Abstract
Background
Myocarditis is a dreaded and unpredictable complication of immune checkpoint inhibitors (ICI). We sought to determine whether routinely measured biomarkers could be helpful in monitoring for ICI myocarditis.
Objectives
The authors examined biomarker trends of patients on ICI and their association with the incidence of ICI myocarditis and outcomes.
Methods
We conducted an observational cohort study of adults who received at least one dose of ICI at Michigan Medicine between June 2014 and December 2021 and underwent systematic serial testing for aspartate aminotransferase (AST) and alanine aminotransferase (ALT), creatine phosphokinase (CPK), and lactate dehydrogenase during ICI therapy.
Results
Among 2,606 patients (mean age 64 ± 13 years; 60.7% men), 27 (1.0%) were diagnosed with ICI myocarditis. At diagnosis, patients with myocarditis had an elevated high-sensitivity troponin T (100%), ALT (88.9%), AST (85.2%), CPK (88.9%), and lactate dehydrogenase (92.6%). Findings were confirmed in an independent cohort of 30 patients with biopsy-confirmed ICI myocarditis. A total of 95% of patients with ICI myocarditis had elevations in at least 3 biomarkers compared with 5% of patients without myocarditis. Among the noncardiac biomarkers, only CPK was associated (per 100% increase) with the development of myocarditis (HR: 1.83; 95% CI: 1.59-2.10) and all-cause mortality (HR: 1.10; 95% CI: 1.01-1.20) in multivariable analysis. Elevations in CPK had a sensitivity of 99% and specificity of 23% for identifying myocarditis.
Conclusions
ICI myocarditis is associated with changes in AST, ALT, and CPK. An increase in noncardiac biomarkers during ICI treatment, notably CPK, should prompt further evaluation for ICI myocarditis.
Key Words: ALT, AST, biomarkers, CPK, immune checkpoint inhibitor, immunotherapy, myocarditis, troponin
Abbreviations and Acronyms: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatine phosphokinase; hsTnT, high-sensitivity troponin T; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; LDH, lactate dehydrogenase; ULN, upper limit of normal
Central Illustration
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of various malignancies.1 ICIs are monoclonal antibodies that target either cytotoxic T lymphocyte antigen 4, programmed cell death protein 1, or programmed cell death ligand 1 and modulate the host’s immune response against cancerous cells.2 The development and widespread use of ICIs have led to improved outcomes with a generally better tolerated side-effect profile compared with other therapies.3,4
Immune-related adverse events (irAEs), while infrequent, are a direct result of the mechanism of action of ICIs, with disinhibition of T cells leading to a complex cascade of dysregulation of immune self-tolerance that can occur in almost any organ system.5 The most common irAEs include dermatitis, myositis, colitis, and endocrinopathies such as thyroiditis.4,6 Myocarditis is the most severe cardiovascular manifestation of irAEs, with an estimated incidence of 1% to 2%.5,7, 8, 9, 10, 11 Presentations of ICI myocarditis range from asymptomatic elevation in cardiac biomarkers found on routine screening and electrocardiogram changes to fulminant heart failure and complete heart block leading to death.8,9 Despite its low incidence, ICI myocarditis has one of the highest fatality rates of all classes of irAEs, with a reported mortality upwards of 50%, highlighting the critical need for early diagnosis and management strategies.4,12, 13, 14, 15
Monitoring for and diagnosing ICI myocarditis is challenging. The current practice of monitoring for irAEs relies on serial measurement of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), creatine phosphokinase (CPK), and lactate dehydrogenase (LDH) levels during ICI therapy. Whether changes in these biomarker levels can identify patients with possible ICI myocarditis is unclear. To address this question, we leveraged the University of Michigan ICI registry, a large observational cohort of over 2,600 patients who received ICI for the treatment of various malignancies and in whom granular data were systematically collected, including demographics and clinical characteristics, presenting symptoms, cardiovascular testing, longitudinal biomarker data, and outcomes.
Methods
Study design and population
We conducted an observational cohort study of all adult (≥18 years of age) patients who had received treatment with single or dual ICI at Michigan Medicine between June 2014 and December 2021. Patients were included if they had received at least 1 dose of ICI (ipilimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, avelumab, or cemiplimab) for the treatment of any malignancy (n = 2,606). Data of patients on ICI therapy who did not have myocarditis were retrospectively collected (n = 2,579). These patients were identified using DataDirect, a self-serve tool which allows access to clinical data, including medication administration data, from all patients who received healthcare at Michigan Medicine since 2005.
Patients diagnosed with ICI myocarditis were prospectively collected as part of the University of Michigan ICI Cardiotoxicity registry established in 2018 (n = 25). In addition, we reviewed medical records of all patients on ICI therapy who had at least 1 measured high-sensitivity troponin T (hsTnT) level above the 99th upper reference limit of normal, or who had the term “myocarditis” in any document to identify additional cases of myocarditis (n = 2). We replicated findings in an independent cohort of 30 patients with definite ICI myocarditis from the University Hospitals of Pitié-Salpêtrière (Assistance Publique–Hôpitaux de Paris; Sorbonne, Paris, France) between 2018 and 2020.
Approval for the study was obtained from the respective Institutional Review Boards of the University of Michigan and University Hospitals of Pitié-Salpêtrière under waivers of informed consent.
Definitions and data collection
Patients were identified as having myocarditis if a clinical diagnosis was made by the cardiovascular consult team. For the purpose of this study, we classified myocarditis as definite, probable, or possible based on previously published definitions (Supplemental Table 1).13,16 We collected cancer-specific variables included the primary cancer diagnosis requiring ICI treatment and the ICI treatment regimen with average total dose of each ICI received per person. Demographic data and clinical characteristics, treatment course, and longitudinal biomarker data including ALT, AST, CPK, LDH, and hsTnT levels were collected. ALT, AST, CPK, and LDH are measured per institutional clinical guidelines on a weekly basis in all patients receiving ICI at Michigan Medicine for the duration of ICI therapy. For the myocarditis cases, we collected data on clinical presentation, presence of additional irAEs, biomarkers, electrocardiograms, echocardiography, treatments administered for myocarditis, and in-hospital outcomes. Systematic postdischarge serial hsTnT measurements were performed in patients with ICI myocarditis as part of a protocol implemented in January 2021. IrAEs were defined according to American Society of Clinical Oncology’s clinical guidelines and graded according to the Common Terminology Criteria for Adverse Events.17 All grade I to IV Common Terminology Criteria for Adverse Events events were included. Immune-related hepatitis was defined as an asymptomatic or symptomatic elevated serum AST/ALT to greater than the upper limit of normal (ULN) with or without a change in bilirubin. Myositis was defined based on the presence of muscle weakness and/or pain with elevated muscle enzymes (ie, CPK). We collected data on all-cause mortality for all patients through review of the electronic medical record in addition to the National Death Index, with the last known alive time based on the date of the latest follow-up visit. Data were entered into REDCap (Research Electronic Data Capture), a secure HIPAA (Health Information Portability and Accountability Act)-compliant Web-based application, using a standardized data collection form.
