Central Illustration
Key Words: heart failure, immunotherapy, myocarditis
Abbreviations and Acronyms: ACR, acute cellular rejection; CNI, calcineurin inhibitor; CTLA-4, cytotoxic T-lymphocyte-associated antigen-4; EMB, endomyocardial biopsy; ICI, immune checkpoint inhibitor; ISHLT, International Society for Heart and Lung Transplantation; LVEF, left ventricular ejection fraction; mTOR, mammalian target of rapamycin; NT-proBNP, N-terminal pro–B-type natriuretic peptide; PD-1, programmed cell death protein-1; PET, positron emission tomography; SOT, solid organ transplant; TTE, transthoracic echocardiogram
Immune checkpoint inhibitors (ICIs) are a transformative group of monoclonal antibodies that target immune regulatory cell-surface proteins and disinhibit antitumor T-cell response. The cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitor ipilimumab and the programmed cell death protein-1 (PD-1) inhibitors nivolumab and pembrolizumab are the most widely used ICIs among those available. Although effective, ICIs are associated with a range of immune-related adverse effects that may affect any organ. ICI-related myocarditis occurs in 0.06% to 1% of patients treated with ICIs and carries a mortality rate of up to 40%.1
Patients with solid organ transplants (SOT) are at a significantly higher risk of developing de novo malignancies through their use of chronic immunosuppressive therapy. However, there are scant data on the use of ICIs in this population as they were excluded from the major clinical trials due to concerns that alloreactive T-cell activation may also promote loss of graft tolerance.
Here, we present 3 orthotopic heart transplant recipients that later developed cancer and were treated with ICIs as per our institutional protocol. As per our protocol, we monitor for allograft rejection and ICI-related myocarditis with weekly troponin levels as well as serum N-terminal pro hormone B-type natriuretic peptide (NT-proBNP), transthoracic echocardiogram (TTE), and endomyocardial biopsy (EMB) 1-2 weeks after each cycle of treatment. If all are stable after 3 months, TTE and EMB intervals are extended to 6-week intervals and then to every 3 months if no pathology is identified after 6 months. If there is no evidence of allograft rejection or ICI-related myocarditis by 12 months, patients return to their baseline monitoring protocols. Of note, to our knowledge, this is the first reported use of the PD-1 inhibitor cemiplimab in this population.
Patient 1
A 74-year-old man received an orthotopic heart transplant in 2010 for a familial dilated cardiomyopathy. In 2012, he developed a right temporal cutaneous squamous cell carcinoma that was treated with wide local excision, but had numerous local and nodal recurrences from 2017 to 2020 requiring extensive surgery and radiotherapy. In late 2020, a right middle lobe lung lesion was identified on surveillance positron emission tomography (PET) scan and was presumed to be metastatic squamous cell carcinoma. He received stereotactic radiotherapy and was commenced on cemiplimab every 3 weeks, which was stretched to every 4 weeks after 13 cycles. His troponin, NT-proBNP, and left ventricular ejection fraction (LVEF) remained within normal limits, and no significant allograft rejection was detected on serial EMB. He has received 19 cycles thus far with an excellent clinical response.
Patient 2
A 59-year-old woman received an orthotopic heart transplant in 2009 for an idiopathic dilated cardiomyopathy. In the decade following transplantation, she had no significant episodes of allograft rejection. In late 2018, she was diagnosed with pseudomyxoma peritonei for which she received hyperthermic intraperitoneal chemotherapy and cytoreductive surgery with good clinical response. Two years later, she was diagnosed with a scalp melanoma (BRAF/NRAS wild-type) with several in-transit metastases and fluorodeoxyglucose (FDG)-avid left supraclavicular lymph nodes on PET scan. The primary lesion and satellites were treated with wide local excision, and she underwent radiotherapy to her scalp, parotid gland, and neck. PET scan 3 months later identified a large central liver lesion that was confirmed on biopsy to be a metastatic melanoma. She initiated treatment with 6-week–interval dosing of pembrolizumab and transitioned from tacrolimus to everolimus. She received 8 cycles of pembrolizumab, during which time the melanoma demonstrated excellent clinical response with complete metabolic remission by late 2020. Six months after starting pembrolizumab, surveillance EMB demonstrated a moderate interstitial lymphocytic infiltration composed predominantly of CD8+ lymphocytes with lesser numbers of CD4+ T lymphocytes. Although it was noted that a CD8+-dominant lymphocytic myocardial infiltrate may be seen as a complication of ICI therapy, moderate acute cellular rejection (ACR) (International Society for Heart and Lung Transplantation [ISHLT] Grade 2R) was considered equally likely. C4d immunoreaction was negative. Subsequent workup revealed an everolimus level of 1.6 μg/L, troponin I of 60 ng/L (normal level <16 ng/L), and an NT-proBNP of 2,176 ng/L (normal level <900 ng/L). TTE was unremarkable. Pembrolizumab was withheld, she was treated with pulsed intravenous methylprednisolone, and everolimus dose was increased. She had biochemical and histological improvement in her presumed allograft rejection, and pembrolizumab was recommenced 1 month later. She had no subsequent abnormal EMBs but died later that year following complications from her pseudomyxoma peritonei.
