate for each sample was added to a 96-well plate in triplicate, followed by adding 150 μL of 15 mM L-DOPA (Sigma-Aldrich, Sydney, Australia) solution. 1% Triton X-100 buffer was used as a blank. The absorbance was measured at 490 nm at a 1 min interval for 60 min using the FLUOstar Omega multi-mode plate reader (BMG Labtech, Ortenberg, Germany). Units of TYR activity was calculated as the change in absorbance at 490 nm/min/mg total protein. The amount of protein for each cell sample was determined by Lowry protein estimation (Parvez et al., 2007).
2.11. Melanin assay
Melanin content was measured as described previously (Chung et al., 2019) with some modifications. B16F0 cells (100,000) were seeded in a 6-well plate and incubated at 37 °C/5% CO2 overnight. On the following day, cells were treated with the extracts for 3 days in the presence of 0.1 μM α-MSH (Sigma-Aldrich, Sydney, Australia). Then, cells were collected, washed, and resuspended in 150 μL of 1N NaOH, and the cell suspension incubated at 60 °C for 1 h. Melanin (Sigma-Aldrich, Sydney, Australia) standards were prepared in 1N NaOH at 80 μg/mL, 40 μg/mL, 20 μg/mL, 10 μg/mL 5 μg/mL and 2.5 μg/mL of melanin standard solutions using 1N NaOH. Then 100 μL of each melanin standard was transferred to a 96-well plate in triplicate to obtain a standard curve. The cell extracts were diluted 1/2 using 1N NaOH, and 100 μL of the diluted cell extracts were transferred to appropriate wells of a 96-well plate in duplicate. The 96-well plate was mixed in the SpectraMax M3 plate reader (Molecular Devices, California, United States) for 30 s, and the absorbance was measured at 405 nm. The melanin amount of each sample was calculated using the melanin standard curve. Lowry protein estimation was used to determine the amounts of total protein in each cell sample. Therefore, the μg of melanin per mg of total protein was calculated for each cell sample (Chung et al., 2019).
2.12. Lowry protein estimation
BSA was used as the standard and 10 mg/mL of BSA (Sigma-Aldrich, NSW, Australia) solution was used to prepare 2 mg/mL, 1 mg/mL, 0.5 mg/mL, 0.25 mg/mL and 0.125 mg/mL BSA solutions by serial dilution. Then 10 μL of each BSA solution was transferred to a 96-well plate in triplicate to obtain a standard curve. The cell extracts were diluted 1/20 and 10 μL of the diluted cell extracts were transferred to appropriate wells of a 96-well plate in triplicate. The DC protein assay kit (Bio-Rad) was used to determine the protein concentration. Firstly, 20 μL of reagent S was mixed with 1 mL of reagent A. Then 25 μL of this mixture was added to each well and incubated for 1 min at room temperature. Then, 200 μL of reagent B was added to each well. The 96-well plate was mixed in the SpectraMax M3 plate reader (Molecular Devices) for 5 min and then left in the dark for 15 min. The absorbance was measured at 750 nm. The protein concentration of each sample was calculated using the BSA standard curve (An et al., 2019).
GraphPad Prism 9 software was used to analyze the data of cell-based assays. All the experiments were performed at least three times (three biological repeats) and the results represent the mean value of all biological repeats with the standard error of the mean (mean ± SEM). One-way ANOVA was used followed by Dunnett's test. A p value less than 0.05 (p < 0.05) was considered significant.
3. Results
3.1. Skin lightning TCM
A comprehensive literature review on Chinese Pharmacopoeia, compendium of Materia Medica as well as German Plant Drug Analysis suggested that more than 120 TCM are used for skin lightning, anti-aging, anti-TYR, and anti-hyperpigmentation purposes. Among the 120 samples, 10 are commonly used in formulations, including M. alba, G. uralensis, R. rugosa, R. canina, R. chinensis, P. lactiflora, T. terrestris, C. atratum, B. striata, and C. asiatica. Detailed information of the 10 TCM material, including their names in Chinese character, common names, family, their medicinal properties and plant parts, are summarized in Table 1. These 10 herbal materials were chosen for the current study.
Table 1.
