Abstract
We previously reported that the CdtB polypeptide of Escherichia coli cytolethal distending toxin (CDT) shares significant pattern-specific homology with mammalian type I DNases. In addition, the DNase-related residues of CdtB are required for cellular toxicity. Here we demonstrate that purified CdtB converts supercoiled plasmid DNA to relaxed and linear forms and promotes cell cycle arrest when combined with an E. coli extract containing CdtA and CdtC. CdtB alone had no effect on HeLa cells, however; introduction of the polypeptide into HeLa cells by electroporation resulted in cellular distension, chromatin fragmentation, and cell cycle arrest, all of which are consequences of CDT action. In contrast to these findings, purified CdtBH154A lacked both DNA-nicking and cell cycle arrest activities. These results suggest a functional relationship between DNase-related residues in CdtB and CDT biological activity.
Cytolethal distending toxins (CDTs) are secreted proteins produced by a number of unrelated pathogenic microbes. To date, CDT production has been reported for some isolates of Escherichia coli (8, 13, 14, 16) Shigella spp. (8, 11), Campylobacter spp. (6, 10, 15) Actinobacillus actinomycetemcomitans (17, 18), Haemophilus ducreyi (4), and a number of Helicobacter spp. (2, 20). CDTs are characterized by their capacity to inhibit cellular proliferation by inducing an irreversible cell cycle block at the G2/M transition (1, 3, 13, 19). CDTs of all strains are composed of three polypeptides, CdtA, CdtB, and CdtC, having molecular masses of approximately 30, 32, and 20 kDa, respectively. Although the role of the individual proteins in cellular toxicity is not firmly established, genetic and biochemical evidence suggests that all three polypeptides are required for CDT activity (14, 16, 18; C. A. Elwell, V. Aragon, and L. A. Dreyfus, Abstr. 97th Gen. Meet. Am. Soc. Microbiol., abstr. B-253, P. 72, 1997).
Recently, it was demonstrated that the CdtB polypeptide bears a striking pattern-specific homology to mammalian type I DNase enzymes. Mutational analysis of CdtB indicated that five DNase I-related amino acids representing catalytic and metal ion binding residues resulted in either a total loss of or a significant reduction in CdtB-related DNase and cell cycle arrest activities (5). Thus, the apparent DNase activity present in crude CdtB preparations correlates with cellular toxicity; however, CdtB itself was insufficient to promote cell cycle arrest. In this report, we extend our recent findings by further assessing the role of CdtB in cellular toxicity.
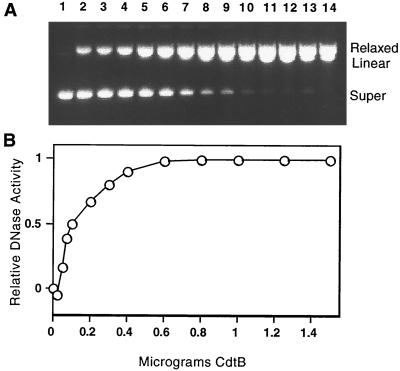
The E. coli CDT-II cdtB and cdtBH154A genes were amplified from the CdtB- and CdtBH154A-encoding templates, pG3 and pG3-CdtBH154A, respectively (5), using the following 5′ and 3′ amplimers: 5′-ATCTCGAGCGATTTAACTGATTTTCG-3′ and 5′-TACTGCAGTTATCGTCTGGATACG-3′. The amplimers were designed such that following flush-end cloning into the SrfI site of pCRScript (Stratagene, La Jolla, Calif.), the fragments could be excised and subcloned directly into the XhoI and PstI sites of the pBAD/HisB expression vector (Invitrogen, Carlsbad, Calif.). In addition, the 5′ amplimer was designed such that the first 18 amino acids of CdtB, corresponding to the putative signal peptide of