Statistical analysis
Categorical variables were summarized as counts and percentages and compared using Fisher exact or chi-square tests. Continuous variables were summarized as mean ± SD or median (IQR) for normally and non-normally distributed data, respectively. Continuous data were compared using unpaired t tests or Mann-Whitney U tests dependent on normality. We calculated the cumulative incidence of myocarditis as the number of myocarditis cases per the total number of patients who received ICIs between 2014 and 2021. The overall incidence rate of myocarditis (cases per 10,000 person-years) and 95% CI was calculated with follow-up time beginning at the start of ICI therapy. We additionally calculated the yearly incidence of myocarditis. To identify risk factors for myocarditis, we compared demographic and clinical variables between patients with and without myocarditis using standard summary statistics, as myocarditis often occurs shortly after beginning ICI treatment, with the vast majority of patients developing myocarditis within 60 days. Although the competing risk of death was unlikely given the short timeline of development of myocarditis, we also examined the association between clinical characteristics and incident myocarditis using univariable Cox proportional hazards models as a sensitivity analysis to estimate HRs and 95% CIs.
We compared biomarker trends (ALT, AST, CPK, LDH) for myocarditis cases and control subjects by plotting the average biomarker concentrations over 12 months following initiation of ICI therapy. To characterize changes in biomarker levels relative to the time of myocarditis diagnosis, we plotted the average biomarker concentrations 30 days prior and after the development of myocarditis using locally weighted scatterplot smoothing 95% CIs with the ggplot2 package in RStudio (Version 2022.02.0). We additionally plotted longitudinal hsTnT levels for myocarditis cases (n = 10) who underwent serial hsTnT measurements postdiagnosis of myocarditis as of January 2021.
We assessed the association between longitudinal changes in biomarker levels (ALT, AST, CPK, and LDH) and time to myocarditis and all-cause mortality using partly conditional survival models to calculate HRs and 95% CIs.18 Multivariable models were adjusted for age, sex, body mass index, smoking history, and history of hypertension, type 2 diabetes, heart failure, and coronary artery disease. The proportional hazards assumption was evaluated using Schoenfeld residuals for time-varying biomarker changes. We also examined the association between the maximum value of each biomarker during ICI therapy and all-cause mortality. The maximum value of each biomarker was categorized as normal, 1× to 1.99× the ULN, 2.0× to 3× the ULN, and >3× the ULN. We calculated the sensitivity and specificity of each separate biomarker for identifying myocarditis using the following cutoff values: greater than the ULN, >2× the ULN, >3× the ULN, and >4× the ULN. A 2-sided P value <0.05 was used to determine statistical significance. All analyses were performed using R version 4.1.0 (R Foundation for Statistical Computing).
Results
Clinical characteristics and outcomes
A total of 2,606 adult patients (mean age 64 ± 13 years; 60.7% men) received at least 1 dose of ICI between June 2014 and December 2021. Of these patients, 27 (1.0%) or 0.14 (95% CI: 0.09-0.21) cases per 10,000 person-years had a diagnosis of myocarditis attributed to ICI therapy, of which 5 cases were classified as definite, 4 as probable, and 18 as possible (Supplemental Table 2). The overall number of patients who received ICI therapy and the incidence of ICI myocarditis increased steadily each year from 2014 to 2021 (Supplemental Figure 1). The median time from the first dose of ICI to diagnosis of myocarditis was 28.0 (range, 1-209) days, with 15 (55.6%) patients developing myocarditis within 30 days after the start of ICI therapy.
Compared with patients without myocarditis, patients with myocarditis were more likely to be older (mean age 67 vs 64 years; P = 0.016), be male (74.1% vs 60.5%; P = 0.043), and have a higher prevalence of coronary artery disease (44.4% vs 19.0%; P = 0.004) and heart failure (29.6% vs 9.8%; P = 0.003) (Table 1). There were no significant differences in levels of ALT, AST, LDH, or CPK prior to ICI therapy (Table 1). Results were similar when examining time to myocarditis in unadjusted Cox proportional hazards modeling (Supplemental Table 3). The most common diagnoses among patients treated with ICIs were malignant melanoma (40.4%) and metastatic lung cancer (24.5%), which were in similar proportions between patients with and without ICI myocarditis (Table 1). Pembrolizumab monotherapy was administered in 50.3% of patients, and 14.6% received dual ICI therapy. Of the patients that developed myocarditis, none completed their initially planned treatment. Two (7.4%) patients resumed treatment after myocarditis after a median of 1.3 (IQR: 0.4-1.9) years. Most patients with myocarditis had evidence of noncardiac irAEs (88.9%) such as a clinical diagnosis of hepatitis, myositis, or elevated AST, ALT, and CPK (Supplemental Tables 4 and 5). Of the 24 patients with elevated AST/ALT or CPK, 9 (37.5%) had a clinical diagnosis of hepatitis and 12 (50.0%) had clinically significant myositis. Details on the course of the hospitalization for ICI myocarditis and treatments are provided in Supplemental Tables 4 to 6. In-hospital mortality of patients with ICI myocarditis was 22.2%.
Table 1.
