Central Illustration
Key Words: cardiomyopathy, colorectal cancer, diagnosis, treatment, immunotherapy, myocarditis
Abbreviations and Acronyms: ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; PD-1, programmed cell death receptor-1
Immune checkpoint inhibitor (ICI) myocarditis is an immune-related adverse event (irAE), in which activated T-lymphocytes infiltrate the myocardium and can cause cardiac biomarker elevation, cardiomyopathy, arrhythmias, or even cardiogenic shock/death.1 Although ICI myocarditis typically occurs soon after initiating checkpoint therapy, cases of delayed ICI myocarditis postimmunotherapy (>90 days) have not been well characterized in the literature, despite their associations with higher rates of major adverse cardiac events.1 We report a case of fulminant late-onset ICI myocarditis that occurred after more than 2 years since starting immunotherapy with pembrolizumab for metastatic colorectal adenocarcinoma, highlighting the need for long-term vigilance for immune-related cardiotoxicity.
Case
A 48-year-old woman with a family history of Lynch syndrome and no prior cardiac history presented with a palpable abdominal mass and unintentional 10-pound weight loss. Biopsy of an ileocecal mass revealed an invasive, poorly differentiated non-small cell carcinoma. She was diagnosed with stage IV colorectal adenocarcinoma with hepatic and retroperitoneal lymph node metastases. Consistent with her Lynch syndrome and germline MSH2 mutation, the patient’s tumor had microsatellite instability with deficient MSH2 and MSH6 by immunohistochemistry. Despite initial treatment with traditional chemotherapy, follow-up positron emission tomography–computed tomography scans showed progression of disease, and thus she was initiated on the programmed cell death receptor-1 (PD-1) ICI, pembrolizumab. At completion of the study protocol, she received 52 cycles of pembrolizumab over 2 years. Approximately 1 year later after initiating pembrolizumab, she demonstrated a complete radiographic response with no evidence of disease on computed tomography scan. Her major side effects from pembrolizumab included new-onset hypothyroidism, and arthralgias and myalgias that developed 4 months after initiating therapy but resolved upon therapy completion without need for glucocorticoid treatment. A cardiovascular evaluation was not performed at this time or during her ICI treatment course.
Nine months after her last dose of pembrolizumab, she presented to the emergency department after experiencing 5 days of intermittent fevers, nausea, vomiting, and chest pain. In the emergency department, she had a blood pressure of 96/73 mm Hg, heart rate of 109 beats/min, and an elevated lactate of 3.9 mmol/L (reference range 0.5-1.9 mmol/L). She received 4 L of intravenous fluids and empiric broad spectrum antibiotics, and was directly admitted to the intensive care unit. A bedside transthoracic echocardiogram revealed significant biventricular systolic dysfunction, global hypokinesis, and severely reduced left ventricular systolic function. Laboratory findings were significant for serum troponin I >90 ng/mL (greater than assay limits; reference range 0-0.04 ng/mL), raising concern for acute coronary syndrome. Of note, her electrocardiogram before starting pembrolizumab revealed sinus rhythm with premature ventricular complexes. However, her electrocardiogram on admission to the intensive care unit demonstrated a wide complex ventricular rhythm with atrioventricular dissociation consistent with ventricular tachycardia. She was treated with 220-J synchronized, biphasic direct current cardioversion and amiodarone. She then underwent coronary catheterization, which demonstrated no obstructive coronary disease. Left ventriculography revealed a left ventricular ejection fraction of 10% with global hypokinesis. Right heart cardiac catheterization revealed a right atrial pressure of 25 mm Hg, pulmonary capillary wedge pressure of 26 mm Hg, and a cardiac index of 1.1 L/min/m2. An intra-aortic balloon pump was inserted, and she was started on dobutamine. Given her immunotherapy history and the lack of reasonable alternative diagnosis, her cardiogenic shock was attributed to possible ICI-mediated myocarditis. She was treated with 1 g of methylprednisolone and transferred to Stanford hospital for consideration of venoarterial extracorporeal membrane oxygenation. On arrival, her ionotropic requirements increased despite intra-aortic balloon pump support, and she developed ventricular tachycardia and cardiac arrest. Despite repeat electrical cardioversions and multiple rounds of cardiopulmonary resuscitation, she was unable to be successfully resuscitated.