10 TCM commonly used as skin-lightning agents.
| No | Chinese name | Common name | Botanical name | Family | Reported property (Lee et al., 1997) | plant part |
|---|---|---|---|---|---|---|
| 1 | 桑叶 | Mulberry leaf | Morus alba L. | Moraceae | Skin lightning | Leaf, fruit |
| 2 | 甘草 | Liquorice | Glycyrrhiza uralensis Fisch. ex DC. | Leguminosae | Skin lightning, freckle removing | Root |
| 3 | 玫瑰花 | Rose flower | Rosa rugosa Thumb | Rosaceae | Skin lightning | Flower |
| 4 | 玫瑰果 | Dog rose | Rosa canina L. | Rosaceae | Skin lightning | Fruit |
| 5 | 五倍子 | Chinese gall | Rhus chinensis Mill. | Anacardiaceae | Skin lightning products | Fruit |
| 6 | 芍药 | White peony | Paeonia lactiflora Pall | Paeoniaceae | Skin lightning | Root |
| 7 | 蒺藜 | Tribulus | Tribulus terrestris L. | Zygophyllaceae | Anti-abTYR activity | Fruit |
| 8 | 白薇 | Swallowwort | Cynanchum atratum L. | Asclepiadaceae | Anti-aging, skin lightning | Root |
| 9 | 白芨 | Bletilla | Bletilla striata (Thunb.) Rchb.F. | Orchidaceae | Anti-ageing | Stem, leaf |
| 10 | 积雪草 | Centella | Centella asiatica (L.) Urb | Umbelliferae | Skin lightning products | Whole plant |
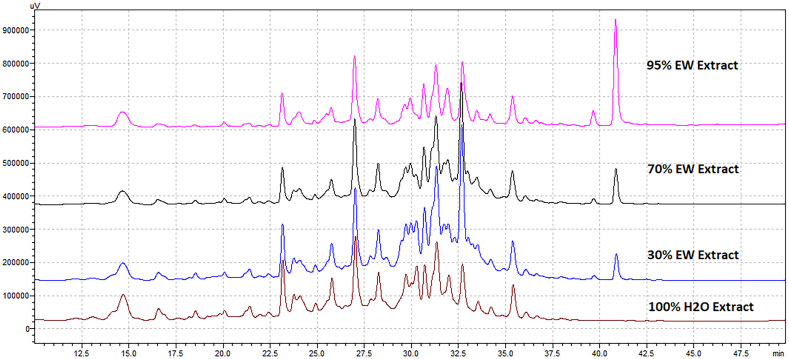
Traditionally, TCMs are prepared by water decoction in boiled water and wine tincture soaking in 70% alcohol. These extraction methods are widely used due to their simple application, low cost and proven medicinal efficacy (Kim et al., 2012). To mimic the traditional preparation methods, TCM plant materials were extracted with solvents comprising different ratios of ethanol and water, namely 95% ethanol, 70% ethanol, 30% ethanol and 0% ethanol water solutions. The extracts were then analysed by HPLC for their chemical compositions and tested against abTYR for their efficacy. Figure 1 displayed the HPLC chromatograms of the 4 extracts from R. rugosa. The results (Figure 1) showed that the extracts had similar chemical profiles judged by number of peaks in HPLC chromatograms. The activity evaluation of the 4 extracts suggested that 95% ethanol attained 99.5% inhibition against abTYR, higher than those of the 3 other extracts (between 80%-85% inhibition). The same analysis was carried out for M. alba, observing similar results. Therefore, 95% ethanol was chosen for TCM extraction.
Figure 1.
HPLC chromatograms of 4 different extracts from R. rugosa. Waters Xtterra C18 reverse phase column (250 × 4.6mm, 5μm) was used with gradient elution of 0.1% formic acid water solution and methanol. The gradient elution was from 0% methanol to 100% methanol in 70 min at the flow rate of 1 mL/min.
