Abstract
Objective
This study aimed to compare the use of a powered instrument (PI) and ultrasonic curettage device (ULCD) with intraoperative blood loss (IOBL), drain volume (DV), calculated blood loss (CBL), and hidden blood loss (HBL) in orthognathic surgery.
Methods
We included 163 patients who underwent bimaxillary surgery in our department. CBL was calculated from the preoperative and postoperative hemoglobin levels using the “hemoglobin balance method.” CBL is an indicator of the amount of perioperative blood loss. HBL was calculated by subtracting IOBL and DV from CBL.
Results
The PI group consisted of 61 patients (17 males and 44 females, age: 24.9 ± 9.5 years), and the ULCD group consisted of 102 patients (40 males and 62 females, age: 23.1 ± 7.8 years). In the PI group, the median IOBL, DV, CBL, and HBL were 540.0 (interquartile range [IQR] 380.0–670.0), 113.0 (IQR 77.0–147.0), 1000.0 (IQR 751.4–1248.6), and 285.8 (IQR 151.0–476.4) ml, respectively. In the ULCD group, the median IOBL, DV, CBL, and HBL were 327.5 (IQR 200.0–455.0), 105.5 (IQR 75.3–136.0), 759.5 (IQR 594.9–944.2), and 294.2 (IQR 120.8–456.9) ml, respectively. IOBL and CBL were significantly reduced with ULCD use, but no significant differences were observed in DV and HBL.
Conclusions
This study showed that IOBL decreased with ULCD use, resulting in a decrease in CBL. Conversely, bleeding parameters (DV and HBL), which reflect the amount of bleeding that occurs after wound closure, did not show a decrease with ULCD use.
Keywords: Orthognathic surgery, Ultrasonic curettage device, Intraoperative blood loss, Calculated blood loss, Hidden blood loss
Orthognathic surgery, Ultrasonic curettage device, Intraoperative blood loss, Calculated blood loss, Hidden blood loss.
1. Introduction
Although orthognathic surgery is generally considered a safe procedure with few intraoperative complications, massive intraoperative and perioperative blood loss has been reported to have various adverse effects on patients [1, 2, 3, 4]. Orthognathic surgery is an elective surgery, and therefore, its associated risks need to be minimized while ensuring high-quality treatment. There have been many studies on the prevention and reduction of bleeding during orthognathic surgery [2, 3, 4, 5]. With this background, ultrasonic curettage devices are used to avoid the risk of injury to blood vessels, nerves, and soft tissues. The use of ultrasonic curettage device (ULCD) has been shown to decrease intraoperative blood loss (IOBL) in orthognathic surgery [6, 7, 8].
Most previous studies have focused on IOBL in orthognathic surgery, which is usually calculated by subtracting the volume of irrigation fluid used from the total volume of fluid in the suction canister [2, 9, 10, 11]. In other various clinical trials, the weight of the surgical gauze and throat pack have also been added [3, 12, 13]. However, these methods cannot account for the amount of bleeding into the tissue cavity or postoperative bleeding. The drainage volume (DV) is also measured to ascertain the amount of postoperative bleeding, but it does not reflect the amount of bleeding in areas where the drain is obstructed or ineffective. In other words, visible blood loss (VBL) may underestimate the actual amount of blood loss attributed to surgery [3, 13]. Therefore, calculated blood loss (CBL) is an indicator of the amount of perioperative blood loss. This method estimates the amount of blood loss based on the patient's estimated blood volume and blood parameters, such as hemoglobin and hematocrit, and various related formulas [14]. CBL has been shown to better capture the amount of undetected blood loss related to the timing of the measurement [3, 4, 14].
Hidden blood loss (HBL) is also used as the parameter to obtain specific information regarding the amount of undetected blood loss, including the amount of blood loss that occurs following wound closure [3]. HBL is already established as a reliable adjunct in various surgical specialties, such as orthopedics and spine surgery [15, 16]. Furthermore, the increased risk of wound infection, delayed wound healing, and prolonged postoperative rehabilitation has been discussed in association with HBL [17].
Although absolute blood loss, rather than relative blood loss (RBL) of a patient's total blood volume (PTBV), is actively investigated for assessing surgery-related blood loss [1, 18, 19, 20, 21], some studies have introduced the concept of patient-specific RBL as an index, which is calculated as the percentage of intraoperative blood loss divided by the estimated total preoperative blood volume [2, 3]. This patient-specific index may provide a more accurate representation of acceptable blood loss.
As mentioned earlier, some reports [6, 7, 8] have shown that IOBL is reduced with ULCD, but it is unclear whether CBL and HBL are reduced.
This study focuses on surgery-related bleeding in orthognathic surgery and aims to evaluate absolute blood loss and RBL using different indices, such as VBL, IOBL, CBL, and HBL, to determine the impact of ULCD use on each blood loss parameter.
2. Methods
This retrospective study included 163 patients aged ≥16 years with jaw deformities who underwent combined surgery of Le Fort I osteotomy and bilateral sagittal split osteotomy in our department from January 1, 2016, to September 30, 2021. Additionally, the patients must have had blood tests taken preoperatively and on the morning of the first postoperative day, which were necessary to calculate the CBL. Since July 2018, our department has been performing all osteotomies in orthognathic surgery with ULCD (SonopetⓇ UST-2001, Stryker, USA) serrated aggressive knife tips instead of Powered Instruments (PI), such as rotating bars or saws.
Patients were thus divided into two groups according to the surgery date. The PI group consisted of 61 patients (17 males and 44 females) who underwent surgery from January 2016 to June 2018 and utilized rotating bars or saws, whereas the ULCD group consisted of 102 patients (40 males, 62 females) who underwent surgery from July 2018 to September 2021. The mean age at surgery ±standard deviation was 24.9 ± 9.5 years in the PI group and 23.1 ± 7.8 years in the ULCD group.