Clinical Characteristics of Patients With ICI Myocarditis and Control Subjects
| Patients With Myocarditis (n = 27) | Control Subjects (n = 2,579) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age at the start of ICI, y | 67.4 ± 17.5 | 63.5 ± 13.2 | 0.016 |
| Male | 20 (74.1) | 1,560 (60.5) | 0.043 |
| Race | 0.92 | ||
| Caucasian | 27 (100.0) | 2,348 (91.0) | |
| African American | 0 (0.0) | 104 (4.0) | |
| Asian | 0 (0.0) | 37 (1.4) | |
| American Indian or Alaska Native | 0 (0.0) | 7 (0.3) | |
| Other | 0 (0.0) | 53 (2.1) | |
| Unknown | 0 (0.0) | 22 (0.9) | |
| Ethnicity | 0.92 | ||
| Non-Hispanic or Latino | 27 (100.0) | 2,490 (96.5) | |
| Hispanic or Latino | 0 (0.0) | 32 (1.2) | |
| Unknown | 0 (0.0) | 56 (2.1) | |
| Past medical history | |||
| Hypertension | 15 (55.6) | 1,603 (62.2) | 0.78 |
| Hyperlipidemia | 14 (51.9) | 1,158 (44.9) | 0.77 |
| Type 2 diabetes mellitus | 7 (25.9) | 590 (22.9) | 0.93 |
| Peripheral vascular disease | 3 (11.1) | 161 (6.2) | 0.58 |
| Coronary artery disease | 12 (44.4) | 490 (19.0) | 0.004 |
| Heart failure | 8 (29.6) | 253 (9.8) | 0.003 |
| Stroke | 4 (14.8) | 238 (9.2) | 0.61 |
| Atrial flutter/fibrillation | 7 (25.9) | 366 (14.2) | 0.22 |
| Cardiovascular risk factors | |||
| History of smoking | 17 (63.0) | 1,330 (51.6) | 0.57 |
| BMI, kg/m2 | 29.1 ± 6.4 | 28.1 ± 6.4 | 0.84 |
| Cancer diagnoses | |||
| Melanoma | 13 (48.1) | 1,040 (40.3) | 0.41 |
| Lung cancer | 6 (22.2) | 632 (24.5) | 0.78 |
| Urothelial | 3 (11.1) | 400 (15.5) | 0.53 |
| Kidney | 1 (3.7) | 218 (8.5) | 0.38 |
| Gastric | 1 (3.7) | 44 (1.7) | 0.42 |
| Hepatocellular carcinoma | 1 (3.7) | 81 (3.1) | 0.87 |
| Other | 3 (11.1) | 158 (6.1) | 0.28 |
| Treatment | |||
| Ipilimumab + nivolumab | 5 (18.5) | 375 (14.5) | 0.58 |
| Ipilimumab | 0 (0.0) | 151 (6.2) | 0.40 |
| Pembrolizumab | 16 (59.3) | 1,294 (51.6) | 0.44 |
| Nivolumab | 5 (18.5) | 568 (30.4) | 0.82 |
| Atezolizumab | 0 (0.0) | 255 (10.0) | 0.10 |
| Durvalumab | 1 (3.7) | 72 (2.8) | 0.55 |
| Avelumab | 0 (0.0) | 0 (0.0) | 0.99 |
| Cemiplimab | 0 (0.0) | 0 (0.0) | 0.99 |
| Number of ICI infusions | 2.41 ± 2.32 | 8.71 ± 9.55 | <0.001 |
| ICI dose, total mg | |||
| Ipilimumab | 400 (340-1,040) | 1,023 (633.5-1,600) | 0.10 |
| Pembrolizumab | 400 (200-600) | 1,000 (400-2,400) | <0.001 |
| Nivolumab | 480 (240-720) | 1,200 (675-2,400) | 0.013 |
| Atezolizumab | — | 4,800 (2,400-9,600) | — |
| Durvalumab | 1,480 (1,480-1,480) | 7,820 (3,915-16,120) | 0.13 |
| Baseline laboratory values | |||
| CPK, IU/L | 74 (46-127) | 69 (44-120) | 0.25 |
| ALT, IU/L | 22 (16-33) | 23 (18-33) | 0.96 |
| AST, IU/L | 25 (20-33) | 22 (21-28) | 0.47 |
| LDH, IU/L | 287 (207-361) | 209 (175-275) | 0.09 |
Values are mean ± SD, n (%), median (IQR). Control subjects are defined as patients who did not develop myocarditis before death or censoring at last known follow-up. All therapies are included in counts; patients could have received more than 1 type of therapy.
ALT = alanine aminotransferase; AST = aspartate aminotransferase; BMI = body mass index; CPK = creatine phosphokinase; ICI = immune checkpoint inhibitor; LDH = lactate dehydrogenase.
A total of 1,212 (46.5%) patients died during follow-up over a median of 1.5 (IQR: 0.5-2.8) years, or 6.3 (95% CI: 6.0-6.7) deaths per 10,000 person-years. Kaplan-Meier survival estimates at year 1 were 50% (95% CI: 33%-73%) and 66% (95% CI: 64%-68%) for patients who did and did not develop myocarditis, respectively (log-rank P = 0.10). In a time-dependent Cox model adjusting for age, sex, body mass index, smoking, hypertension, coronary artery disease, and heart failure, the occurrence of myocarditis was associated with a higher risk of all-cause death (HR: 1.66; 95% CI: 1.01-2.74). However, patients who survived the myocarditis hospitalization had similar long-term survival compared with patients without ICI myocarditis (Supplemental Figure 2).
Biomarker trends after ICI therapy initiation
We noted differences in the trends of biomarker levels between patients who had myocarditis and those that did not. Approximately 10.0% to 30.0% of patients without myocarditis had at least one elevated level of ALT (30.0%), AST (30.0%), LDH (15.1%), or CPK (10.7%) during the first 12 months of therapy, with average peak concentrations much lower than in patients who developed myocarditis (Table 2). Only 128 (4.9%) of patients without myocarditis had elevations in at least 3 biomarkers during the first 12 months of therapy.
Table 2.
Biomarker Elevations During 12 Months of ICI Therapy
| Patients With Myocarditis (n = 27) |
Control Subjects (n = 2,579) |
|||||
|---|---|---|---|---|---|---|
| Elevated | Time to First Elevated Biomarker (d) | Average Peak Level (IU/L) | Elevated | Time to First Elevated Biomarker (d) | Average Peak Level (IU/L) | |
| ALT | 24 (88.9) | 23 (9-36) | 162 ± 410 | 774 (30.0) | 28 (11-84) | 61 ± 112 |
| AST | 23 (85.2) | 21 (9-33) | 84 ± 110 | 776 (30.0) | 22 (0-77) | 46 ± 60 |
| LDH | 25 (92.6) | 24 (21-26) | 329 ± 186 | 390 (15.1) | 22 (0-84) | 317 ± 141 |
| CPK | 24 (88.9) | 21 (21-24) | 489 ± 610 | 275 (10.7) | 63 (20-217) | 152 ± 204 |
Values are n (%), median (IQR), or mean ± SD. Reference values: ALT, 49 IU/L; AST, 34 IU/L; LDH, 240 IU/L; CPK, 240 IU/L.