the CDT-II CdtB (14), were deleted. Subcloning the CdtB fragments into pBAD/HisB resulted in an in-frame amino-terminal fusion with a vector-encoded 5′-hexahistidine peptide sequence and an epitope tag sequence (14 amino acids). The DNA sequences of the cloned cdtB and cdtBH154A genes were verified, and the constructs were transferred to E. coli TOP10 (ΔaraBC ΔaraC araD139). The pBAD/HisB carrying cdtB was expressed, and the His6-tagged CdtB protein was purified from a cytoplasmic extract by nickel-nitrilotriacetic acid (NTA)-agarose chromatography (Fig. 1). Purified His6-tagged CdtB was assessed for DNase activity by a plasmid-nicking reaction essentially as described by Pan et al. (12). The reaction mixture contained supercoiled pGEM-7Zf(+) (50 μg/ml), 5 mM MgCl2, 5 mM CaCl2, and 25 mM HEPES (pH 7.4) in a volume of 20 μl. The reaction mixture, with the reaction initiated by the addition of CdtB, was incubated at 37°C for 1 h. The reaction was stopped by the addition of 5 μl of stop buffer (50 mM EDTA, 50 mM EGTA, 0.25% bromphenol blue, and 30% glycerol). Plasmid DNA was separated by agarose gel electrophoresis (1%), stained with ethidium bromide, and photographed using an Alpha Imager 2000 Documentation and Analysis System. The gel image was analyzed using the Kodak Digital Science 1D Image Analysis Software (Eastman Kodak Co., Rochester, N.Y.). This system provides a quantitative assessment of DNA present in a gel band visualized in an ethidium bromide-stained gel. DNA-nicking activity was assessed by the disappearance of supercoiled pGEM-7Zf(+) DNA as a function of CdtB. The results of this analysis are shown in Fig. 2. A relative DNase activity value of 1 represents the total disappearance of the supercoiled form. Specific activity was defined as the number of micrograms of DNA converted per minute per milligram of protein (12). The specific activity of CdtB was determined from the results shown in Fig. 2 using the 50% completion point (relative DNase activity = 0.5) and the interpolated amount of CdtB (0.1 μg). The specific DNA-nicking activity of CdtB was 166.7 μg min−1 mg−1. This is approximately 0.01% of the activity reported for purified human or bovine DNase I (12). DNase activity was absent in a Ni-NTA-purified control preparation derived from a strain harboring the native pBAD/His vector (data not shown).
FIG. 1.
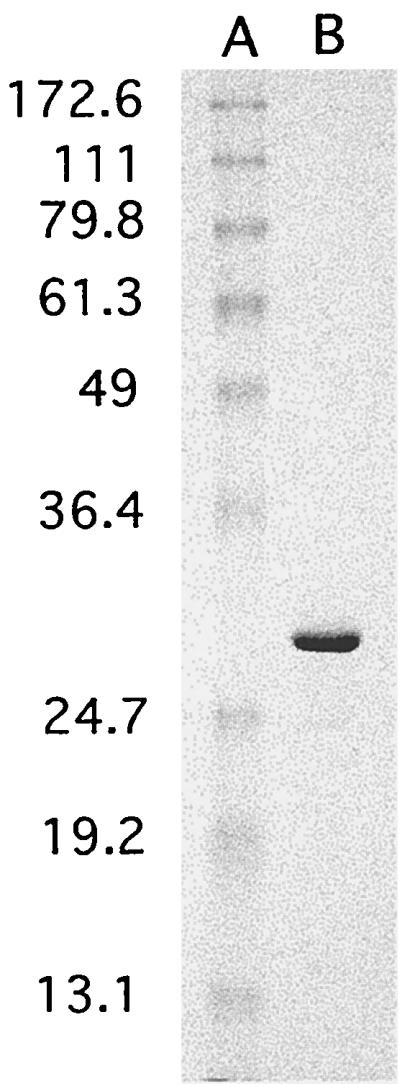
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of purified CdtB. Purified CdtB and molecular mass standards were subjected to SDS-PAGE (12% acrylamide gel) followed by staining with Coomassie brilliant blue. A, molecular size markers (sizes [in kDa] shown at left); B, 6 μg of CdtB.
FIG. 2.
DNase activity of CdtB. (A) Increasing amounts of purified CdtB incubated with supercoiled pGEM7zf(+) (Promega) as described in the text. Lane 1, no His6-tagged CdtB; lanes 2 to 14, 0.025, 0.05, 0.07, 0.1, 0.2, 0.3, 0.4, 0.6, 0.8, 1.25, 1.5, 2.0, and 2.5 μg, respectively, of His6-tagged CdtB. (B) Relative DNAse activity (a value of 1.0 represents the total disappearance of supercoiled substrate DNA). The agarose gel (1% agarose) was stained with ethidium bromide and visualized by UV transillumination. The numeric data used to generate the activity curve shown in panel B was generated as described in the text.