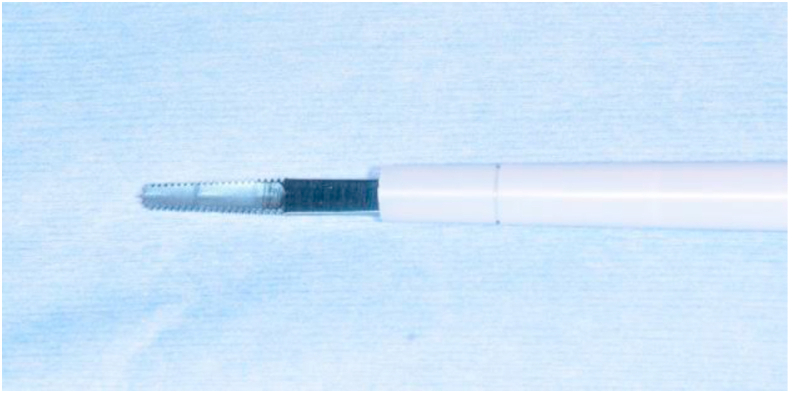
2.1. Serrated aggressive knife tip of an ultrasonic curettage device
The SonopetⓇ ULCD consists of an ultrasonic handpiece connected to a base control module (UST-2001) that regulates the machine's irrigation rate, aspiration, and ultrasound power parameters. The serrated aggressive knife tip blade has serrations on both sides and at the tip, with a 0.8 mm thick and 12.4 mm long blade [8] (Figure 1). Connected to a handpiece, this tip oscillates non-rotationally up to 25,000 times per second with a maximum longitudinal amplitude of up to 0.3 mm [8]. The device allows 30–100% amplitude settings [8]. We set it to an amplitude of 70–80%, which is suitable for bone cutting. The tip heats up during use and must be cooled using room-temperature saline solution, which flows from the dedicated tip cover connected to the handpiece to the blade; however, no suction is available while using this tip. The length of the dedicated tip cover can be adjusted in three steps if required. We use this tip in orthognathic surgery by shortening the length of the dedicated tip cover by one step and setting the saline irrigation volume to 25–30 mL/min.
Figure 1.
Serrated aggressive knife tip. The blade of serrated aggressive knife tip is 12.4 mm long and 0.8 mm thick, with serrations on both sides and at the tip of the blade.
2.2. Anesthesia
General anesthesia, using a combination of remifentanil and propofol, was administered intravenously, with the addition of sevoflurane inhalation if indicated. Nasotracheal intubation was performed for all patients, and surgical gauze was packed around the tube in the oropharynx. After anesthesia induction, local anesthesia (lidocaine 1%, epinephrine 1:200,000) was administered intraorally to the surgical site before the mucosal incision was performed; 10 mL was used at the maxilla surgical site and at the bi-mandibular surgical site each. The mean arterial pressure was maintained at 60–80 mmHg.
Preoperatively, intravenous antibiotics (cefmetazole sodium; 1000 mg) were given. When surgery exceeded 3 h, an additional dose of cefmetazole sodium (1000 mg) was administered as is routine. Patients allergic to penicillin and cephem antibiotics were administered clindamycin (600 mg). No antifibrinolytic agents were administered.
2.3. Surgical procedures
The planning method was based on cephalometric analysis and computed tomographsy (CT), and the surgical plan was developed in collaboration with the orthodontist. The surgeon performed a model surgery and after finalizing the surgical plan, an intraoperative positioning splint was fabricated.
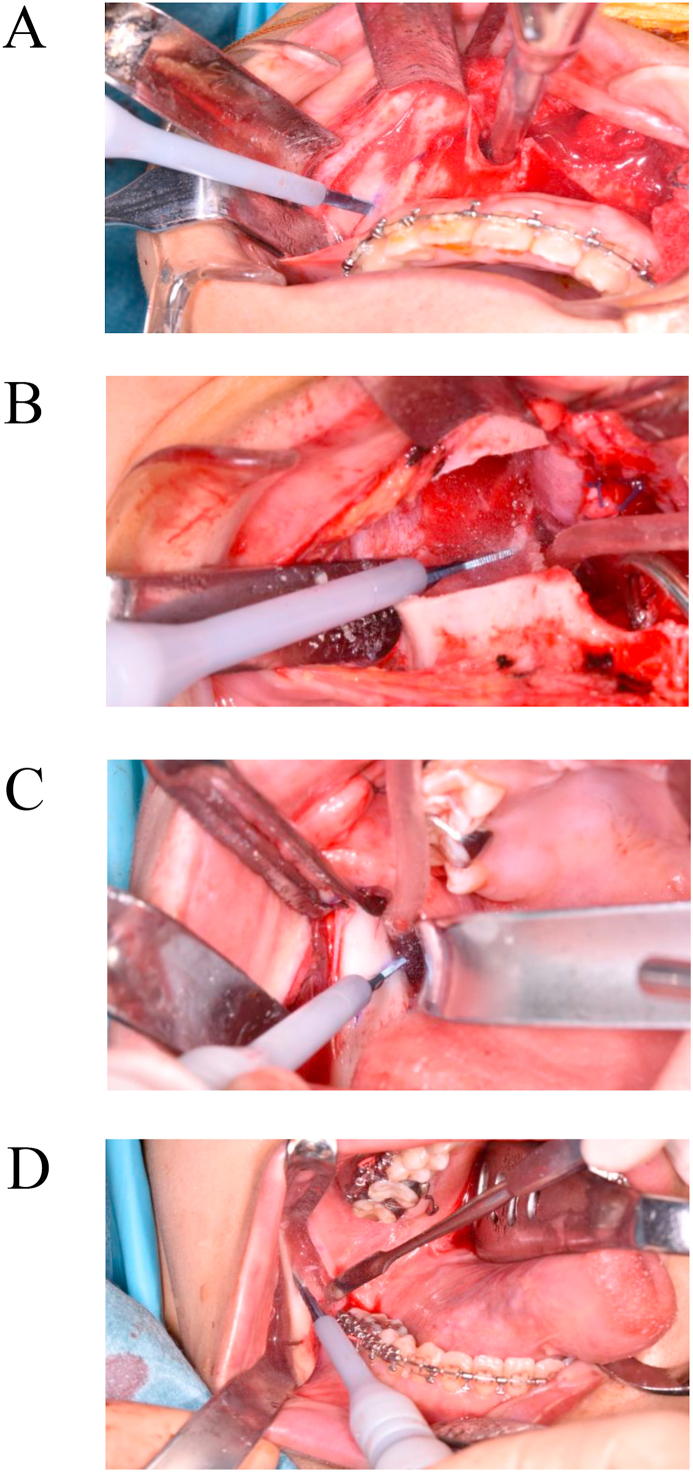
Le Fort I osteotomy was performed according to Bell's technique [22]; BSSO was performed following the Obwegeser-Dal Pont technique using the Hunsuck and Epker modifications [23, 24]. In the PI group, a reciprocating saw was used for horizontal osteotomy of the maxilla in Le Fort I osteotomy. Some rotating burs (such as Lindemann bur and round bur) were used for cutting cortical bone in BSSO. Conversely, in the ULCD group, the Sonopet® ULCD serrated aggressive knife tip was used for all osteotomies and bone deletions in the maxilla and the mandible. Horizontal osteotomies in the maxilla were performed with the sides of the blade in a continuous sweeping maneuver while moving the tip back and forth (Figure 2A); the bone around the descending palatine artery and the pterygoid process was removed with the tip (Figure 2B). The cortical bone in the mandible was cut with the cutting edge on the side of the blade (Figure 2C, D). When separation of the inferior alveolar neurovascular bundle from the proximal spicule was necessary, the bone surrounding the neurovascular bundle was removed using the tip of the blade, and the neurovascular bundle was separated from the proximal spicule [8].
Figure 2.