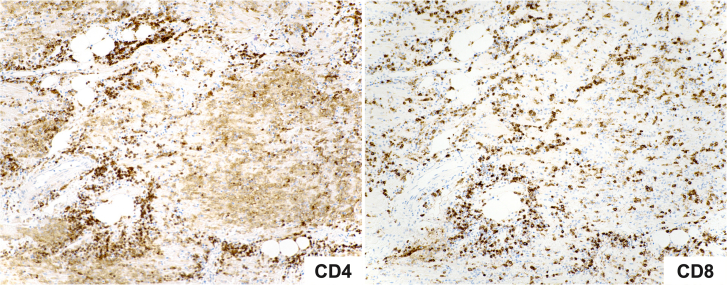
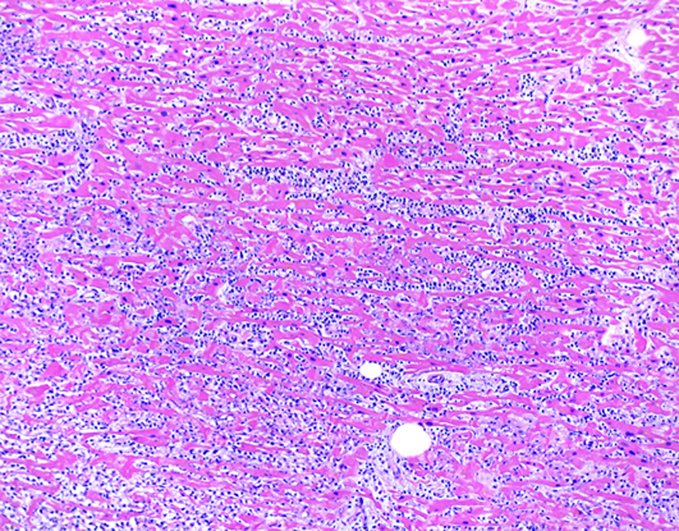
Autopsy revealed an enlarged heart that weighed 440 g (>99th percentile), with mild 4-chamber dilation and a small pericardial effusion. Coronary arteries had minimal atherosclerosis. Sections of the myocardium demonstrated myocyte necrosis and dense chronic inflammatory infiltrates composed predominantly of lymphocytes and monocytes (Figure 1). Immunohistochemical staining revealed immune subpopulations of CD3+ T lymphocytes, with a slight predominance of CD8+ cytotoxic T cells over CD4+ helper T cells, as well as scattered CD163+ macrophages (Figure 2). A PD-L1 stain was performed on the myocardial sections, which demonstrated strong cytoplasmic staining in cardiomyocytes with lymphocytic infiltration and necrosis, consistent with myocarditis. Endomyocardial tissue culture from the left ventricle was negative for bacterial and fungal growth. To exclude the possibility of a viral myocarditis, viral polymerase chain reaction and culture was performed on myocardial tissue, which were negative for influenza (A, A-H1, A-H3, H1N1, B), respiratory syncytial virus, parainfluenza virus (types 1-4), human metapneumovirus, cytomegalovirus, adenovirus, enterovirus, coxsackievirus, and human herpesviruses (HHV1 and HHV2). The remainder of her autopsy did not reveal pathologic findings of noncardiovascular irAE.
Figure 1.