Abbreviations as in Table 1.
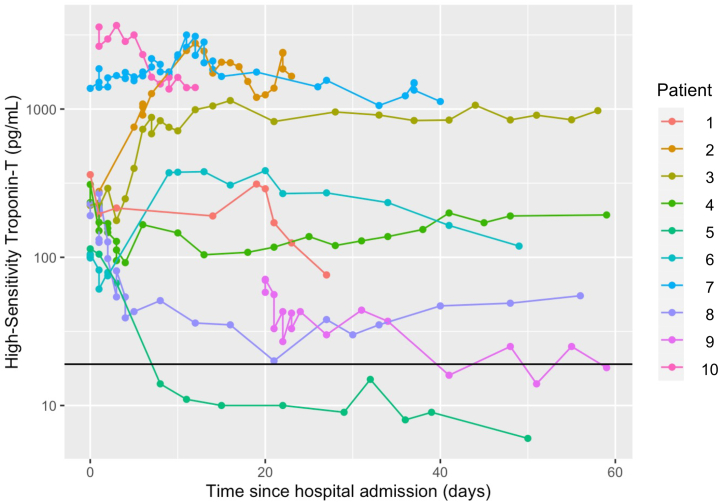
The majority of patients who developed myocarditis had an elevated hsTnT (100%), ALT (88.9%), AST (85.2%), CPK (88.9%), and LDH (92.6%) at the time of diagnosis of myocarditis (Table 2). Among the 3 patients without a rise in CPK, 2 had elevations in AST, ALT, and LDH and 1 patient had an elevation in just LDH. The median hsTnT level at the time of diagnosis was 393 (IQR: 110-1,323) pg/mL. Of the 10 patients diagnosed since January 2021 with serial hsTnT measurements after admission for myocarditis, all but 1 had persistently elevated troponin for up to 60 days (Figure 1).
Figure 1.
Serial High-Sensitivity Troponin T Levels Among Selected Immune Checkpoint Inhibitor–Induced Myocarditis Cases
Serial high sensitivity troponin T plotted for 10 patients beginning at hospital admission for myocarditis. Patients were included if diagnosed after January 1, 2021. The black reference line indicates upper limit of normal (19 pg/mL). All but 1 patient had persistently elevated troponin for up to 60 days after diagnosis of myocarditis, likely reflecting persistent immune activation by immune checkpoint inhibitors or chronic myocarditis.
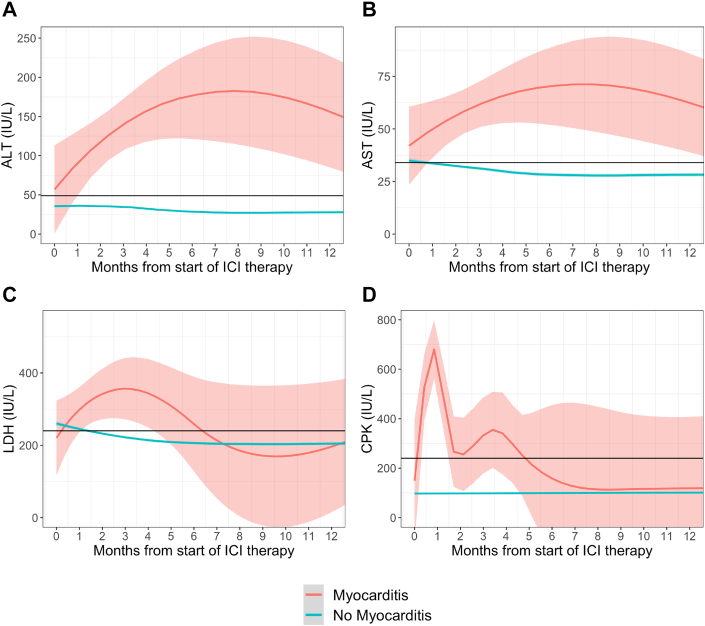
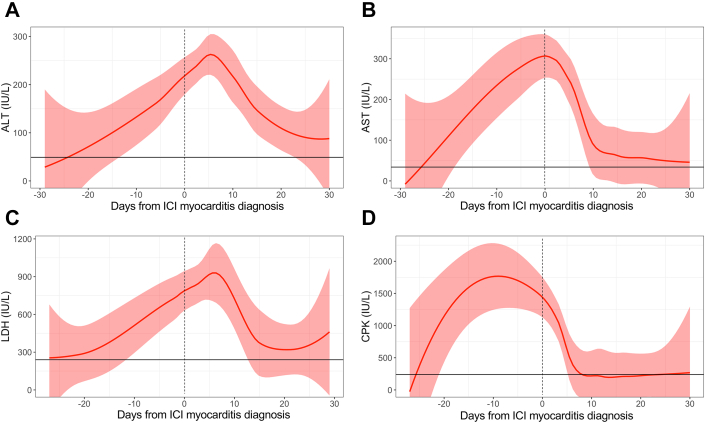
Patients with ICI myocarditis had higher average concentrations of ALT, AST, LDH, and CPK over 12 months after ICI therapy initiation compared with patients without myocarditis (all P < 0.001) (Figure 2). The median time to the first level of elevated biomarker after starting ICI therapy ranged from 21 to 24 days in patients with myocarditis (Table 2). On average, increases in the levels of the nontroponin biomarkers were noted within 30 days prior to hospitalization for myocarditis (Figure 3). CPK peaked the fastest, with a median time to peak concentration of -2 (IQR: -4 to 6) days prior to the diagnosis of myocarditis. Concentrations declined precipitously, returning to normal levels after a median of 17 (IQR: 7 to 22) days. ALT and AST levels peaked at a median of 4 (IQR: 1 to 26) and 0 (IQR: 0 to 6) days after diagnosis of ICI myocarditis, respectively, and returned to after a median of 43 (IQR: 18 to 78) days. LDH concentrations peaked after a median of 11 (IQR: 0 to 43) days after diagnosis of myocarditis returned to normal levels after a median of 159 (IQR: 79 to 217) days. Posthospitalization, CPK returned to normal in all patients, while a subset of patients had persistently elevated AST (33.0%), ALT (15.0%) and LDH (15.0%) at follow-up.
Figure 2.