Patient 3
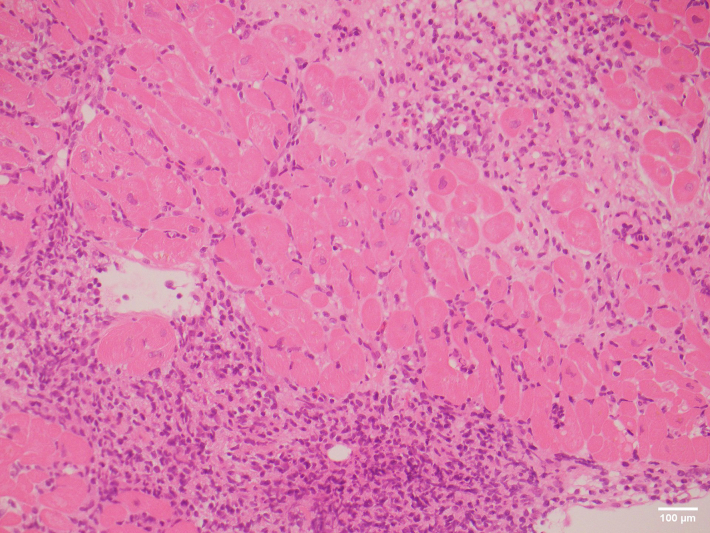
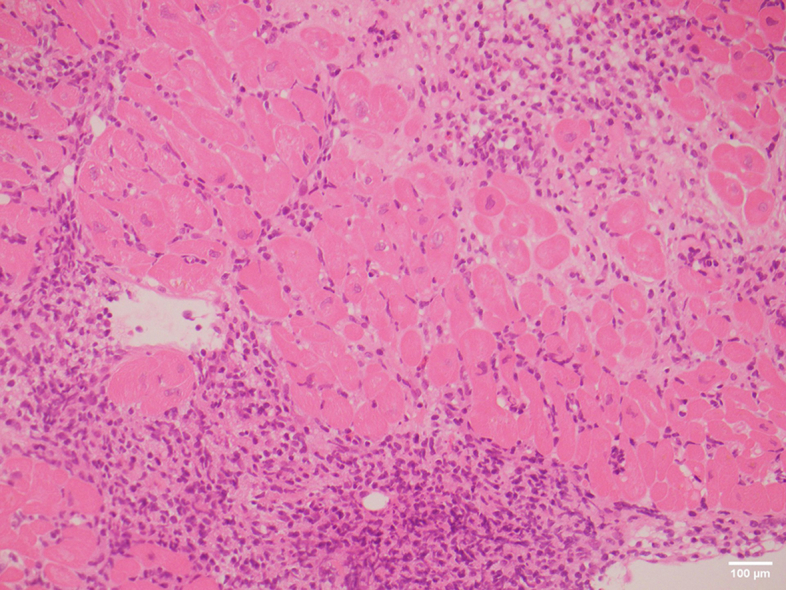
A 65-year-old man received an orthotopic heart transplant in 2018 for arrhythmogenic right ventricular cardiomyopathy. His initial immunosuppression regimen included everolimus, tacrolimus, mycophenolate mofetil, and prednisolone. He had a single episode of moderate ACR (ISHLT Grade 2R) diagnosed on routine EMB 6 months post-transplant in the context of subtherapeutic tacrolimus levels. In January 2020, he was diagnosed with a left calf melanoma (NRAS Q61R mutant). The following month, he developed numerous cutaneous and subcutaneous in-transit metastases in his left leg, left groin, suprapubic area, and right thigh. PET scan demonstrated FDG-avid inguinal and external iliac lymph nodes that fine needle aspirate confirmed to be metastatic melanoma. He received radiotherapy to all affected areas, as well as 2 cycles of temozolomide. Restaging PET in June 2020 demonstrated progression of subcutaneous and lymph node disease, as well as liver metastases. His tacrolimus dose was reduced, and he was commenced on ipilimumab. One week later, surveillance EMB demonstrated a florid endocardial and myocardial inflammatory cell infiltrate composed of CD8+ T lymphocytes, predominantly with histiocytes and an occasional eosinophil, that formed localized clusters in the interstitium with scant areas of myocyte loss (Figures 1 and 2). This was consistent with severe ICI-related myocarditis. The differential diagnosis, thought to be less likely on pathological grounds, was ACR although the changes seen were difficult to classify in terms of those expected for ISHLT Grade 2R or Grade 3R ACR. Further investigation revealed an everolimus level of 2.1 μg/L, tacrolimus level of 1.1 μg/L, troponin I of 17 ng/L (normal level <26 ng/L), and an LVEF of 59% on TTE. Ipilimumab was ceased, and he was treated with pulsed methylprednisolone. The patient was not rechallenged with an ICI, and a palliative approach was taken for the remainder of his care. He died 2 months later from a community-acquired pneumonia.