Mutations in the CdtB gene which alter DNase-related active site residues yield CDT preparations devoid of or greatly reduced in cell cycle arrest activity (5). We determined that the functional defect of CDT preparations from strains harboring mutant CdtB alleles was completely restored by the addition of a crude preparation of native (secreted) CdtB (5). Here we compared purified His6-tagged CdtB to a crude periplasmic extract from a CdtB-only construct expressing native CdtB for the ability to complement the defect of an inactive CDT preparation (CDT-CdtBH154A) (5). A polymyxin B extract of E. coli BL21 harboring pCDT-CdtBH154A was prepared as previously described (5) and designated CdtA-CdtC. After dialysis against 25 mM Tris (pH 7.4)–25 mM NaCl, 1 ml of the extract containing 300 μg of protein was mixed with various quantities of either native or His6-tagged CdtB. After 30 min of incubation at room temperature, the toxin preparations were added to 5 × 105 HeLa cells seeded 18 h prior to toxin addition in 100-mm-diameter culture dishes. Toxin-treated HeLa cells, cultured in 5 ml of Dulbecco's modified Eagle's medium (DMEM), were incubated for 48 h in an atmosphere containing 5% CO2 and then prepared for cell cycle analysis by flow cytometry (5). The results indicated that His6-tagged CdtB possessed a level of biological activity similar to that of native CdtB (Table 1). The addition of as little as 2.5 μg of native CdtB to the mutant CDT-CdtBH154A extract resulted in a significant rise in the percentage of cells blocked at the G2/M interface (29.9% compared to 15.8% for the control [Table 1]). Similar results were obtained with His6-tagged CdtB; however, unlike native CdtB, which yielded a maximal arrest of 90% of cells in G2/M, pure CdtB induced a response which appeared to level off at 60% of cells in G2/M. HeLa cells receiving native, or purified, CdtB alone did not arrest. Identical results were obtained when HeLa cells received the CDT-CdtBH154A extract only, indicating that CdtB was required for cellular toxicity but was not sufficient to mediate the response in the absence of CdtA and CdtC. The inability of purified His6-tagged CdtB to fully complement the biological defect of the CDT-CdtBH154A extract may be a consequence of the presence of the amino-terminal epitope and histidine tag sequences (14 amino acids) on this polypeptide. It is possible that this peptide sequence affects the interaction of pure CdtB with CdtA and/or CdtC. Attempts to remove the histidine epitope tag from CdtB resulted in degradation of CdtB (data not shown).
TABLE 1.
| CDT preparation | Amt of toxin added (μg) | No. (%) of cells inc:
|
||
|---|---|---|---|---|
| G0/G1 | S | G2/M | ||
| CdtA-CdtC + CdtB | 0 | 48.5 | 35.7 | 15.8 |
| 1.0 | 33.3 | 39.9 | 26.8 | |
| 2.5 | 36.4 | 33.7 | 29.9 | |
| 5.0 | 34.6 | 34.9 | 30.5 | |
| 10 | 7.2 | 16.4 | 76.4 | |
| 25 | 4.2 | 12.0 | 83.8 | |
| 50 | 2.3 | 7.5 | 90.2 | |
| CdtBd | 50 | 57.4 | 26.5 | 16.1 |
| CdtA-CdtC + His6-tagged CdtB | 0 | 48.5 | 35.7 | 15.8 |
| 1.0 | 41.0 | 37.8 | 21.2 | |
| 2.5 | 35.2 | 28.4 | 36.4 | |
| 5.0 | 30.4 | 28.6 | 41 | |
| 10 | 23.6 | 31.8 | 44.6 | |
| 25 | 15.4 | 25.2 | 59.4 | |
| 50 | 19.9 | 28.1 | 52.0 | |
| His6-tagged CdtBd | 50 | 55.3 | 28.1 | 16.6 |
The CDT preparation was a periplasmic extract from a strain producing the CDT-CdtBH154A mutant toxin (5). The preparation is designated CdtA-CdtC since it contains wild-type levels of these subunits and a nonfunctional CdtB. Each cell preparation received 1 ml of the CDT-CdtBH154A periplasmic extract containing 300 μg of protein.