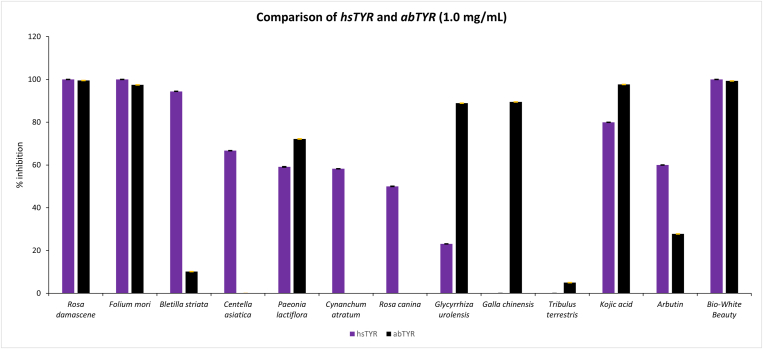
3.2. Inhibitory activity against abTYR and hsTYR
The 95% ethanolic extracts of 10 TCM were tested against abTYR and hsTYR activities at 1 mg/mL in triplicates using enzymatic assays. Dose response experiments were not performed due to limited (and hugely expensive) supply of hsTYR. The results (Table 2) showed some interesting trends. For hsTYR, extracts from R. rugosa, M. alba and B. striata exhibited >90% inhibition, extracts from C. asiatica, P. lactiflora, C. atratum and R. canina had moderate (between 45-67%) inhibitory activity, and the remaining three from G. uralensis, R. chinensis and T. terrestris presented minimal (∼20%) or no activity. For abTYR, extracts from R. rugosa, M. alba, G. uralensis and R. chinensis had >85% inhibition, P. lactiflora displayed moderate activity (72%), and the remaining 5 extracts showed minimal (<10%) or no inhibition. Overall, 7 out of 10 TCM exhibited relatively strong activity against hsTYR in comparison to only 5 against abTYR.
Table 2.
Anti-TYR and antioxidant activities of 10 TCM extracts at 1 mg/mL.
| TCM | hsTYR (% inhibition ±SEM) | abTYR (% inhibition ±SEM) | Total Antioxidant Capacity (μM/mg ± SEM) |
|---|---|---|---|
| Rosa rugosa Thumb | 100.00 ± 0.01 | 99.50 ± 0.01 | 3006.70 ± 0.02 |
| Morus alba L. | 100.00 ± 0.01 | 97.50 ± 0.02 | 232.40 ± 0.001 |
| Bletilla striata (Thunb.) Rchb.F. | 94.40 ± 0.06 | 10.20 ± 0.01 | 472.01 ± 0.04 |
| Centella asiatica (L.) Urb | 66.70 ± 0.01 | NA1 | 229.50 ± 0.04 |
| Paeonia lactiflora Pall | 59.10 ± 0.11 | 72.20 ± 0.09 | 1024.85 ± 0.01 |
| Cynanchum atratum L. | 58.30 ± 0.01 | NA1 | 427.41 ± 0.02 |
| Rosa canina L. | 50.00 ± 0.11 | NA1 | 224.10 ± 0.03 |
| Glycyrrhiza uralensis Fisch. ex DC. | 23.10 ± 0.01 | 88.90 ± 0.01 | 218.35 ± 0.04 |
| Rhus chinensis Mill. | NA1 | 89.50 ± 0.01 | 7403.45 ± 0.02 |
| Tribulus terrestris L. | NA1 | 5.00 ± 0.01 | 12.08 ± 0.11 |
| Kojic acid | 80.0 ± 0.01 | 97.7 ± 0.01 | NT2 |
| Arbutin | 60.0 ± 0.01 | 27.8 ± 0.01 | NT2 |
NA1: not active; NT2: not tested.
The comparison of activity between hsTYR and abTYR (Figure 2) showed little correlation between the two enzymes. Of the 10 TCM extracts, three extracts from R. rugosa, M. alba and P. lactiflora showed comparable activity against both hsTYR and abTYR, six (C. atratum, B. striata, G. urolensis, R. chinensis, R. canina, and C. asiatica) were active against either hsTYR or abTYR, while one (T. terrestris) had minimum/no activity against both mushroom and human TYR activities. The most active TCM were R. rugosa and M. alba with >95% inhibition against both enzymes at 1 mg/mL.
Figure 2.
Anti-hsTYR and abTYR activity of 10 TCM extracts at 1 mg/mL.
3.3. Antioxidant activity
The antioxidant activity of the 10 TCM extracts were also evaluated given its link to TYR activity (Lee and Kim, 1995; Bernard and Berthon, 2000; Cai et al., 2004; Wong et al., 2006). The results (Table 2) showed that 9 TCM extracts which were active against abTYR and/or hsTYR also exhibited some antioxidant activity with TAC values ranging from 200 to 7500 μM/mg. R. chinensis, which inhibited abTYR by 89.5% at 1 mg/mL, had the highest antioxidant activity with a TAC of 7403.45 μM/mg. R. rugosa, which showed 100% and 99.5% inhibition against hsTYR and abTYR, also exhibited strong antioxidant activity with a TAC of 3006.5 μM/mg. T. terrestris induced the lowest TAC value of 12.08 μM/mg, it also had minimum/no activity against either abTYR or hsTYR.