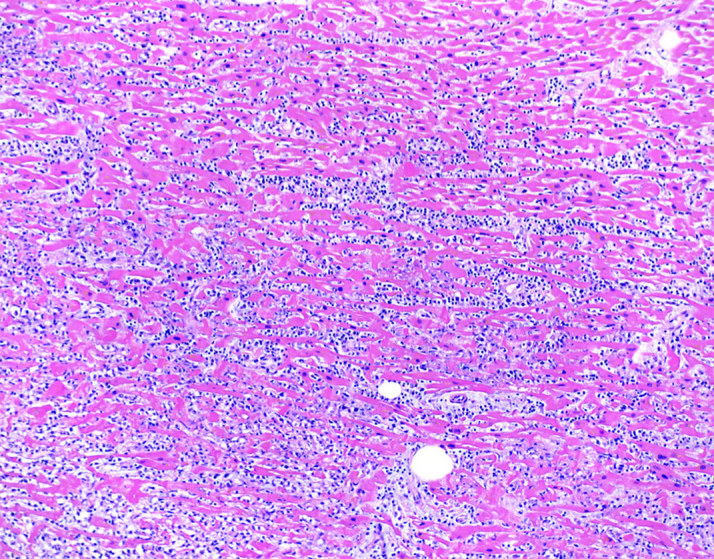
Post-Mortem Histology of the Left Ventricular Free-Wall
Histological findings by hematoxylin and eosin stain show diffuse lymphocytic and monocytic infiltration of myocardium, expanding the interstitial spaces (×100).
Figure 2.
Immunohistochemical Stains Identifying T-Cell Populations
CD4 stains the T-helper cell population (left) and CD8 stains the cytotoxic T-cell population (right). The CD8 staining demonstrates a slight excess of cytotoxic T cells over T-helper cells (×100).
Discussion
ICI are monoclonal antibodies that block inhibitory T-cell signaling pathways, including PD-1/PD-L1 and CD80/ cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) utilized by tumors to evade immune surveillance. By inhibiting immune checkpoints, ICI allow T lymphocytes to activate and target tumor cells. Although ICI have revolutionized cancer therapy, the resultant hyperactivated immune response can precipitate autoimmune disorders, including fulminant ICI myocarditis. The estimated prevalence of ICI myocarditis is approximately 1.14% of patients, but is rising due to increasing recognition by clinicians.1 Although ICI myocarditis is uncommon, it has a high rate of mortality with reports between 40% to 50% of cases.2, 3, 4
ICI myocarditis typically occurs within the early phase of initiating therapy (<90 days).5 The median time of onset for ICI myocarditis has been reported to be 34 days after initiation of ICI therapy, and most commonly after the first or third infusion.1 The latest presentation of ICI myocarditis reported in literature occurred at approximately 500 days after initiating ICI.1 In our patient, ICI myocarditis occurred at 987 days (141 weeks) after initiating ICI and after receiving 52 infusions of pembrolizumab, which is more than twice the length of time previously reported. Additionally, she had been off ICI for 33 weeks prior to the development of ICI myocarditis, the longest time reported in literature to our knowledge. Delayed immune-related events occurring after discontinuation of ICI therapy are becoming a recognized phenomenon.6 Importantly, for cardiac irAE, these delayed immune-related events have been associated with higher rates of left ventricular systolic dysfunction and heart failure, highlighting the importance of earlier recognition and diagnosis.5 Prospective troponin surveillance screening for ICI myocarditis has successfully detected early cases, and implementation remains an open area of investigation for earlier detection of cardiotoxicity.7
Active hypotheses for the pathophysiology behind ICI myocarditis include T cells targeting cardiac-specific antigens or shared antigens between tumor and heart. Common antigens have been demonstrated by sequencing of CDR3 regions of T-cell receptors, revealing shared sequences between tumor and cardiac and skeletal muscle.8 With our patient’s defective DNA mismatch repair genes (Lynch syndrome and germline MSH2 mutation), frameshift mutations in tumor generate a high burden of neoantigens, which results in a more robust T-cell response.9 Although specific mechanistic studies are necessary, it is plausible that just as neoantigens enhance immunogenicity against tumor, a neoantigen with cross-reactivity to myocardium could stimulate a profound autoimmune response in the heart. Because defective DNA repair continually creates neoantigens, prolonged T-cell stimulation occurs, which may allow for a late-onset autoimmunity. It is possible that our patient had also developed low-grade, subclinical myocarditis around this time, which may have been detected with further cardiac work-up. Our patient had also experienced autoimmune thyroiditis early in treatment, suggestive of a predisposition to autoimmunity that became unmasked by checkpoint inhibition. Understanding the mechanisms of ICI myocarditis can inform long-term risk stratification as well as potential therapies against ICI myocarditis. In patients who achieve control of tumor from immunotherapy, it is important to recognize late-onset cases of ICI myocarditis, and the role of extended vigilance for irAE requires further investigation.