The CdtB preparation was either an extract from an E. coli strain producing only the native CdtB (CdtB) (5) or purified His6-tagged CdtB. The quantity of pure His6-tagged CdtB was estimated by UV absorption; the quantity of native CdtB was estimated by comparison to purified known amounts of pure CdtB in a quantitative Western blot (5).
The data shown are representative of at least three repeats of the experiment. The variances in the percentages of cells in the cell-cycle positions did not exceed 10%.
CdtB or His6-tagged CdtB added in the absence of CdtA-CdtC.
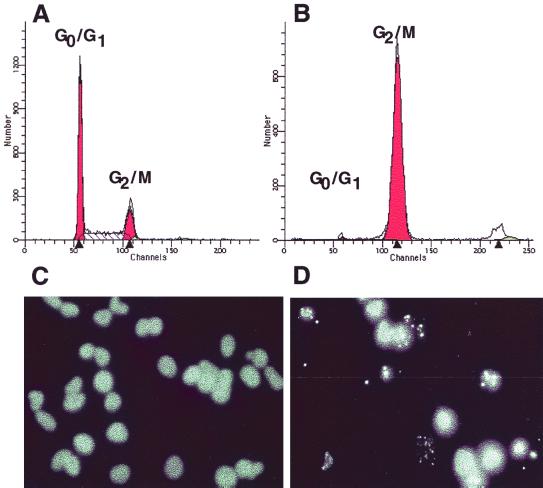
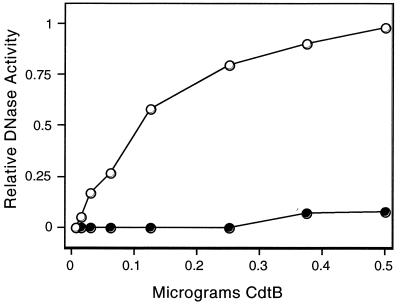
Previously obtained results describing the contribution of DNase catalytic residues in CdtB action (5) raise the possibility that CdtB alone may be sufficient for cell cycle arrest if introduced inside cells. Since electroporation is a simple method for passively delivering macromolecules to cells, we examined the effect of introducing His6-tagged CdtB into HeLa cells by this technique. HeLa cells were trypsinized from culture dishes, washed twice in phosphate-buffered saline (PBS) and resuspended at 107 cells per ml in PBS. Pure His6-tagged CdtB (or buffer alone) was incubated with 106 cells in a total volume of 0.2 ml for 10 min at room temperature. The cells were electroporated as described by Li and Benezra (9), immediately washed from the cuvette in prewarmed complete DMEM, and plated in 100-mm-diameter culture dishes containing 5 ml of complete DMEM. Following 24 h of incubation at 37°C in 5% CO2, the cells were prepared for flow cytometry (5). Alternatively, the electroporated cells were divided evenly into 4-well Lab-Tek chamber slides and examined for chromosomal abnormalities following staining with 4′,6-diamidino-2-phenylindole (DAPI) as described by Whitehouse et al. (19). His6-tagged CdtB introduced into HeLa cells by electroporation arrested the cell population at G2/M and resulted in chromatin fragmentation in approximately 50% of His6-tagged CdtB-treated cells (Fig. 3B and D). In contrast to these results, HeLa cells electroporated in the absence of CdtB displayed a normal cell cycle distribution and intact nuclei (Fig. 3A and C). In addition to cell cycle arrest, direct introduction of CdtB into HeLa cells resulted in elongation and distension identical to that observed following CDT treatment (data not shown). Morphological changes were not observed in the absence of CdtB and thus were not a function of the electroporation procedure. Since electroporation was an efficient method for introducing CdtB into HeLa cells, we determined the minimal amount of toxin required to intoxicate cells in this manner. The results (Table 2) indicated that 25 ng of CdtB was sufficient to induce significant cell cycle arrest when electroporated into HeLa cells (52%). With the biological specific activity of CdtB defined as the amount that would result in 50% of cells blocked at the G2/M boundary following electroporation, the specific activity of this CdtB preparation would therefore be approximately 25 ng. In the absence of electroporation, the addition of 100 μg of pure CdtB to HeLa cells had no effect on cells, again indicating that CdtB translocation is required for toxin-mediated cell cycle arrest. To assess whether the biological action of His6-tagged CdtB was related to the DNase I homology of this polypeptide, we tested purified His6-tagged CdtBH154A for cell cycle arrest activity. Electroporation of HeLa cells with as much as 2.5 μg of purified His6-tagged CdtBH154A failed to result in cell cycle arrest (Table 2). Purified His6-tagged CdtBH154A was also tested for DNA-nicking activity as described for His6-tagged CdtB. As shown in Fig. 4, the His6-tagged CdtBH154A protein lacked the DNA-nicking activity associated with His6-tagged CdtB.