3.4. Inhibitory activity against mmTYR and melanin production
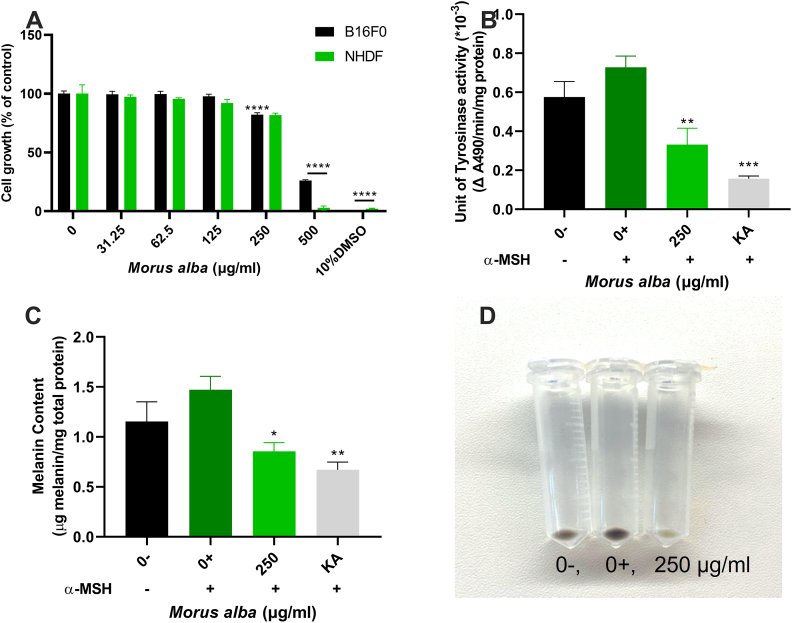
Pigmented mouse melanoma cells, the B16F0 cell line series, were used for cell-based TYR activity studies. Normal NHDF cells were also included for cytotoxicity assay. Given B16F0 cells became less pigmented as subculture continues, α-melanocyte stimulating hormone (α-MSH) was added to stimulate melanogenesis and increase melanin content in cells. It is worth noting that mouse melanoma cells B16F0 co-treated with α-MSH have been widely used as a possible cellular testing system in the literature (Kim et al., 2012; Pillayar et al., 2018; Fogal et al., 2015). The two most active extracts, M. alba and R. rugosa, were chosen for the cell-based assay.
Firstly, the effect of M. alba on cell growth was assessed. B16F0 and NHDF cells were treated with 0, 31.25, 62.5, 125, 250 and 500 μg/mL M. alba extract for 3 days, then the cell growth was assessed using resazurin proliferation assays to determine the optimal concentration that could be used in subsequent assays. The results (Figure 3A) suggested that cell growth was not affected by M. alba extract at the concentrations of 31.25, 62.5, and 125 μg/mL. However, cell growth was moderately reduced (15%) at 250 μg/mL for mouse melanoma B16F0 but severely (>70%) at 500 μg/mL for NHDF cells. A concentration of 250 μg/mL M. alba extract was therefore selected for cell-based mmTYR and melanin assays.
Figure 3.
M. alba treatment of B16F0 cells and NHDF. A, Resazurin cell proliferation assays. B16F0 mouse melanoma cells and normal human dermal fibroblasts (NHDF) were treated with 0, 31.25, 62.5, 125, 250 and 500 μg/mL of M. alba extract for 3 days to assess its effect on the cell growth. Cell growth is presented as a percentage of untreated cells. B, Tyrosinase activity. C, melanin content. D, and cell pellet photo were determined after 3 days when cells were co-treated with 0, 250 μg/mL M. alba extract and 0.1 μM α-MSH. Cells with growth media (0-) were used for comparison with the α-MSH treated cells (0+). Kojic acid (KA, 500 μg/mL) was used as positive control. Mean ± SEM (n = 3) of three independent experiments, each performed in triplicate are shown. One-way ANOVA followed by Dunnett’s test was employed. ∗P < 0.05, ∗∗∗∗P < 0.0001.
B16F0 cells were co-treated with 250 μg/mL M. alba and 0.1 μM α-MSH for 3 days. Cells with growth media (0-) were used for comparison with the α-MSH treated cells (0+). The results showed that M. alba significantly reduced mmTYR activity (Figure 3B) by 60% and melanin content (Figure 3C) by 50% after 3 days of treatment with 250 μg/mL extract, compared to the control (0+). The colour of the cell pellet (Figure 3D) was considerably paler in the treated group than those of the controls (0- and 0+).