Longitudinal Biomarkers After ICI Comparing Patients With and Without Myocarditis
Average concentration of biomarkers over 12 months beginning at the start of immune checkpoint inhibitor (ICI) therapy for patients with (red) and without (blue) myocarditis for the following biomarkers: (A) alanine aminotransferase (ALT) (reference: 49 IU/L), (B) aspartate aminotransferase (AST) (reference: 34 IU/L), (C) lactate dehydrogenase (LDH) (reference: 240 IU/L), and (D) creatine phosphokinase (CPK) (reference: 240 IU/L). Patients with myocarditis had higher average concentrations of ALT, AST, LDH, and CPK over 12 months after ICI therapy initiation compared with patients without myocarditis.
Figure 3.
Longitudinal Biomarkers 30 Days Prior to and After Myocarditis
Locally weighted scatterplot smoothing and 95% CIs of biomarker levels 30 days prior to and after diagnosis of myocarditis for (A) ALT (reference: 49 IU/L), (B) AST (reference: 34 IU/L), (C) LDH (reference: 240 IU/L), and (D) CPK (reference: 240 IU/L). Time 0 represents the time of myocarditis diagnosis. On average, increases in the levels of the nontroponin biomarkers preceded hospitalization for myocarditis, with CPK peaking the fastest prior to hospitalization. Elevations in these biomarkers may indicate the presence of myocarditis and should prompt further evaluation. Abbreviations as in Figure 2.
In a separate cohort of 30 patients diagnosed with definite ICI myocarditis from the University Hospitals of Pitié-Salpêtrière between 2018 and 2020, we similarly found that elevations in ALT (100%), AST (96.7%), CPK (83.3%), and LDH (96.7%) were common at the time of diagnosis (Supplemental Table 7). When examining both cohorts of cases, patients with more severe myocarditis (grade ≥3) tended to have higher peak levels of the aforementioned biomarkers compared with patients with milder forms of myocarditis (grade <3). All patients had at least 1 noncardiac biomarker level elevated, with 95% having at least 3 biomarkers with levels above the ULN (Table 3, Central Illustration, Supplemental Table 8). Patients without elevations in CPK did not die during hospitalization and were often asymptomatic or with mild symptoms (88.0%). Conversely, all patients that died during hospitalization had elevations in all biomarkers.
Table 3.
Peak Biomarker Concentrations and Biomarker Elevation by Myocarditis Grade
| Grade I-II (n = 11) | Grade ≥III (n = 16) | |
|---|---|---|
| Peak concentration | ||
| AST, IU/L | 118 (80-171) | 202 (106-328) |
| ALT, IU/L | 84 (55-321) | 212 (65-342) |
| LDH, IU/L | 347 (321-622) | 594 (395-672) |
| CPK, IU/L | 418 (376-1,648) | 1,253 (260-2,603) |
| Biomarker elevation | ||
| 1-2 biomarkers | 0 (0.0) | 1 (6.3) |
| ≥3 biomarkers elevated | 11 (100.0) | 15 (93.7) |
Values are median (IQR) or n (%). Myocarditis was graded according to the American Society of Clinical Oncology guidelines. Reference values: ALT, 49 IU/L; AST, 34 IU/L; LDH, 240 IU/L; CPK, 240 IU/L.
Abbreviations as in Table 1.
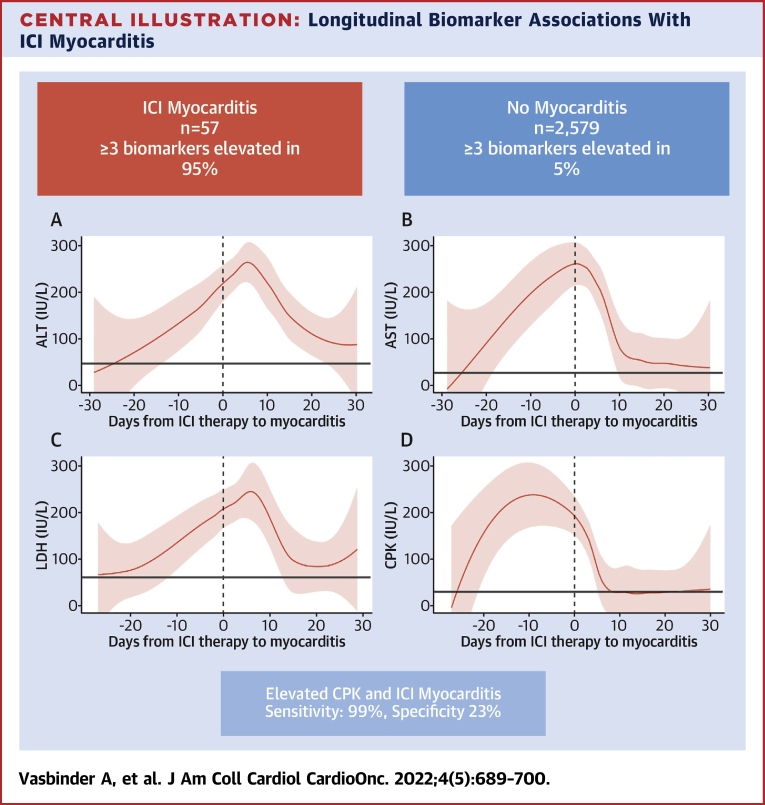
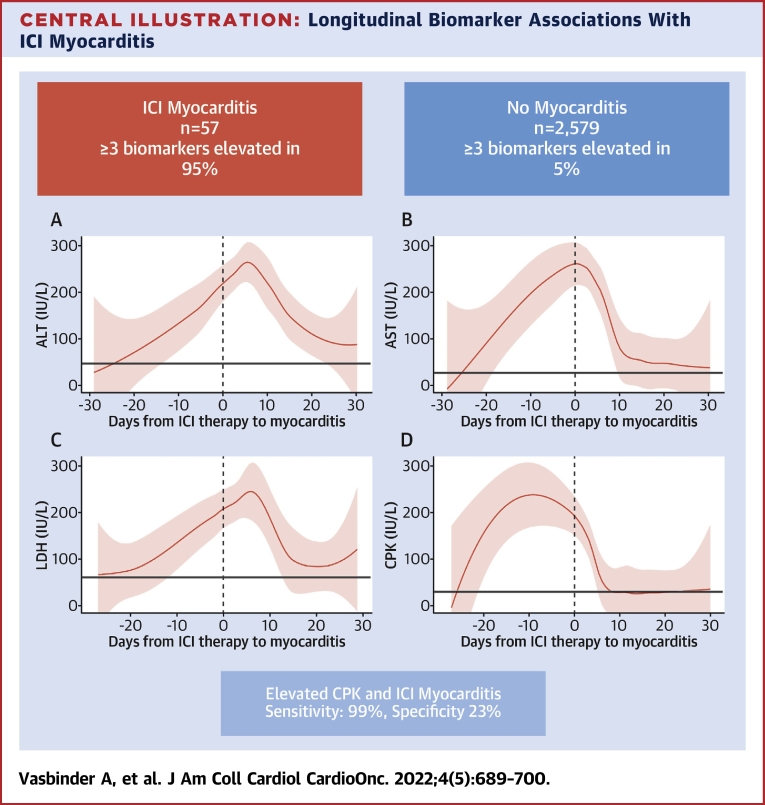
Central Illustration.