FIG. 3.
Cell cycle arrest and chromatin fragmentation of HeLa cells. Shown are cell cycle distribution histograms representing data obtained from the analysis of 10,000 HeLa cells electroporated in buffer alone (A) or in the presence of 100 μg of CdtB (B), as well as chromatin fragmentation determined by DAPI staining and fluorescence microscopy 24 h following electroporation in buffer alone (C) or in the presence of 100 μg of CdtB (D). The cell cycle distribution was determined by flow cytometry on a FACSCalibur (Bectin Dickinson) flow cytometer, and the data were analyzed with the ModFit LT software package (Verity Software).
TABLE 2.
Cell cycle distribution following electroporation of purified wild-type and mutant CdtB into HeLa cellsa
| Toxin and amt (μg) | No. (%) of cells in:
|
||
|---|---|---|---|
| G0/G1 | S | G2/M | |
| None | 48.1 | 31.5 | 20.4 |
| His6-tagged CdtB | |||
| 0.010 | 45.7 | 26.5 | 27.8 |
| 0.025 | 33.3 | 14.8 | 51.9 |
| 0.050 | 23.2 | 10.4 | 66.4 |
| 0.100 | 16.6 | 7.9 | 75.5 |
| 0.250 | 8.0 | 8.4 | 83.6 |
| 0.500 | 3.1 | 6.0 | 90.9 |
| 1.000 | 3.5 | 2.7 | 93.8 |
| 100b | 44.1 | 30.1 | 25.8 |
| His6-tagged CdtBH154A | |||
| 0.050 | 46.0 | 31.1 | 22.9 |
| 0.500 | 42.7 | 32.3 | 25.0 |
| 2.500 | 45.2 | 29.4 | 25.4 |
The data shown are representative of at least three repeats of the experiment. The variances in the percentages of cells in the cell cycle positions did not exceed 10%.
Purified CdtB was added but cells were not electroporated.
FIG. 4.
DNase activity of His6-tagged CdtB versus that of His6-tagged CdtBH154A. His6-tagged CdtB and His6-tagged CdtBH154A were expressed and purified in the same experiment and tested for DNase activity as described in the text and the legend to Fig. 2. Open circles, His6-tagged CdtB; closed circles, His6-tagged CdtBH154A.
Despite an apparent requirement for CdtA and CdtC, once introduced into the cell, His6-tagged CdtB induced the entire spectrum of CDT toxicity. These data suggested that CdtA and/or CdtC participates in the cellular binding and translocation of CdtB. Moreover, delivery of His6-tagged CdtB directly into the cell resulted in a 1,000-fold reduction in the effective dose, the amount of CdtB which results in 50% of cells blocked in G2, as compared to the level seen when His6-tagged CdtB was combined with functional CdtA and CdtC proteins (0.025 μg versus 25 μg, respectively). The explanation of this difference is presently unknown, but it may reflect the efficiency of CdtB uptake by electroporation relative to CdtA- and/or CdtC-mediated delivery. Alternatively, the level of CdtA and CdtC present in the CDT-CdtBH154A extract may be limiting. Attempts to increase the level of CdtA and CdtC in complementation experiments, however, failed to affect the level of toxicity for HeLa cells. Cell cycle arrest following the direct introduction of CdtB also suggests that a cytoplasmic intermediate step is likely to occur in the normal pathway of CDT-mediated toxicity. This observation may also suggest that CdtA and CdtC are not necessary for intracellular events leading to cell cycle arrest, such as the intracellular processing or trafficking of CdtB. Moreover, we demonstrate that CdtB bearing the H154A mutation, previously shown to be devoid of CDT activity, does not result in cell cycle arrest when introduced into HeLa cells by electroporation.