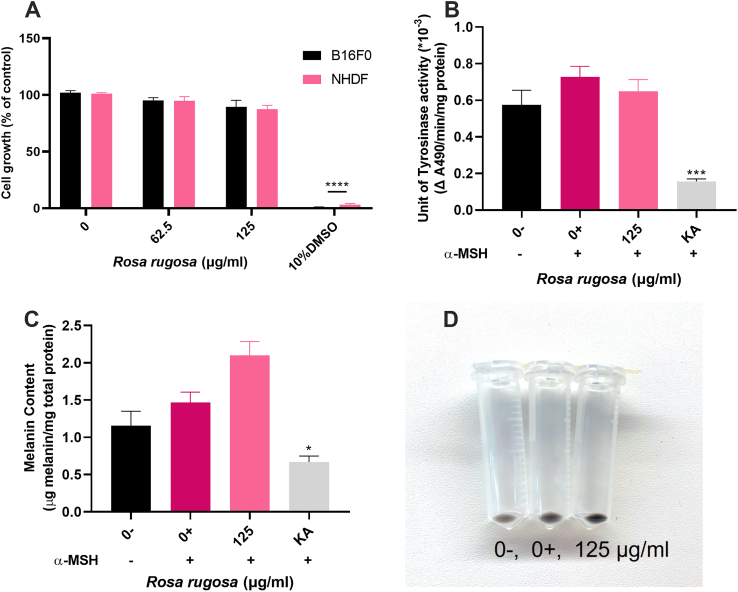
Using the same methodology, R. rugosa was also tested against B16F0 and NHDF cells. Resazurin proliferation assays showed that R. rugosa did not affect cell growth at the concentrations of 62.5 and 125 μg/mL but was toxic at higher concentrations (Figure 4A). Hence a concentration of 125 μg/ml was chosen for further TYR and melanin assays. A small, although not statistically significant, inhibition on mmTYR activity was observed (Figure 4B) when cells were treated with 125 μg/mL extract for 3 days, although melanin levels were slightly increased in the cells (Figure 4C and D).
Figure 4.
R. rugosa treatment of B16F0 cells and NHDF. A, Resazurin cell proliferation assays. B16F0 mouse melanoma cells and normal human dermal fibroblasts (NHDF) were treated with 0, 62.5 and 125 μg/mL of R. rugosa extract for 3 days to assess its effect on the cell growth. Cell growth is presented as a percentage of untreated cells. B, Tyrosinase activity. C, melanin content. D, and cell pellet photo were determined after 3 days when cells were co-treated with 0, 125 μg/mL R. rugosa extract and 0.1 μM α-MSH. Cells with growth media (0-) were used for comparison with the α-MSH treated cells (0+). Kojic acid (KA, 500 μg/mL) was used as positive control. Mean ± SEM (n = 3) of three independent experiments, each performed in triplicate are shown. One-way ANOVA followed by Dunnett’s test was employed. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗∗P < 0.0001.
4. Discussion
The comprehensive herbal medicine system has been recorded in Chinese Pharmacopeia for decades, with the number of plant species increasing from 78 in the first version to 1146 in the 2005 version. Based on such accumulated knowledge, many TCM formulae have been used to treat diseases clinically and will continue to play a significant role in the mainstream health system in China and around the world. However, the efficacies of TCM have been a topic of discussion and intensive research have been/are being conducted to validate their health benefit using biological assays.
A comprehensive review in Chinese Pharmacopoeia has shown that around 120 TCM have been frequently used in skin care products for freckle removing, anti-aging and skin lightning. Several recent reviews on medicinal plants (Gragnani et al., 2014; Hsieh et al., 2015) also highlighted the importance of TCM as source of anti-skin pigmentation agents. Our investigation concentrated on 10 commonly used skin lightning TCM. Two enzymatic assays against abTYR and hsTYR and a cell-based assay using mouse melanoma B16F0 cell line were established and used to evaluate the depigmentation activity of the 10 TCM. Our results showed that 7 out of 10 TCM had significant activity against hsTYR (>50% inhibition at 1 mg/mL), while 6 TCM showed >50% inhibition against abTYR when tested at the same concentration. When evaluated in cells, M. alba, one of the most active extracts (>97% inhibition against both hs and abTYR at 1 mg/mL), significantly reduced mmTYR activity and melanin content in mouse melanoma B16F0 cells at 250 μg/mL. The results demonstrate the efficacy of the TCM not only in enzymatic assay but also in a biological system. R. rugosa, an equally active TCM (∼100% inhibition against both emzymes at 1 mg/mL), showed little effect on mmTYR activity. However, the extract slightly increased melanin production in cells at the concentration of 125 μg/mL. It is noteworthy that the extract from R. rugosa showed significant toxicity at higher doses (>125 μg/mL). Therefore, higher concentrations could not be used in B16F0 cells which limits further investigation on mmTYR activity and melanin production. Overall, our results validated the traditional use of 7 TCM herbs as skin lightning agents, they reveal a remarkable convergence between thousands of years of traditional knowledge and biological activities attained through modern drug screening technology.