Le Fort I osteotomy and BSSO using serrated aggressive knife tip. A: Bone cutting from lateral maxillary buttress to ipsilateral piriform rim at Le Fort I osteotomy. B: Bone removal around descending palatal artery at Le Fort I osteotomy. C: Horizontal cortical bone cutting inside the mandibular ramus at BSSO. D: Cortical bone cutting of the external oblique ridge of the mandibular ramus at BSSO.
All procedures were performed by several surgeons with a skilled surgeon (surgeons A, C, H, I, and K were board-certified trainers, surgeons B, D, F, and G were board-certified specialists, and surgeons E and J were board-certified members in Oral and Maxillofacial Surgery of the Japanese Society of Oral and Maxillofacial Surgeons) (Table 1). No cases of multipiece Le Fort I osteotomy were included.
Table 1.
The number of operations performed by each of the surgeons involved.
| Surgeon | A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PI (n = 61) | 50 | 4 | 3 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| ULCD (n = 102) | 80 | 8 | 0 | 0 | 0 | 4 | 3 | 2 | 2 | 2 | 1 |
| Overall (n = 163) | 130 | 12 | 3 | 2 | 2 | 4 | 3 | 2 | 2 | 2 | 1 |
PI, Powered instrument; ULCD, Ultrasonic curettage device.
Operative time, which was defined as the time from the start of the incision to the end of the suture, was measured in both groups.
2.4. Preoperative and postoperative procedures
Autologous blood donation of 400 ml or 800 ml was prepared for all patients at least 72 h prior to surgery, and autologous blood was transfused intraoperatively at the discretion of the anesthesiologist. Continuous infusion of fluids (60–80 ml/h) was administered until 9:00 am the day after surgery.
2.5. Visible blood loss (VBL)
Visible blood loss (VBL) VBL was defined as the sum of the blood loss in intraoperative blood loss (IOBL) and drainage volume (DV) [3]. IOBL was the amount of blood loss starting from mucocutaneous incision to wound closure, calculated by subtracting the amount of saline used to clean the surgical field from the amount of fluid in the suction tube, and taking the sum of the pre-and post-operative weight difference of the surgical gauze. Continuous suction drains were placed on both sides of the mandible and were removed on the first postoperative day; then, DV was the total amount of blood loss from the drains.
2.6. Calculated blood loss (CBL)
Calculated blood loss (CBL) was calculated by the “hemoglobin balance method” (Table 1) based on the hemoglobin levels measured preoperatively and on the morning of the first postoperative day [3, 14]. The patient's total blood volume (PTBV) was calculated by applying Nadler's formula (Table 1) [3].
2.7. Hidden blood loss (HBL)
Hidden blood loss (HBL) was defined as the difference between VBL and CBL [3]. Therefore, it includes the amount of undetected blood loss during the procedure and the amount of blood loss occurring from wound closure to the morning of the first postoperative day. HBL was calculated by subtracting VBL from CBL (HBL = CBL-VBL) (Table 2) [3].
Table 2.
Formulae used to estimate CBL and HBL.
| Equation | Index |
|---|---|
| HBL (ml)=CBL – VBL | HBL (ml): Hidden blood loss |
| CBL (ml): Calculated blood loss | |
| VBL ml): visible blood loss = intraoperative bleeding + drainage volume | |
| Hemoglobin balance method | |
| CBL (ml) = 1000 × (Hbloss/Hbpre) | Hbpre: preoperative hemoglobin level (g/L) |
| Hbloss = BV × (Hbpre – Hbpost) × 0.001 + Hbt | Hbpost: postoperative hemoglobin level (g/L) |
| Hbt: total volume of blood transfusion (g) | |
| Estimation of total blood volume | |
| PTBV = 0.3669 × H3 + 0.03219 × W + 0.6041 (Male) | PTBV (ml): Patient's total blood volume |
| PTBV = 0.3561 × H3 + 0.03308 × W + 0.183 (Female) | H: height in metres |
| W: weight in kilograms | |
2.8. Relative blood loss (RBL)
Relative blood loss (RBL) (%), the percentage of blood loss to PTBV, was calculated as BL/PTBV × 100 for the three blood loss parameters (IOBL, CBL, and HBL) [3].