Figure 1.
Hematoxylin and Eosin–Stained Endomyocardial Biopsy
Sections showed extensive sheet infiltration of the interstitium by lymphocytes and histiocytes with an occasional eosinophil.
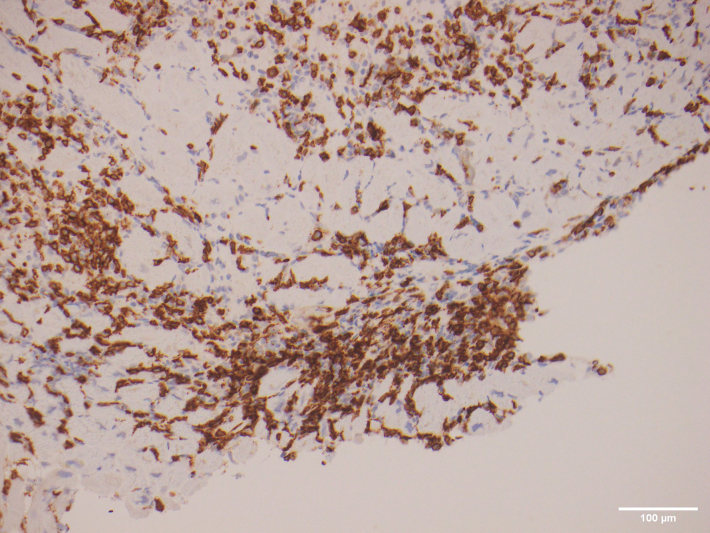
Figure 2.
Immunoperoxidase-Stained Endomyocardial Biopsy
Sections showed infiltrating lymphocytes that were predominantly CD8+.
Discussion
The theoretical risk of ICI-associated allograft rejection in patients with SOT appears to translate clinically based on case report data. A recent systematic review of 86 case reports of patients with SOT treated with ICIs found that 33 patients (39.8%) developed biopsy-proven allograft rejection after commencing ICI therapy, and of these, 71% went on to develop end-stage organ failure.2 The implications of this differ between organ types, with cardiac allograft failure likely to be a life-limiting complication, compared with renal allograft failure where organ support with hemodialysis is available. As such, ICIs should only be considered in heart transplant recipients when no other viable cancer treatment options are available.
There have been 12 cases of ICI use in heart transplant recipients reported in the literature. All received PD-1 inhibitor monotherapy, and 4 (33.3%) developed allograft rejection.3, 4, 5, 6, 7, 8 There are few documented cases of combination PD-1 and CTLA-4 inhibitor therapy in SOT recipients, despite it being first-line treatment in several cancer subtypes, including metastatic melanoma. Patients 2 and 3, as detailed in the preceding text, were treated with monotherapy due to theoretical concern for an increased risk of allograft rejection with a second agent. Current evidence is conflicting on the comparative risk of allograft rejection with PD-1 and CTLA-4 inhibitors as mono- or sequential therapy in this context.2 Appropriate patient selection may help mitigate some of this risk, with increasing duration since transplant and previous episodes of rejection having been identified as potential risk factors for allograft rejection with ICI use.2
Prophylactic up-titration of immunosuppression may also help reduce the risk of rejection; however, the inverse relationship between immunosuppression and tumor response must be considered.7 ICI therapy is dependent on an effective T-cell response that is dampened by the immunosuppressive agents commonly used in heart transplant. In a systematic review of 39 SOT patients who received ICIs,9 those that received single-agent calcineurin inhibitor (CNI) therapy had the lowest rate of allograft rejection (11%) of any immunosuppression regimen, but also the lowest tumor response (25%). Conversely, single-agent corticosteroid regimens in this context have resulted in the greatest tumor response, but the highest rate of allograft rejection.9 Mammalian target of rapamycin (mTOR) inhibitors, which inhibit IL-2 further downstream than CNIs and corticosteroids, offer some promise. In vitro and case report data suggest that mTOR inhibitors both promote CD4 T-cell proliferation and maintain T regulatory cell function, thereby enabling the ICI antitumor response while preserving allograft tolerance, respectively. With the available data, we suggest that a combination immunosuppressive therapy regimen that includes an mTOR inhibitor and corticosteroids, without a CNI, may balance tumor response and allograft rejection most effectively.