Melanogenesis is an oxygen-dependent process, which consists of a sequence of oxidation reactions accompanied by the generation of ROS. Research has shown that extracts containing high level of antioxidants, such as flavonoids and phenolic compounds, also exhibit anti-melanogenesis effects (Lee and Kim, 1995; Bernard and Berthon, 2000; Cai et al., 2004). However, our research showed no clear correlation between anti-TYR and antioxidant activities, with only three TCM extracts (R. rugosa, R. chinensis and P. lactiflora) exhibiting significant TAC. Further chemical investigation is required to identify the chemical composition of the TCM extracts and hence shed light on their antioxidant potential.
For years, research aiming at developing skin depigmentation agents relied heavily on abTYR, largely due to the difficulties in hsTYR expression and purification. It has been assumed that agents identified through abTYR assays should be potential TYR inhibitors for human-directed applications. Recent advances in protein recombinant expression and purification technologies have allowed the production of hsTYR, leading to the determination of the 3D structure of mammalian TYR. Studies on amino acid sequence and active binding sites on hsTYR, however, disclosed some remarkable differences compared to abTYR (Fogal et al., 2015; Mann et al., 2018). A considerable pool of abTYR inhibitors show minimal effect against the human enzyme (Kolbe et al., 2013). This phenomenon was also mirrored in our current study. Of the 10 TCM, only 3 had comparable activity between abTYR and hsTYR, while 6 extracts were active against one or the other. The results suggest that a sole abTYR measurement should never lead to a direct assumption towards hsTYR activity (Solano et al., 2006; Lai et al., 2018; Pillayar et al., 2018). An appropriate array of tests should be performed when developing depigmentation agents for human application, using not only abTYR but also mammalian TYR, in an isolated form or in cells or cell free extracts.
Declarations
Author contribution statement
Tina Liu: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Yaoyin Lu: Performed the experiments; Analyzed and interpreted the data.
Kathryn Tonissen, Yunjiang Feng: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Giovanna Di Trapani: Conceived and designed the experiments; Wrote the paper.
William Tang: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors would like to acknowledge Ferngrove Pharmaceutical Pty Ltd for a PhD scholarship for Tina Liu.
References
- An S.M., Kim H.J., Kim J.E., et al. Flavonoids, taxifolin and luteolin attenuate cellular melanogenesis despite increasing tyrosinase protein levels. Phytother Res. 2019;22:1200–1207. doi: 10.1002/ptr.2435. [DOI] [PubMed] [Google Scholar]
- Baurin N., Arnoult E., Scior T., et al. Preliminary screening of some tropical plants for anti-tyrosinase activity. Ethnopharmacol. 2002;82:155–158. doi: 10.1016/s0378-8741(02)00174-5. [DOI] [PubMed] [Google Scholar]
- Bernard P., Berthon Y. Resveratrol: an original mechanism on tyrosinase inhibition. Int. J. Cosmet. Sci. 2000;22:219–226. doi: 10.1046/j.1467-2494.2000.00019.x. [DOI] [PubMed] [Google Scholar]
- Cai Y., Luo Q., Sun M., et al. Antioxidant activity and phenolic compounds of 112 traditional Chinese medical plants associated with anticancer. Life Sci. 2004;74(17):2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callendar V.D., Sharleen S.T., Erica C., et al. Post inflammatory hyperpigmentation. Am. J. Clin. Dermatol. 2011;12(2):87–99. [Google Scholar]
- Chan Y.Y., Kim K.H., Cheah S.H. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. Ethnopharmacol. 2011;137:1183–1188. doi: 10.1016/j.jep.2011.07.050. [DOI] [PubMed] [Google Scholar]