Longitudinal Biomarker Associations With ICI Myocarditis
In this cohort study of patients with and without myocarditis, almost all patients with myocarditis had elevations in (A) alanine aminotransferase (ALT), (B) aspartate aminotransferase (AST), (C) lactate dehydrogenase (LDH), and (D) creatine phosphokinase (CPK), compared with only 5% of patients without myocarditis. All biomarkers began to rise prior to the diagnosis of myocarditis with CPK rising the fastest and peaking prior to hospitalization of myocarditis. Elevations in CPK had the best performance, with a sensitivity and specificity of 99% and 23%, respectively. Elevations in these biomarkers should prompt further evaluation for immune checkpoint inhibitor (ICI) myocarditis.
Associations between longitudinal biomarkers, incident myocarditis, and all-cause mortality
When examined as continuous variables (per 100% increase) and in unadjusted analysis, changes in all noncardiac biomarkers after initiating ICI therapy were associated with incident ICI myocarditis (Table 4). After adjustment for demographics and clinical risk factors, only changes in CPK were associated with the development of myocarditis. Each doubling in CPK from baseline was associated with an 83.0% increased risk in incident myocarditis (HR: 1.83: 95% CI: 1.59-2.10; P = 0.007) (Table 4).
Table 4.
Associations Between Changes in Biomarkers and Incident Myocarditis
| Unadjusted |
Adjusteda |
|||
|---|---|---|---|---|
| HR (95% CI) per 100% Increase in Level | P Value | HR (95% CI) per 100% Increase in Level | P Value | |
| ALT | 1.65 (1.50-1.81) | <0.001 | 0.99 (0.90-1.10) | 0.99 |
| AST | 1.50 (1.30-1.73) | <0.001 | 0.92 (0.80-1.06) | 0.82 |
| LDH | 3.09 (1.86-5.13) | 0.016 | 1.26 (0.66-2.42) | 0.88 |
| CPK | 2.21 (1.95-2.51) | <0.001 | 1.83 (1.59-2.10) | 0.007 |
Abbreviations as in Table 1.
Adjusted for age, sex, BMI, smoking history, history of hypertension, type 2 diabetes, heart failure, and coronary artery disease.
Similarly, only increases in CPK (per 100% increase in level) were associated with all-cause mortality (Table 5). After adjustment, each doubling in CPK from baseline was associated with a 10% increased risk of death from any cause (HR: 1.10; 95% CI: 1.01-1.20; P = 0.029). Maximum LDH concentrations greater than the ULN and CPK concentrations >3× the ULN during ICI therapy were associated with a greater risk of all-cause mortality (Table 5). ALT and AST concentrations were not associated with all-cause mortality.
Table 5.
Associations Between Biomarker Elevation During ICI Therapy and All-Cause Mortality
| Unadjusted |
Adjusteda |
|||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| ALT | ||||
| Per 100% increase | 0.99 (0.94-1.04) | 0.64 | 1.00 (0.99-1.03) | 0.96 |
| Normal | 1.00 (reference) | 1.00 (reference) | ||
| 1-2× ULN | 0.98 (0.86-1.12) | 0.78 | 0.97 (0.85-1.10) | 0.62 |
| >2-3× ULN | 1.02 (0.92-1.14) | 0.66 | 1.01 (0.90-1.12) | 0.92 |
| >3× ULN | 1.11 (1.00-1.2 | 0.049 | 1.09 (0.98-1.21) | 0.11 |
| AST | ||||
| Per 100% increase | 1.03 (0.96-1.10) | 0.38 | 1.04 (0.98-1.11) | 0.18 |
| Normal | 1.00 (reference) | 1.00 (reference) | ||
| 1-2× ULN | 1.00 (0.86-1.17) | 0.98 | 1.01 (0.87-1.18) | 0.87 |
| >2-3× ULN | 0.91 (0.81-1.02) | 0.10 | 0.91 (0.81-1.02) | 0.11 |
| >3× ULN | 0.96 (0.86-1.08) | 0.53 | 0.96 (0.85-1.08) | 0.48 |
| LDH | ||||
| Per 100% increase | 1.10 (0.98-1.24) | 0.10 | 1.13 (0.99-1.28) | 0.07 |
| Normal | 1.00 (reference) | 1.00 (reference) | ||
| 1-2× ULN | 1.09 (1.01-1.17) | 0.022 | 1.10 (1.03-1.19) | 0.008 |
| >2-3× ULN | 1.41 (1.24-1.60) | <0.001 | 1.41 (1.24-1.60) | <0.001 |
| >3× ULN | 1.54 (1.37-1.75) | <0.001 | 1.58 (1.39-1.78) | <0.001 |
| CPK | ||||
| Per 100% increase | 1.10 (1.01-1.20) | 0.035 | 1.10 (1.01-1.20) | 0.029 |
| Normal | 1.00 (reference) | 1.00 (reference) | ||
| 1-2× ULN | 0.98 (0.87-1.10) | 0.75 | 0.97 (0.86-1.09) | 0.56 |
| >2-3× ULN | 0.96 (0.87-1.05) | 0.34 | 0.94 (0.86-1.04) | 0.22 |
| >3× ULN | 1.20 (1.10-1.31) | <0.001 | 1.20 (1.10-1.31) | <0.001 |
ULN = upper limit of normal; other abbreviations as in Table 1.
Model adjusted for age, sex, BMI, smoking history, history of hypertension, type 2 diabetes, heart failure, and coronary artery disease.
Lastly, levels of AST, ALT, and CPK above the ULN exhibited a sensitivity ranging from 94% to 99% and a specificity ranging from 3% to 23% (Table 6), with CPK levels having the best diagnostic performance (sensitivity of 99%, specificity of 23%) (Central Illustration). Combining CPK with AST or ALT did not meaningfully improve the sensitivity or specificity for ICI myocarditis.