Differentiating cardiac allograft rejection from ICI-related myocarditis can be difficult as they are mechanistically and clinically similar. If low immunosuppression levels are detected at the time of abnormal EMB, as was the case with Patient 2, this may make allograft rejection more likely. EMB has potential use in differentiating ICI-related myocarditis from ICI-associated rejection. ICI-related myocarditis is classically associated with an abundance of CD8+ T cells interspersed with CD4+ T cells and macrophages,1,10 though most documented cases have been either severe or fulminant, with less known about the histopathology in lower grade disease. Conversely, lower grades (ISHLT Grade 1R or 2R) of ICI-associated allograft rejection are usually perivascular and CD4+ lymphocyte predominant, with or without myocyte fiber scalloping by lymphocytes. This process is thus distinguishable from ICI-related myocarditis. However, at higher grades (ISHLT Grade 3R), the extent of lymphocyte infiltration, myocyte destruction, and relative increase in CD8+ lymphocytes and histiocytes makes accurate distinction less likely on pathology grounds alone. With careful clinical correlation, we suggest that if broad clusters of interstitial CD8+ lymphocytes and histiocytes are present, in the absence of widespread interstitial perivascular neutrophils, edema, and red cell extravasation, a diagnosis of ICI-related myocarditis can be made. Larger-scale quantitative analysis comparing the relative amounts of CD4+ and CD8+ T cells on EMB in higher grade disease may help delineate these diagnoses in future.
We have outlined our monitoring protocol and highlighted some of the challenges of differentiating cardiac allograft rejection from ICI-related myocarditis. More research is needed to determine the optimal immunosuppression regimen for these patients, as well as to identify the patients at greatest risk of developing these potentially fatal complications.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Johnson D.B., Balko J.M., Compton M.L., et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.d'Izarny-Gargas T., Durrbach A., Zaidan M. Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: a systematic review. Am J Transplant. 2020;20:2457–2465. doi: 10.1111/ajt.15811. [DOI] [PubMed] [Google Scholar]
- 3.Qin R., Salama A.K. Report of ipilimumab in a heart transplant patient with metastatic melanoma on tacrolimus. Melanoma Manag. 2015;2:311–314. doi: 10.2217/mmt.15.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gastman B., Ernstoff M. Tolerability of immune checkpoint inhibition cancer therapy in a cardiac transplant patient. Ann Oncol. 2016;27:2304–2305. doi: 10.1093/annonc/mdw293. [DOI] [PubMed] [Google Scholar]
- 5.Kittai A.S., Oldham H., Cetnar J., Taylor M. Immune checkpoint inhibitors in organ transplant patients. J Immunother. 2017;40:277–281. doi: 10.1097/CJI.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 6.Grant M.J., DeVito N., Salama A.K. Checkpoint inhibitor use in two heart transplant patients with metastatic melanoma and review of high-risk populations. Melanoma Manag. 2018;5:MMT10. doi: 10.2217/mmt-2018-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daud A., Mehra M.R., Siu A., et al. Immune checkpoint inhibitors in heart or lung transplantation: Early results from a registry initiative. J Heart Lung Transplant. 2020;39:604–606. doi: 10.1016/j.healun.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Owonikoko T.K., Kumar M., Yang S., et al. Cardiac allograft rejection as a complication of PD-1 checkpoint blockade for cancer immunotherapy: a case report. Cancer Immunol Immunother. 2017;66:45–50. doi: 10.1007/s00262-016-1918-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel-Wahab N., Safa H., Abudayyeh A., et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer. 2019;7:106. doi: 10.1186/s40425-019-0585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atallah-Yunes S.A., Kadado A.J., Kaufman G.P., Hernandez-Montfort J. Immune checkpoint inhibitor therapy and myocarditis: a systematic review of reported cases. J Cancer Res Clin Oncol. 2019;145:1527–1557. doi: 10.1007/s00432-019-02927-x. [DOI] [PubMed] [Google Scholar]