Our previous data describing the loss of CDT activity following mutagenesis of key DNase-related residues in CdtB suggests that nuclease activity may be essential for cellular toxicity. Here we demonstrate that purified His6-tagged CdtB possesses DNA-nicking activity that is not present in His6-tagged CdtBH154A. Although the level of DNase activity associated with CdtB was approximately 10,000-fold less than that of purified human or bovine DNase I, the activity was totally absent in the biologically inert His6-tagged CdtBH154A. These data suggest that the DNA-nicking activity of His6-tagged CdtB is at least associated with the cell cycle arrest activity of this polypeptide, if not required for its action. It is possible that CdtB triggers the mitotic DNA damage checkpoint by directly damaging chromosomal DNA as a function of the associated nicking activity. Alternatively, CdtB may indirectly affect chromatin or DNA stability by virtue of its apparent capacity to bind DNA, resulting in indirect DNA damage and leading to arrest at the G2/M boundary. Our previous mutational analysis of CdtB (5) and data presented here suggest that chromosomal DNA is an intracellular target for CDT action. A mechanism of CDT action involving nuclear targeting and chromosomal damage would represent a unique mode of action for a microbial product. The events following the introduction of CdtB and cell cycle arrest are currently under investigation.
Acknowledgments
This work was supported by a grant from the Missouri Research Board.
REFERENCES
- 1.Aragon V, Chao K, Dreyfus L A. Effect of cytolethal distending toxin on F-actin assembly and cell division in Chinese hamster ovary cells. Infect Immun. 1997;65:3774–3780. doi: 10.1128/iai.65.9.3774-3780.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chien C C, Taylor N S, Ge Z, Schauer D B, Young V B, Fox J G. Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. J Med Microbiol. 2000;49:525–534. doi: 10.1099/0022-1317-49-6-525. [DOI] [PubMed] [Google Scholar]
- 3.Comayras C, Tasca C, Pérès S Y, Ducommun B, Oswald E, De Rycke J. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect Immun. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cope L D, Lumbley S, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elwell C A, Dreyfus L A. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol Microbiol. 2000;37:952–963. doi: 10.1046/j.1365-2958.2000.02070.x. [DOI] [PubMed] [Google Scholar]
- 6.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb Pathog. 1988;4:115–126. doi: 10.1016/0882-4010(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 7.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Escherichia coli isolates from clinical material. Microb Pathog. 1988;4:103–113. doi: 10.1016/0882-4010(88)90052-6. [DOI] [PubMed] [Google Scholar]
- 8.Johnson W M, Lior H. Production of shiga toxin and a cytolethal distending toxin (CLDT) by serogroups of Shigella spp. FEMS Microbiol Lett. 1987;48:235–238. [Google Scholar]
- 9.Li Y, Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274:246–248. doi: 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- 10.Ohya T, Tominaga K, Nakazawa M. Production of cytolethal distending toxin (CLDT) by Campylobacter fetus subsp. fetus isolated from calves. J Vet Med Sci. 1993;55:507–509. doi: 10.1292/jvms.55.507. [DOI] [PubMed] [Google Scholar]
- 11.Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathog. 1995;18:167–172. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 12.Pan C Q, Ulmer J S, Herzka A, Lazarus R A. Mutational analysis of human DNaseI at the DNA binding interface: implications for DNA recognition, catalysis, and metal ion dependence. Protein Sci. 1998;7:628–636. doi: 10.1002/pro.5560070312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peres S Y, Marches O, Daigle F, Nougayrede J P, Herault F, Tasca C, De Rycke J, Oswald E. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 14.Pickett C L, Cottle D L, Pesci E C, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect Immun. 1994;62:1046–1051. doi: 10.1128/iai.62.3.1046-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickett C L, Pesci E C, Cottle D L, Russell G, Erdem A N, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB genes. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott D A, Kaper J B. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shenker B J, McKay T, Datar S, Miller M, Chowhan R, Demuth D. Actinobacillus actinomyecetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J Immunol. 1999;162:4773–4780. [PubMed] [Google Scholar]
- 18.Sugai M, Kawamoto T, Peres S Y, Ueno Y, Komatsuzawa H, Fujiwara T, Kurihara H, Suginaka H, Oswald E. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun. 1998;66:5008–5019. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitehouse C A, Balbo P B, Pesci E C, Cottle D L, Mirabito P M, Pickett C L. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young V B, Chien C C, Knox K A, Taylor N S, Schauer D B, Fox J G. Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum. J Infect Dis. 2000;182:620–623. doi: 10.1086/315705. [DOI] [PubMed] [Google Scholar]