Table 6.
Sensitivity and Specificity of the Noncardiac Biomarkers in Identifying Myocarditis
| ALT |
AST |
CPK |
LDH |
|||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | |
| >ULN | 0.94 (0.82-1.00) | 0.03 (0.02. 0.03) | 0.97 (0.86-1.00) | 0.06 (0.05-0.07) | 0.99 (0.94-1.00) | 0.23 (0.21-0.25) | 0.85 (0.62-0.99) | 0.11 |
| >2× ULN | 0.82 (0.65-1.00) | 0.06 (0.05-0.07) | 0.95 (0.84-1.00) | 0.12 (0.11-0.13) | 0.98 (0.90-1.00) | 0.23 (0.21-0.25) | 0.40 (0.11-0.70) | 0.56 |
| >3× ULN | 0.71 (0.47-0.94) | 0.12 (0.10-0.13) | 0.91 (0.72-1.00) | 0.35 (0.33-0.36) | 0.92 (0.76-0.99) | 0.31 (0.28-0.34) | — | — |
| >4× ULN | 0.71 (0.47-0.94) | 0.34 (0.32-0.36) | 0.71 (0.48-0.94) | 0.41 (0.38-0.43) | 0.76 (0.56-0.95) | 0.54 (0.51-0.57) | — | — |
Discussion
We leveraged a large observational cohort study of over 2,600 consecutive patients who received ICIs for the treatment of malignancy and underwent systematic testing for AST, ALT, CPK, and LDH to assess biomarker trends in patients who developed ICI myocarditis and those who did not. Our findings provide insight regarding the usefulness of the aforementioned biomarkers in monitoring for ICI myocarditis. Notably, ICI myocarditis was associated with elevations in AST, ALT, LDH, and CPK levels in the majority of patients, indicating that myocarditis co-occurs with other irAEs. All ICI myocarditis patients had abnormal hsTnT levels, suggesting that low hsTnT levels could rule out ICI myocarditis and isolated elevation in cardiac troponin is unlikely to be due to acute ICI myocarditis. Survivors of ICI myocarditis had levels of AST, ALT, and hsTnT that remained elevated for months, while CPK levels rapidly declined after initiation of treatment. Thus, an isolated troponin elevation in asymptomatic patients on ICI therapy could indicate subacute or chronic myocarditis. Among the noncardiac biomarkers, increases in CPK during ICI treatment were independently associated with the development of myocarditis and all-cause mortality. We replicated our findings in a cohort of 30 consecutive patients with definite ICI myocarditis, providing external validity to our conclusions. Overall, these data provide valuable guidance for the development of monitoring strategies in patients receiving immunotherapy.
Diagnosing ICI myocarditis is challenging and relies on the interpretation of a combination of presenting clinical symptoms, electrocardiogram, biomarker, cardiovascular imaging, and occasional endomyocardial biopsy.19,20 Myocarditis often presents with nonspecific signs and symptoms. Common symptoms, such as dyspnea, fatigue, and weakness, can also occur due to malignancy or other treatment-related adverse effects such as anemia, or physical deconditioning.21 While the incidence of ICI myocarditis is generally low, its high mortality warrants establishing a cost-effective screening strategy for early identification and treatment. A unique characteristic of ICI myocarditis is its co-occurrence with evidence of other irAEs.4,6,9,16,22 In this study, all patients had an elevated noncardiac biomarker prior to the diagnosis of acute ICI myocarditis, with 95.0% having at least 3 elevated biomarkers. Conversely, only 5.0% of patients without myocarditis had elevated biomarkers. Thus, the absence of elevated ALT, AST, CPK, and LDH could assist clinicians in ruling out myocarditis. We have found that an elevated CPK alone had a 99% sensitivity and 23% specificity for the diagnosis of acute ICI myocarditis. The high sensitivity of these markers and the fact that they are already routinely measured make them ideal screening tools for ICI myocarditis. Thus, evidence of elevated ALT, AST, and CPK should trigger the measurement of cardiac biomarkers—and if abnormal, prompt clinicians to conduct a thorough evaluation for acute myocarditis or initiate more frequent cardiac monitoring while minimizing treatment disruptions. The co-occurrence of elevated cardiac biomarkers with AST, ALT, and CPK should increase the suspicion a patient is presenting with myocarditis vs other causes of troponin elevations. This is particularly important when deciding whether to perform additional resource intensive testing such as magnetic resonance imaging or initiate costly therapeutics, such as abatacept. Early identification of myocarditis is crucial to risk stratify and initiate treatment as if often escalates quickly even in the absence of symptoms.
We found that levels of the aforementioned biomarkers, including hsTnT, remain elevated for months after diagnosis of ICI myocarditis, despite corticosteroid use and immunomodulatory therapy. These elevations have been previously documented in case reports, and likely reflect the persistent activation of the immune system or chronic myocarditis.23,24 The clinical significance of these persistent elevations is unclear. We have found that patients with ICI myocarditis who were discharged alive have similar long-term survival as those without ICI myocarditis despite persistent elevations in biomarkers, putting into question the clinical impact of these findings. Indeed, the most common cause of death among patients with myocarditis who survived the initial hospitalization for myocarditis was disease progression, rather than cardiovascular causes. Among the studied biomarkers, CPK levels exhibited the most rapid decline following initiation of immunosuppressive therapies and may be a useful test to track the effectiveness of immunosuppression.
Study strengths
This is one of the largest cohorts of patients receiving ICIs who were characterized by detailed medical chart review, rather than by reliance on billing data. By including all patients receiving ICIs, we were able to provide a more accurate estimate of the incidence of ICI myocarditis. Given that our institutional protocol dictates the serial measurement of several biomarkers in patients receiving ICI, we can characterize and compare longitudinal trends in relevant biomarkers in patients with and without myocarditis. As of January 2021, we have adopted systematic long-term serial measurement of hsTnT in patients with a diagnosis of ICI myocarditis, limiting the impact of selection bias in interpretation of these data. However, given the implementation timeline, they were only available in a subset of patients. Our findings were replicated in a separate cohort of definite ICI myocarditis cases in which serial measurements of biomarkers were obtained. This is also the first study to compare mortality rates with a cohort of patients receiving ICIs that did not develop myocarditis, while previous assessments of mortality were limited to case series.14,15,22
Study limitations
Notably, the retrospective nature of the study precluded systematic pre-ICI or myocarditis cardiovascular testing. Troponin levels were not systematically tested in patients without ICI, precluding comparison of troponin trends between patients with and without ICI myocarditis. Without routine monitoring of troponin, it is possible that subclinical cases of myocarditis could be missed. Additionally, troponin I, for which testing is not available at the University of Michigan, may be more appropriate for the diagnosis of myocarditis given its higher specificity for cardiac injury.25 Further research is needed to determine the clinical utility of routinely measuring troponin in all patients receiving ICIs.