- Chang T.S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials. 2012;5(9):1661–1685. [Google Scholar]
- Chen D.L., Xu D.L., Lin H. Antioxidant of total flavonoids extracted from Polygonatum Odoratum in vivo and in vitro. Pharm. Today. 2008;18:13–14. [Google Scholar]
- Choi S.Y., Bin B.H., Kim W., et al. Exposure of human melanocytes to UVB twice and subsequent incubation leads to cellular senescence and senescence-associated pigmentation through the prolonged p53 expression. Dermatol Sci. 2018;90:303–312. doi: 10.1016/j.jdermsci.2018.02.016. [DOI] [PubMed] [Google Scholar]
- Chung S., Lim G.J., Lee J.Y. Quantitative analysis of melanin content in a three -dimensional melanoma cell culture. Sci. Rep. 2019;9:780. doi: 10.1038/s41598-018-37055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curto E.V., Kwong C., Hermersdorfer H., et al. Inhibitors of mammalian melanocyte tyrosinase: in vitro comparison of alkyl esters of gentisic acid with other putative inhibitors. Biochem. Pharmacol. 1999;57:663–672. doi: 10.1016/s0006-2952(98)00340-2. [DOI] [PubMed] [Google Scholar]
- Del Marmiol V., Beermann F. Tyrosinase related proteins in mammalian pigmentation. FEBS Lett. 1996;381:165–168. doi: 10.1016/0014-5793(96)00109-3. [DOI] [PubMed] [Google Scholar]
- D’Mello S., Finlay G.J., Baguley B.C., et al. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016;17(7):1144. doi: 10.3390/ijms17071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinska M., Winfield P.T., Young K.L., et al. The TYRP1-medicated protection of human tyrosinase activity does not involve stable interaction of tyrosinase domains. Pigm. Cell Melanoma Res. 2019;32:753–765. doi: 10.1111/pcmr.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraisamy A., Narayanaswamy N., Balakrishnan K.P. Antioxidant and anti-tyrosinase activity of some medicinal plants. Res. J. Pharmacogn. Phytochem. 2011;3(2):86–90. [Google Scholar]
- Fogal S., Carotti M., Giaretta L., et al. Human tyrosinase produced in insect cells: a landmark for the screening of new drugs addressing its activity. Mol. Biotechnol. 2015;57:45–57. doi: 10.1007/s12033-014-9800-y. [DOI] [PubMed] [Google Scholar]
- Fu G.Q., Ma P.C., Wu Q.X., et al. Inhibition effects of ethanolic extracts from 196 kind of Chinese herbs on tyrosinase. Chin. J. Dermatol. 2003;36:103–106. [Google Scholar]
- Gragnani A., Cornick S.M., Chominski V., Noronha SMRd., Noronha S.A., Ferreira L.M. Review of major theories of skin aging. Adv. Aging Res. 2014;3(4):265–284. [Google Scholar]
- Hearing V.J., Jimenez M. Analysis of mammalian pigmentation at the molecular level. Pigm. Cell Res. 1989;2:75–85. doi: 10.1111/j.1600-0749.1989.tb00166.x. [DOI] [PubMed] [Google Scholar]
- Hsieh T., Chang Y., Liu B. Effect of extracts of traditional Chinese medicines on anti-tyrosinase and antioxidant activities. J. Med. Plants Res. 2015;9(48):1131–1138. [Google Scholar]
- Jimenez-Cervantes C., Garcia-Borron J.C., Valverde P., et al. Tyrosinase isoenzymes in mammalian melanocytes- biochemical characterization of two melanosomal tyrosinase from B16 mouse melanoma. Eur. J. Biochem. 1993;217:549–556. doi: 10.1111/j.1432-1033.1993.tb18276.x. [DOI] [PubMed] [Google Scholar]
- Kim J., Lee K.T. Inhibitory effects of ramulus mori extracts on melanogenesis. Cosmet. Toilet. 1998;113(10):65–70. [PubMed] [Google Scholar]
- Kim M., Park J., Song K., et al. Screening of plant extracts for human tyrosinase inhibiting effects. Int. J. Cosmet. Sci. 2012;34:202–208. doi: 10.1111/j.1468-2494.2012.00704.x. [DOI] [PubMed] [Google Scholar]
- Kolbe L., Mann T., Gerwat W., et al. 4-n-Butylresorcinol, a highly effective tyrosinase inhibitor for the topical treatment of hyperpigmentation. Eur Acad Dermatol Venereol. 2013;27:19–23. doi: 10.1111/jdv.12051. [DOI] [PubMed] [Google Scholar]