2.9. Statistical analysis
Statistical analyses were conducted using the BellCurve for Excel (Social Survey Research Information Corporation, Tokyo, Japan), which is an add-in software for Excel statistical evaluation., and comparisons between the two groups for independent samples were studied using the Brunner–Munzel test. The Brunner–Munzel test was designed to detect differences between groups without making any assumptions regarding the shape or continuity of the underlying distribution [25]. It is also less susceptible to outliers and is considered to work well with a sample size of ≥10 [25]. Spearman's correlation coefficient was used to evaluate the correlation between operative time, age, body mass index (BMI), and each blood loss parameter (IOBL, CBL, and HBL). A p-value of <0.05 was defined as the cut-off value for statistical significance.
3. Results
The mean BMI and PTBV at surgery were 21.2 ± 3.0 kg/m2 and 3785.1 ± 660.0 ml in the PI group and 20.9 ± 2.5 kg/m2 and 3816.1 ± 701.9 ml in the ULCD group, respectively, with no significant differences between the two groups. The mean operative time was 226.6 ± 44.6 min in the PI group and 251.4 ± 53.6 min in the ULCD group, a statistically significant difference (p = 0.0017) (Table 3).
Table 3.
Details of the comparison based on the osteotomy device.
| Powered instrument (n = 61) |
Ultrasonic curettage device (n = 102) |
p-value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (y) | 24.9 ± 9.5 | 23.1 ± 7.8 | 0.3875 |
| BMI | 21.2 ± 3.0 | 20.9 ± 2.5 | 0.5641 |
| PTBV (ml) | 3785.1 ± 660.0 | 3816.1 ± 701.9 | 0.9889 |
| Operative time (min) | 226.6 ± 44.6 | 251.4 ± 53.6 | 0.0017 |
| Blood loss parameters | Median (IQR) | Median (IQR) | p-value |
| IOBL (ml) | 540.0 (380.0–670.0) | 327.5 (200.0–455.0) | <0.001 |
| DV (ml) | 113.0 (77.0–147.0) | 105.5 (75.3–136.0) | 0.3770 |
| VBL (ml) | 658.0 (493.0–812.0) | 445.5 (315.0–551.5) | <0.001 |
| CBL (ml) | 1000.0 (751.4–1248.6) | 759.5 (594.9–944.2) | <0.001 |
| HBL (ml) | 285.8 (151.0–476.4) | 294.2 (120.8–456.9) | 0.5373 |
| RBL-IOBL (%) | 14.4 (10.0–19.9) | 8.5 (5.2–11.9) | <0.001 |
| RBL-CBL (%) | 26.8 (19.5–34.5) | 20.3 (15.8–24.8) | <0.001 |
| RBL-HBL (%) | 7.9 (4.6–13.7) | 7.7 (3.2–11.7) | 0.5613 |
| VBL/CBL (%) | 69.1 (49.7–83.1) | 59.4 (47.1–76.0) | 0.0847 |
SD, standard deviation; IQR, interquartile range; IOBL, intraoperative blood loss; DV, drainage volume; VBL, visible blood loss; HBL, hidden blood loss; CBL, calculated blood loss; RBL, relative blood loss; PTBV, Patient's total blood volume.
3.1. Analysis based on the type of osteotomy device
In the PI group, the median VBL, IOBL, CBL, and HBL were 658.0 (interquartile range [IQR] 493.0–812.0) ml, 540.0 (IQR 380.0–670.0) ml, 1000.0 (IQR 751.4–1248.6) ml, and 285.8 (IQR 151.0–476.4) ml, respectively. In the ULCD group, the median VBL, IOBL, CBL, and HBL were 445.5 (IQR 315.0–551.5) ml, 327.5 (IQR 200.0–455.0) ml, 759.5 (IQR 594.9–944.2) ml, and 294.2 (IQR 120.8–456.9) ml, respectively. VBL, IOBL, and CBL were significantly higher in the PI group than in the ULCD group (p < 0.001). There was no statistically significant difference in HBL values between the two groups (p = 0.5373) (Table 3).
The median RBL of IOBL, CBL, and HBL was 14.4% (IQR 10.0–19.9), 26.8% (IQR 19.5–34.5), and 7.9% (IQR 4.6–13.7) in the PI group and 8.5% (IQR 5.2–11.9), 20.3% (IQR 15.8–24.8), and 7.7% (IQR 3.2–11.7) in the ULCD group, respectively. All RBL parameters except HBL were significantly higher in the PI group than in the ULCD group (p < 0.001) (Table 2). Conversely, the median DV was 113.0 (IQR 77.0–147.0) ml in the PI group and 105.5 (IQR 75.3–136.0) ml in the ULCD group, with no statistically significant difference between the two groups (p = 0.5613). VBL/CBL (%) was 69.1% (IQR 49.7–83.1) and 59.4% (IQR 47.1–76.0) in the PI and ULCD groups, respectively (Table 3).