Conclusions
Although its incidence is rare, ICI myocarditis is associated with poor outcomes. Thus, identifying patients with myocarditis is crucial to provide early intervention and treatment. Acute ICI myocarditis co-occurs with other irAEs. Thus, evidence of possible irAEs such as elevated AST, ALT, and CPK during the first 3 months of ICI therapy should prompt further evaluation for ICI myocarditis, and normal levels of these biomarkers could rule out clinically significant acute ICI myocarditis.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In a large cohort of patients receiving ICI therapy for cancer, myocarditis was commonly preceded by a rise in noncardiac biomarkers, notably AST, ALT, and CPK. Elevations in these biomarkers during ICI therapy should prompt clinicians to screen for myocarditis using high-sensitivity troponin and consider further diagnostic evaluation. Normal levels of AST, ALT, and CPK makes clinically significant acute ICI myocarditis highly unlikely, and other causes of myocardial injury should be investigated.
TRANSLATIONAL OUTLOOK: This study provides insight in guiding the development of biomarker-guided strategies for monitoring of irAEs including myocarditis in patients receiving ICIs.
Funding Support and Author Disclosures
Dr Vasbinder is supported by a National Heart, Lung, and Blood Institute funded postdoctoral fellowship (T32HL007853). Dr Hayek is supported by National Heart, Lung, and Blood Institute (R01HL153384) and the National Institute on Diabetes and Digestive and Kidney Diseases (R01-DK128012). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Vaddepally R.K., Kharel P., Pandey R., Garje R., Chandra A.B. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel) 2020;12:738. doi: 10.3390/cancers12030738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havel J.J., Chowell D., Chan T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133–150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolchok J. Putting the immunologic brakes on cancer. Cell. 2018;175:1452–1454. doi: 10.1016/j.cell.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Wang D.Y., Salem J.-E., Cohen J.V., et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salem J.-E., Manouchehri A., Moey M., et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puzanov I., Subramanian P., Yatsynovich Y.V., et al. Clinical characteristics, time course, treatment and outcomes of patients with immune checkpoint inhibitor-associated myocarditis. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escudier M., Cautela J., Malissen N., et al. Clinical features, management, and outcomes of immune checkpoint inhibitor–related cardiotoxicity. Circulation. 2017;136:2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 8.Mahmood S.S., Fradley M.G., Cohen J.V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moslehi J.J., Salem J.-E., Sosman J.A., Lebrun-Vignes B., Johnson D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drobni Z.D., Alvi R.M., Taron J., et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142:2299–2311. doi: 10.1161/CIRCULATIONAHA.120.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amiri-Kordestani L., Moslehi J., Cheng J., et al. Cardiovascular adverse events in immune checkpoint inhibitor clinical trials: a U.S. Food and Drug Administration pooled analysis. J Clin Oncol. 2018;36:3009. [Google Scholar]
- 12.Mir H., Alhussein M.M., Alrashidi S., et al. Cardiac complications associated with checkpoint inhibition: a systematic review of the literature in an important emerging area. Can J Cardiol. 2018;34 8:1059–1068. doi: 10.1016/j.cjca.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Bonaca M.P., Olenchock B.A., Salem J.-E., et al. Myocarditis in the setting of cancer therapeutics. Circulation. 2019;140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Kindi S.G., Oliveira G.H. Reporting of immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;392:382–383. doi: 10.1016/S0140-6736(18)31542-3. [DOI] [PubMed] [Google Scholar]
- 15.Makunts T., Saunders I.M., Cohen I.V., et al. Myocarditis occurrence with cancer immunotherapy across indications in clinical trial and post-marketing data. Sci Rep. 2021;11 doi: 10.1038/s41598-021-96467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palaskas N., Lopez-Mattei J., Durand J.B., Iliescu C., Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider B.J., Naidoo J., Santomasso B.D., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39:4073–4126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 18.Zheng Y., Heagerty P.J. Partly conditional survival models for longitudinal data. Biometrics. 2005;61:379–391. doi: 10.1111/j.1541-0420.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 19.Power J.R., Alexandre J., Choudhary A., et al. Electrocardiographic manifestations of immune checkpoint inhibitor myocarditis. Circulation. 2021;144:1521–1523. doi: 10.1161/CIRCULATIONAHA.121.055816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmann L.H., Cautela J., Palaskas N., et al. Clinical strategy for the diagnosis and treatment of immune checkpoint inhibitor–associated myocarditis: a narrative review. JAMA Cardiol. 2021;6:1329–1337. doi: 10.1001/jamacardio.2021.2241. [DOI] [PubMed] [Google Scholar]
- 21.Azeem Khan M., Florou V., Swami U. Immunotherapy and fatigue: what we know and what we don't know. Oncotarget. 2021;12:719–720. doi: 10.18632/oncotarget.27946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atallah-Yunes S.A., Kadado A.J., Kaufman G.P., Hernandez-Montfort J. Immune checkpoint inhibitor therapy and myocarditis: a systematic review of reported cases. J Cancer Res Clin Oncol. 2019;145:1527–1557. doi: 10.1007/s00432-019-02927-x. [DOI] [PubMed] [Google Scholar]
- 23.Lie G., Weickhardt A., Kearney L., et al. Nivolumab resulting in persistently elevated troponin levels despite clinical remission of myocarditis and myositis in a patient with malignant pleural mesothelioma: case report. Transl Lung Cancer Res. 2020;9:360–365. doi: 10.21037/tlcr.2020.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S., Chan J., Brinc D., et al. Immune checkpoint inhibitor-associated myocarditis with persistent troponin elevation despite abatacept and prolonged immunosuppression. J Am Coll Cardiol CardioOnc. 2020;2:800–804. doi: 10.1016/j.jaccao.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welsh P., Preiss D., Hayward C., et al. Cardiac troponin T and troponin I in the general population. Circulation. 2019;139:2754–2764. doi: 10.1161/CIRCULATIONAHA.118.038529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.