Our case emphasizes the importance of recognizing that ICI myocarditis can present as a delayed irAE after discontinuation of ICI therapy. Although fulminant ICI myocarditis is a well-documented phenomenon, diagnosing it involves a high index of suspicion because presenting symptoms vary widely, and may range from nonspecific symptoms such as fatigue and myalgia, to malignant arrhythmias and cardiogenic shock. This case highlights the importance of awareness, developing early clinical suspicion, and rapid detection of delayed-onset ICI myocarditis to avoid decompensation to fulminant myocarditis. As the indications for immunotherapy continue to grow, recognizing delayed-onset ICI myocarditis will be important as life expectancy after cancer treatment continues to improve.
Funding Support and Author Disclosures
Dr Zhu is supported by the Sarnoff Scholar Career Development Award through the Sarnoff Cardiovascular Research Foundation. Dr Berry has received a speaker honorarium from Merck Pharmaceuticals. Dr Witteles has served on advisory boards for Pfizer, Alnylam, Eidos, Ionis, Intelia, and Janssen. Dr Le has served on advisory boards for Merck, Bristol Myers Squibb, and Janssen; has received research funding from Merck, Bristol Myers Squibb, Aduro Biotech, Curegenix, Medivir, Nouscom, and Abbvie; has received speaking honoraria from Merck; and is an inventor of licensed intellectual property from and managed by Johns Hopkins University. Dr Wu has received research funding from Sanofi; has a patent on induced pluripotent stem cell cardiomyocytes; and has received other expenses from Amgen. Dr Fisher has served on advisory boards for Merck and Genentech and Data Safety Monitoring Boards for Astra Zeneca, Hutchison Pharma and CytomX. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Tomas G. Neilan, MD, MPH, served as Guest Associate Editor. Kathryn J. Ruddy, MD, MPH, served as Guest Editor in Chief.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Mahmood S.S., Fradley M.G., Cohen J.V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D.Y., Salem J.E., Cohen J.V., et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moslehi J.J., Salem J.E., Sosman J.A., Lebrun-Vignes B., Johnson D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Kindi S.G., Oliveira G.H. Reporting of immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;392:382–383. doi: 10.1016/S0140-6736(18)31542-3. [DOI] [PubMed] [Google Scholar]
- 5.Dolladille C., Ederhy S., Allouche S., et al. Late cardiac adverse events in patients with cancer treated with immune checkpoint inhibitors. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couey M.A., Bell R.B., Patel A.A., et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. doi: 10.1186/s40425-019-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waliany S., Neal J.W., Reddy S., et al. Myocarditis surveillance with high-sensitivity troponin I during cancer treatment with immune checkpoint inhibitors. J Am Coll Cardiol CardioOnc. 2021;3:137–139. doi: 10.1016/j.jaccao.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson D.B., Balko J.M., Compton M.L., et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahin I.H., Akce M., Alese O., et al. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br J Cancer. 2019;121:809–818. doi: 10.1038/s41416-019-0599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]