3.2. Analysis based on sex
In the PI group, the mean PTBV was 4605.5 ± 485.7 ml in males and 3468.1 ± 388.8 ml in females, with a statistically significant difference between them (p < 0.001). However, there was no statistically significant difference between males and females according to their age, BMI, or operative time (Table 4). The median IOBL was 580.0 (IQR 470.0–1000.0) ml in males and 525.0 (IQR 368.8–617.5) ml in females, with no statistically significant difference between the two groups (p = 0.1.066). The median CBL was 1213.8 (IQR 974.9–1286.1) ml in males and 924.9 (IQR 701.3–1205.5) ml in females, with a significantly higher value in males (p = 0.0024) (Table 4). The median HBL was 285.3 (IQR 216.1–475.3) ml in males and 289.9 (IQR 133.1–485.8) ml in females, with no statistically significant difference between the two groups (p = 0.8039). RBL for each parameter was not statistically significant between males and females (Table 4). The median DV was 118.0 (IQR 95.0–172.0) ml in males and 109.0 (IQR 76.5–130.5) ml in females, with no significantly higher value in males (p = 0.1715). VBL/CBL (%) was 71.2% (IQR 56.1–83.1) in males and 69.1% (IQR 49.1–83.6) in females.
Table 4.
Details of the comparison based on the gender in the Powered instrument group.
| males (n = 17) |
females (n = 44) |
p-value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (y) | 25.4 ± 10.9 | 24.7 ± 9.0 | 0.9133 |
| BMI | 21.4 ± 3.8 | 21.1 ± 2.6 | 0.8449 |
| PTBV (ml) | 4605.5 ± 485.7 | 3468.1 ± 388.8 | <0.001 |
| Operative time (min) | 228.8 ± 48.7 | 225.7 ± 43.4 | 0.7448 |
| Blood loss parameters | Median (IQR) | Median (IQR) | p-value |
| IOBL (ml) | 580.0 (470.0–1000.0) | 525.0 (368.8–617.5) | 0.1066 |
| DV (ml) | 118.0 (95.0–172.0) | 109.0 (76.5–130.5) | 0.1715 |
| VBL (ml) | 805.0 (555.0–1118.0) | 641.0 (483.0–729.8) | 0.0530 |
| CBL (ml) | 1213.8 (974.9–1286.1) | 924.9 (701.3–1205.5) | 0.0024 |
| HBL (ml) | 285.3 (216.1–475.3) | 289.9 (133.1–485.8) | 0.8039 |
| RBL-IOBL (%) | 11.2 (10.2–21.1) | 15.4 (9.9–19.0) | 0.7840 |
| RBL-CBL (%) | 26.8 (22.2–29.2) | 28.0 (19.4–35.4) | 0.5884 |
| RBL-HBL (%) | 6.9 (4.6–10.7) | 8.1 (4.1–14.5) | 0.4307 |
| VBL/CBL (%) | 71.2 (56.1–83.1) | 69.1 (49.1–83.6) | 0.8283 |
SD, standard deviation; IQR, interquartile range; IOBL, intraoperative blood loss; DV, drainage volume; VBL, visible blood loss; HBL, hidden blood loss; CBL, calculated blood loss; RBL, relative blood loss; PTBV, Patient's total blood volume.
In the ULCD group, the mean PTBV was 4552.2 ± 423.8 ml in males and 3341.2 ± 341.2 ml in females, with a statistically significant difference between them (p < 0.001). However, there was no statistically significant difference between males and females according to their age, BMI, or operative time (Table 5). The median IOBL was 350.0 (IQR 213.8–450.0) ml in males and 305.0 (IQR 175.0–445.0) ml in females. There was no statistically significant difference between the two groups (p = 0.1497). The median CBL was 822.1 (IQR 649.2–1001.0) ml in males and 724.6 (IQR 588.8–866.7) ml in females, with a significantly higher value in males (p < 0.001) (Table 5). The median HBL was 223.8 (IQR 107.1–466.5) ml in males and 308.2 (IQR 148.7–449.6) ml in females, with no statistically significant difference between the two groups (p = 0.0095). RBL was higher in females than in males for each parameter, with statistically significant differences in the CBL and HBL (Table 5). The median DV was 118.5 (IQR 91.3–152.3) ml in males and 100.0 (IQR 68.5–119.8) ml in females, with a statistically significant difference between the two groups (p = 0.0180). VBL/CBL (%) was 68.5% (IQR 47.2–81.9%) in males and 56.%0 (IQR 47.1–71.7%) in females.
Table 5.
Details of the comparison based on the gender in the Ultrasonic curettage device group.
| males (n = 40) |
females (n = 62) |
p-value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (y) | 21.8 ± 5.6 | 26.3 ± 11.2 | 0.6530 |
| BMI | 21.2 ± 2.4 | 20.7 ± 2.6 | 0.0891 |
| PTBV (ml) | 4552.2 ± 423.8 | 3341.2 ± 341.2 | <0.001 |
| Operative time (min) | 259.3 ± 56.8 | 246.3 ± 51.3 | 0.2943 |
| Blood loss parameters | Median (IQR) | Median (IQR) | p-value |
| IOBL (ml) | 350.0 (213.8–450.0) | 305.0 (175.0–445.0) | 0.1497 |
| DV (ml) | 118.5 (91.3–152.3) | 100.0 (68.5–119.8) | 0.0180 |
| VBL (ml) | 485.0 (372.3–575.0) | 412.0 (279.0–524.5) | 0.0404 |
| CBL (ml) | 822.1 (649.2–1001.0) | 724.6 (588.8–866.7) | 0.1213 |
| HBL (ml) | 223.8 (107.1–466.5) | 308.2 (148.7–449.6) | 0.4660 |
| RBL-IOBL (%) | 7.5 (5.0–10.3) | 8.9 (5.7–12.9) | 0.3642 |
| RBL-CBL (%) | 17.8 (14.1–21.2) | 21.6 (17.0–26.3) | 0.0026 |
| RBL-HBL (%) | 5.0 (2.6–10.4) | 9.1 (4.4–13.1) | 0.0153 |
| VBL/CBL (%) | 68.5 (47.2–81.9) | 56.0 (47.1–71.7) | 0.1275 |
SD, standard deviation; IQR, interquartile range; IOBL, intraoperative blood loss; DV, drainage volume; VBL, visible blood loss; HBL, hidden blood loss; CBL, calculated blood loss; RBL, relative blood loss; PTBV, Patient's total blood volume.