- Lai X., Wichers H.J., Soler-Lopez M., et al. Structure and function of human tyrosinase and tyrosinase related proteins. Chem. Eur J. 2018;24:50–53. doi: 10.1002/chem.201704410. [DOI] [PubMed] [Google Scholar]
- Lee K.T., Kim B.J., Kim H.J., et al. Biological screening of 100 plant extracts for cosmetic use (I): inhibitory activities of tyrosinase and DOPA auto-oxidation. Int. J. Cosmet. Sci. 1997;19:291–298. doi: 10.1046/j.1467-2494.1997.171725.x. [DOI] [PubMed] [Google Scholar]
- Lee O., Kim E. Skin lightning. Cosmet. Toilet. 1995;110(10):51–56. [Google Scholar]
- Lee Y.S., Kim D.W., Kim S., et al. Downregulation of NFAT2 promotes melanogenesis in B16 melanoma cells. Anat Cell Biol. 2010;43(4):303–309. doi: 10.5115/acb.2010.43.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann T., Gerwat W., Batzer J., et al. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. Invest Dermatol. 2018;138:1601–1608. doi: 10.1016/j.jid.2018.01.019. [DOI] [PubMed] [Google Scholar]
- McEvilly J.A., Lyengar R., Otwell W.S. Inhibition of enzymatic browning in foods and beverages. Crit. Rev. Food Sci. Nutr. 1992;32:253–273. doi: 10.1080/10408399209527599. [DOI] [PubMed] [Google Scholar]
- Meredith P., Sarna T. The physical and chemical properties of eumelanin. Pigm. Cell Res. 2006;19:572–594. doi: 10.1111/j.1600-0749.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- Nerya O., Vaya J., Musa R., et al. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. Agric Food Chem. 2003;51:1201–1207. doi: 10.1021/jf020935u. [DOI] [PubMed] [Google Scholar]
- Nicolaidou E., Katsambas A.D. Pigmentation disorders: hyperpigmentation and hypopigmentation. Clin. Dermatol. 2014;32(1):66–72. doi: 10.1016/j.clindermatol.2013.05.026. [DOI] [PubMed] [Google Scholar]
- Ortonne J.P., Bissett D.L. Latest insights into skin hyperpigmentation. Investig Dermatol Symp Proc. 2008;13:10–14. doi: 10.1038/jidsymp.2008.7. [DOI] [PubMed] [Google Scholar]
- Parvez S., Kang M., Chung H.S., et al. Natural occurring tyrosinase inhibitors: mechanism and application in skin health, cosmetics, and agriculture industries. Phytother Res. 2007;21:805–816. doi: 10.1002/ptr.2184. [DOI] [PubMed] [Google Scholar]
- Pillayar T., Namasivayam V., Manickam M., et al. Inhibition of melanogenesis: an updated review. Med. Chem. 2018;61:7395–7418. doi: 10.1021/acs.jmedchem.7b00967. [DOI] [PubMed] [Google Scholar]
- Pomeranz S.H. Separation, purification, and properties of the two tyrosinases from hamster melanoma. Biol. Chem. 1963;238:2351–2357. [PubMed] [Google Scholar]
- Rudeekulthamrong P., Kaulpiboon Optimization of a mylomaltase for the synthesis of α-arbutin derivatives as tyrosinase inhibitors. Carbohydr. Res. 2020;494:1–8. doi: 10.1016/j.carres.2020.108078. 108078. [DOI] [PubMed] [Google Scholar]
- Solano F., Briganti S., Picardo M., et al. Hyperpigmentation agents: an updated review on biological, chemical, and clinical aspects. Pigm. Cell Res. 2006;19:550–571. doi: 10.1111/j.1600-0749.2006.00334.x. [DOI] [PubMed] [Google Scholar]
- Wong S.P., Leong L.P., William Koh JH. Antioxidant activities of aqueous extract of selected plants. Food Chem. 2006;99(4):775–783. [Google Scholar]
- Xu Y., Stokes A.H., Freeman W.M., et al. Tyrosinase mRNA is expressed in human substantia nigra. Mol. Brain Res. 1997;45:159–162. doi: 10.1016/s0169-328x(96)00308-7. [DOI] [PubMed] [Google Scholar]
- Xue Y.F., Li Z.B. Effects of qiong-yu-gao on the nervous system in a mouse model of aging. Tradit. Chin. Drug Res. Clin. Pharmacol. 1999;10:159–161. [Google Scholar]
- Zheng W., Wang S.Y. Antioxidant activity and phenolic compounds in selected herbs. Agric Food Chem. 2001;49(11):5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.