3.3. Correlation analysis
Correlations between the operative time, age, BMI, and all the parameters used in the analysis of blood loss were evaluated. The operative time correlated with the IOBL and CBL in both the PI and ULCD groups. Age and BMI did not correlate with each bleeding parameter and RBL in both the PI and ULCD groups (Table 6).
Table 6.
Spearman rank correlations between each blood loss parameter and patient's demographics.
| Powered instrument | IOBL | CBL | HBL |
|---|---|---|---|
| Age | 0.0098 | 0.1126 | 0.0967 |
| BMI | 0.0977 | 0.1622 | 0.0781 |
| PTBV | 0.2420 | 0.2483 | −0.0632 |
| Operative time | 0.3699 | 0.3127 | −0.0353 |
| Ultrasonic curettage device | IOBL | CBL | HBL |
| Age | 0.1012 | 0.1172 | −0.0016 |
| BMI | −0.0227 | −0.0421 | −0.00624 |
| PTBV | 0.1508 | 0.1572 | −0.0448 |
| Operative time | 0.2207 | 0.3609 | 0.1382 |
IOBL, intraoperative blood loss; HBL, hidden blood loss; CBL, calculated blood loss; PTBV, Patient's total blood volume.
Bold letters indicate p < 0.05.
4. Discussion
Although rare, serious, and fatal hemorrhage associated with orthognathic surgery has been reported [1, 2, 8, 26]. In particular, PIs may entrap surrounding soft tissues during orthognathic surgical bone cutting, seriously damaging blood vessels and nerves. Excessive bleeding during orthognathic surgery is caused by damage to large blood vessels, including the sphenopalatine artery, descending palatine artery, pterygoid plexus, maxillary artery, alveolar artery, and facial artery [8, 27]. Against this background, ULCDs have been used in orthognathic surgery to avoid the risk of damage to soft tissues, nerves, and blood vessels [6, 8, 28, 29]. The use of a ULCD has been shown to reduce IOBL in orthognathic surgery [6, 8, 28, 29]. Furthermore, the reduction rate of IOBL in this study was higher than the reduction rate with piezoelectric devices (25–30%) [6, 7]. In contrast, operative time with ULCDs is longer than that with Pis [6, 8, 28, 29]. In the present study, the mean operative time in the ULCD group was significantly longer than that in the PI group. However, the rate of prolonged operative time was approximately 11%, which is lower than the previously reported rate of 35% [7]. The operative time is reduced because this serrated aggressive knife tip is highly efficient in bone-cutting given its unique shape and high output of the device, and it does not require tip replacement [8].
IOBL is calculated by subtracting the amount of perfusate used from the amount of fluid in the suction tube, plus the pre-and postoperative weights related to the surgical swab and throat pack [3]. It is a practical and widely used assessment tool [3]. Unfortunately, IOBL does not take into account the amount of blood stored in the tissue cavity that could not be aspirated during surgery or the amount of bleeding occurring after wound closure. In addition, although the DV is measured to determine the amount of postoperative bleeding, it does not reflect the amount of bleeding at sites where drainage is occluded or ineffective. Therefore, CBL, which represents the amount of blood loss between blood draws based on the patient's estimated circulating blood volume and change in hemoglobin or hematocrit level, is used as an indicator of total blood loss for a given period, including blood loss after wound closure [3, 14, 17]. There are several formulas for CBL calculation, among which the hemoglobin balance method has been widely adopted and is considered more accurate in comparison with other calculation methods [3, 11]. Furthermore, it has been employed in studies on orthognathic surgery [3, 4]. Therefore, the hemoglobin balance method was employed in this study. HBL has also been established and used in surgical specialties, such as orthopedics and spine surgery, as a parameter to obtain specific information on the undetected blood loss, including the amount of blood loss that occurs following wound closure [15, 16]. In these specialties, HBL has been shown to account for a significant proportion of the total blood loss [15, 16] and has also been used to predict postoperative outcomes such as the increased risk of wound infection, delayed wound healing, and prolonged postoperative rehabilitation in association with HBL [17].
In this study, CBL in the ULCD group was significantly reduced by 24% compared to that in the PI group. These results indicate that the use of ULCDs reduces total surgery-related blood loss. On the other hand, DV and HBL did not differ between the ULCD and PI groups. This indicates that the amount of bleeding that occurs after wound closure does not differ between bone-cutting instruments and that the use of ULCDs does not decrease postoperative blood loss. These results indicate that the decrease in CBL with the use of ULCD is due to a decrease in IOBL. On the other hand, the percentage of VBL to CBL in this study was calculated to be 69.1% in the PI group and 59.4% in the ULCD group. These results indicate that the assessment of blood loss by VBL underestimates surgical blood loss, and furthermore, that the ULCD group tends to underestimate surgery-related blood loss compared to the PI group.
The impact of sex on blood loss in orthognathic surgery remains controversial [2, 3, 4]. The results of this study showed that in the PI group, men had significantly higher CBL than women. On the other hand, women had higher RBL than men, although no statistically significant difference was found in IOBL, CBL, and HBL. This indicated that there was no sex-related difference in surgery-related blood loss in the PI group. This result is consistent with studies showing that RBL is comparable regardless of sex [2, 3]. On the other hand, IOBL and CBL in the ULCD group were higher in males than in females, although RBL was higher in females than in males. The reversal of results between absolute and relative assessments in this study may be attributed to the fact that the estimated total circulating blood volume was significantly higher in men than in women. Based on these findings, we believe that the use of relative values in addition to absolute values when evaluating blood loss due to surgery may enable a more accurate assessment and understanding of an individual's blood loss.
In addition to sex, age [2, 30], BMI [2, 30], and operative time [3, 21] have been previously examined as factors affecting blood loss. The present study showed that IOBL and CBL increased with increasing operative time in the PI and ULCD groups, but there was no effect on HBL. This result was consistent with the report of Schwaiger et al. [3]. It is noteworthy that despite the fact that the results of this study showed an increase in IOBL and CBL with increasing operative time, there was a significant decrease in IOBL and CBL in the ULCD group compared to the PI group, despite an increase in operative time. As mentioned earlier, the decrease in CBL in this study was dependent on the decrease in IOBL, which strongly suggests that the use of ULCD strongly indicates the effect of IOBL reduction.
Hypotensive anesthesia is effective in reducing intraoperative blood loss and improving the quality of the operative field in orthognathic surgery, and the combination of regional and hypotensive anesthesia has been shown to further reduce intraoperative blood loss [31]. Since intraoperative blood pressure control and regional anesthesia use are common in both the PI and ULCD groups in this study, we were unable to examine the relationship between the mean arterial pressure and the use of regional anesthesia with each bleeding parameter. In addition, the study was retrospective and did not evaluate the quality of the surgical field, so it was not possible to examine the effect of field quality on operative time and each of the bleeding parameters.
The serrated aggressive knife tip is disposable and more expensive per piece than the powered instrument system saws and bars [8]. However, this ultrasonic blade can perform Le Fort I osteotomy, BSSRO osteotomy, and bone removal with a single tip. Thus, the total cost of orthognathic surgery using this ultrasonic blade is lower than using multiple saws and bars with a powered instrument system [8].
A limitation of this study is that hemodilution due to continuous infusion during the postoperative blood collection period ultimately affects the calculated values. Therefore, it is difficult to compare the results of this study with other reports of CBL and HBL in orthognathic surgery, but since the comparison of cases in our clinical department seems to be equally affected by hemodilution, we believe it is less likely to affect the results of this study. In addition, the age, experience of the surgeon, type of deformity, and amount of maxillary and mandibular surgical mobilization were not matched in this study, so the influence of these differences on the results cannot be excluded.
5. Conclusions
The use of ULCD decreases IOBL in orthognathic surgery. Moreover, the decrease in IOBL was shown to decrease CBL. Conversely, bleeding parameters (DV and HBL), which reflect the amount of bleeding that occurs after wound closure, did not show a decrease with the use of ULCD. Furthermore, the use of multiple bleeding parameters indicated that conventional assessments relying on measurable bleeding underestimated the amount of bleeding.
Declarations
Author contribution statement
Hidenobu Sakuma: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Daichi Hasebe; Ryoko Takeuchi; Taichi Hara; Daisuke Suda; Naoaki Saito; Daisuke Saito: Contributed reagents, materials, analysis tools or data.
Tadaharu Kobayashi: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the ethics committee of Niigata University (approval no. 2021-0227).
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
References
- 1.Kramer F.J., Baethge C., Swennen G., Teltzrow T., Schulze A., Berten J., et al. Intra- and perioperative complications of the Le Fort I osteotomy: a prospective evaluation of 1000 patients. J. Craniofac. Surg. 2004;15:971–977. doi: 10.1097/00001665-200411000-00016. discussion 978. [DOI] [PubMed] [Google Scholar]
- 2.Thastum M., Andersen K., Rude K., Nørholt S.E., Blomlöf J. Factors influencing intraoperative blood loss in orthognathic surgery. Int. J. Oral Maxillofac. Surg. 2016;45:1070–1073. doi: 10.1016/j.ijom.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Schwaiger M., Wallner J., Edmondson S.J., Mischak I., Rabensteiner J., Gary T., et al. Is there a hidden blood loss in orthognathic surgery and should it be considered? Results of a prospective cohort study. J. Cranio-Maxillo-Fac. Surg. 2021;49:545–555. doi: 10.1016/j.jcms.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Schwaiger M., Edmondson S.J., Rabensteiner J., Prüller F., Gary T., Zemann W., et al. Gender-specific differences in haemostatic parameters and their influence on blood loss in bimaxillary surgery. Clin. Oral Invest. 2022;26:3765–3779. doi: 10.1007/s00784-021-04347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mei A., Qiu L. The efficacy of tranexamic acid for orthognathic surgery: a meta-analysis of randomized controlled trials. Int. J. Oral Maxillofac. Surg. 2019;48:1323–1328. doi: 10.1016/j.ijom.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Landes C.A., Stübinger S., Rieger J., Williger B., Ha T.K., Sader R. Critical evaluation of piezoelectric osteotomy in orthognathic surgery: operative technique, blood loss, time requirement, nerve and vessel integrity. J. Oral Maxillofac. Surg. 2008;66:657–674. doi: 10.1016/j.joms.2007.06.633. [DOI] [PubMed] [Google Scholar]
- 7.Spinelli G., Lazzeri D., Conti M., Agostini T., Mannelli G. Comparison of piezosurgery and traditional saw in bimaxillary orthognathic surgery. J. Cranio-Maxillo-Fac. Surg. 2014;42:1211–1220. doi: 10.1016/j.jcms.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Kato Y., Saito N., Niimi K., Saito D., Sakuma H., Hasebe D., et al. A comparison and evaluation of the use of ultrasonic cutting devices with conventional powered instruments in orthognathic surgery. Ann. Oral Maxillofac Surg. 2021;2 [Google Scholar]
- 9.Al-Sebaei M.O. Predictors of intra-operative blood loss and blood transfusion in orthognathic surgery: a retrospective cohort study in 92 patients. Patient Saf. Surg. 2014;8:41. doi: 10.1186/s13037-014-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen K., Thastum M., Nørholt S.E., Blomlöf J. Relative blood loss and operative time can predict length of stay following orthognathic surgery. Int. J. Oral Maxillofac. Surg. 2016;45:1209–1212. doi: 10.1016/j.ijom.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Olsen J.J., Ingerslev J., Thorn J.J., Pinholt E.M., Gram J.B., Sidelmann J.J. Can preoperative sex-related differences in hemostatic parameters predict bleeding in orthognathic surgery? J. Oral Maxillofac. Surg. 2016;74:1637–1642. doi: 10.1016/j.joms.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Ueki K., Marukawa K., Shimada M., Nakagawa K., Yamamoto E. The assessment of blood loss in orthognathic surgery for prognathia. J. Oral Maxillofac. Surg. 2005;63:350–354. doi: 10.1016/j.joms.2004.05.226. [DOI] [PubMed] [Google Scholar]
- 13.Kretschmer W., Köster U., Dietz K., Zoder W., Wangerin K. Factors for intraoperative blood loss in bimaxillary osteotomies. J. Oral Maxillofac. Surg. 2008;66:1399–1403. doi: 10.1016/j.joms.2008.01.060. [DOI] [PubMed] [Google Scholar]
- 14.Gao F.Q., Li Z.J., Zhang K., Sun W., Zhang H. Four methods for calculating blood-loss after total knee arthroplasty. Chin. Med. J. 2015;128:2856–2860. doi: 10.4103/0366-6999.168041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith G.H., Tsang J., Molyneux S.G., White T.O. The hidden blood loss after hip fracture. Injury. 2011;42:133–135. doi: 10.1016/j.injury.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Ogura Y., Dimar J.R., Gum J.L., Crawford C.H., 3rd, Djurasovic M., Glassman S.D., et al. Hidden blood loss following 2- to 3- level posterior lumbar fusion. Spine J. 2019;19:2003–2006. doi: 10.1016/j.spinee.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Liu X., Liu J., Sun G. A comparison of combined intravenous and topical administration of tranexamic acid with intravenous tranexamic acid alone for blood loss reduction after total hip arthroplasty: a meta-analysis. Int. J. Surg. 2017;41:34–43. doi: 10.1016/j.ijsu.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Modig M., Rosén A., Heimdahl A. Template bleeding time for preoperative screening in patients having orthognathic surgery. Br. J. Oral Maxillofac. Surg. 2008;46:645–648. doi: 10.1016/j.bjoms.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Madsen D.E., Ingerslev J., Sidelmann J.J., Thorn J.J., Gram J. Intraoperative blood loss during orthognathic surgery is predicted by thromboelastography. J. Oral Maxillofac. Surg. 2012;70:e547–e552. doi: 10.1016/j.joms.2012.06.182. [DOI] [PubMed] [Google Scholar]
- 20.Faverani L.P., Ramalho-Ferreira G., Fabris A.L., Polo T.O., Poli G.H., Pastori C.M., et al. Intraoperative blood loss and blood transfusion requirements in patients undergoing orthognathic surgery. Oral Maxillofac. Surg. 2014;18:305–310. doi: 10.1007/s10006-013-0415-4. [DOI] [PubMed] [Google Scholar]
- 21.Choi B.K., Yang E.J., Oh K.S., Lo L.J. Assessment of blood loss and need for transfusion during bimaxillary surgery with or without maxillary setback. J. Oral Maxillofac. Surg. 2013;71:358–365. doi: 10.1016/j.joms.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Bell W.H., Mannai C., Luhr H.G. Art and science of the Le Fort I down fracture. Int. J. Adult Orthod. Orthognath. Surg. 1988;3:23–52. [PubMed] [Google Scholar]
- 23.Hunsuck E.E. A modified intraoral sagittal splitting technique for correction of mandibular prognathism. J. Oral Surg. 1968;26:250–253. [PubMed] [Google Scholar]
- 24.Epker B.N. Modifications of the sagittal osteotomy of the mandible. J. Oral Surg. 1977;35:157–159. [PubMed] [Google Scholar]
- 25.Brunner E., Munzel U. The nonparametric BehrensFisher problem: asymptotic theory and a small-sample approximation. Biom. J. 2000;42:17–25. [Google Scholar]
- 26.Teltzrow T., Kramer F.J., Schulze A., Baethge C., Brachvogel P. Perioperative complications following sagittal split osteotomy of the mandible. J. Cranio-Maxillo-Fac. Surg. 2005;33:307–313. doi: 10.1016/j.jcms.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Piñeiro-Aguilar A., Somoza-Martín M., Gandara-Rey J.M., García-García A. Blood loss in orthognathic surgery: a systematic review. J. Oral Maxillofac. Surg. 2011;69:885–892. doi: 10.1016/j.joms.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Ueki K., Nakagawa K., Marukawa K., Shimada M., Yamamoto E. Use of the Sonopet ultrasonic curettage device in intraoral vertical ramus osteotomy. Int. J. Oral Maxillofac. Surg. 2007;36:745–747. doi: 10.1016/j.ijom.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Gilles R., Couvreur T., Dammous S. Ultrasonic orthognathic surgery: enhancements to established osteotomies. Int. J. Oral Maxillofac. Surg. 2013;42:981–987. doi: 10.1016/j.ijom.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Moenning J.E., Bussard D.A., Lapp T.H., Garrison B.T. Average blood loss and the risk of requiring perioperative blood transfusion in 506 orthognathic surgical procedures. J. Oral Maxillofac. Surg. 1995;53:880–883. doi: 10.1016/0278-2391(95)90273-2. [DOI] [PubMed] [Google Scholar]
- 31.Lin S., McKenna S.J., Yao C.F., Chen Y.R., Chen C. Effects of hypotensive anesthesia on reducing intraoperative blood loss, duration of operation, and quality of surgical field during orthognathic surgery: a systematic review and meta-analysis of randomized controlled trials. J. Oral Maxillofac. Surg. 2017;75:73–86. doi: 10.1016/j.joms.2016.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.