Abstract
Background
Immune checkpoint inhibitors (ICIs) are a central part of cancer therapy; however, cardiac complications, such as myocarditis, have the potential for significant morbidity and mortality. Within this population, the clinical significance of longitudinal strain (LS) remains unknown.
Objectives
This study sought to define the changes in LS in ICI-treated patients, and their associations with high-sensitivity troponin I (hsTnI) and myocarditis.
Methods
We conducted a retrospective cohort study of patients who received ICIs at our hospital from April 2017 to September 2021. All patients underwent echocardiography and blood sampling at standardized time intervals. We measured the changes in global and regional LS before and after ICI administration. Age- and sex-adjusted Cox regression analysis was used to evaluate the association between LS and elevations in hsTnI and myocarditis.
Results
In a cohort of 129 patients with a median follow-up period of 170 (IQR: 62-365) days; 6 and 18 patients had myocarditis and hsTnI elevation, respectively. In an age- and sex-adjusted Cox proportional hazards model, an early relative worsening of ≥10% in the basal and mid LS and ≥15% in global LS was associated with hsTnI elevation. Relative reductions in LS were not significantly associated with myocarditis; however, 4 of the 6 patients with myocarditis had relative reduction of ≥10% in the basal LS.
Conclusions
An early worsening in the global and regional LS was associated with increased hsTnI in patients receiving ICIs. Assessment of LS early after ICI administration should be further studied as a strategy for risk stratification of ICI-treated patients.
Key Words: cardiac troponin, echocardiography, immune checkpoint inhibitors, longitudinal strain, myocarditis
Abbreviations and Acronyms: GLS, global longitudinal strain; hsTnI, high-sensitivity troponin I; ICI, immune checkpoint inhibitor; LS, longitudinal strain; MRI, magnetic resonance imaging; TnI, troponin I
Central Illustration
Immune checkpoint inhibitors (ICIs) represent a significant advance in cancer therapy and are increasingly being used.2 However, myocarditis due to ICIs can be a serious adverse event. Although the frequency of ICI-induced myocarditis is low (0.6%-1.14%),1, 2, 3 its severity is high and mortality has been reported to be 36% to 67%.4,5
Despite the provision of expert consensus recommendations and position statements,6, 7, 8, 9 the appropriate follow-up method for patients receiving ICIs still remains to be clarified. In other cardio-oncology populations, such as anthracycline-treated patients, troponin I (TnI) and global longitudinal strain (GLS) are associated with a risk of adverse cardiac events.10,11 Although a subject of debate, it has also been reported that early cardioprotective therapy based on changes in GLS might reduce the incidence of cardiotoxicity.12 In addition, a basal longitudinal strain (LS) reduction in patients treated with anthracyclines has been shown to precede GLS reduction.13 However, the only report on the association between GLS and ICIs demonstrated that major adverse cardiac events are more frequent in patients with myocarditis who have worse GLS.14 The potential utility of early GLS or regional LS as a predictor of cardiac events remains to be determined.
Cardiac troponin is an organ-specific marker and not a disease-specific marker. However, it is the most widely used biomarker for myocarditis; furthermore, it has high sensitivity for diagnosing myocarditis due to ICIs.2 The role of troponin as a risk predictor in this population is also an area of active research.
We hypothesized that patients with myocarditis and TnI elevation might manifest changes in myocardial deformation early in the course of ICI therapy.15 Because echocardiography for patients receiving ICI therapy is performed routinely and at standardized time intervals for patients treated at our hospital, we investigated whether early GLS and regional LS changes predict myocarditis and TnI elevation.
Methods
Ethics approval
This study conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the ethics committee of the International University of Health and Welfare Mita Hospital (approval number 5-21-12). The requirement for informed consent was waived due to the retrospective nature of the study.
Study participants
This retrospective cohort study included patients who received ICI therapy at the International University of Health and Welfare Mita Hospital, Japan, between April 2017 and September 2021. The following patients were excluded: 1) those without routine follow-up by a cardiologist; 2) those who did not receive transthoracic echocardiography at baseline or on days 8 to 14; and 3) those for whom GLS or local LS at baseline or on days 8 to 14 was not analyzable. In our hospital, all patients treated with ICIs are regularly followed up by cardiologists.
Procedure for follow-up of patients receiving ICIs
Patients treated with ICIs at our hospital are followed according to a standardized clinical protocol. As shown in Figure 1, patients receiving ICIs were evaluated by a cardiologist before ICI administration during week 1 (days 1-7), week 2 (days 8-14), and week 3 (days 15-21), after 60 days, and every 3 months after ICI administration. In general, patients with ICI therapy received medical examination, blood tests, electrocardiography, and chest radiography examination on visit days. Echocardiography was performed before ICI administration, after 2 weeks, after 60 days, and then every 3 months until 3 moths after the final ICI administration. Blood tests included the measurement of TnI, brain natriuretic peptide, D-dimer, creatine kinase, and creatine kinase-myocardial band levels. Patients were followed up by cardiologists until 3 months after the final ICI cycle. In some situations, cardiologists may decide to shorten the frequency of testing and perform additional tests based on individual symptoms or objective findings.
Figure 1.
Follow-Up Schedule and Clinical Routine Tests for Patients Receiving ICIs
Prospecting screening protocol for patients treated with immune checkpoint inhibitors (ICIs) was shown. The follow-up schedule and examinations (B-type natriuretic peptide [BNP], troponin I, D-dimer, creatine kinase [CK], creatine kinase-myocardial band [CK-MB], chest radiography, electrocardiogram [ECG]) in patients receiving ICI therapy are shown. TTE = transthoracic echocardiography
Evaluation and definition of clinical variables
We collected data from patients’ electronic medical records including age, sex, body mass index, coexisting disease, cardiac biomarker levels, and echocardiographic parameters. Cancer-specific covariates including the type, prior anthracycline use, radiation therapy, and ICI type were extracted. Hypertension was defined as a systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg or receipt of antihypertensive medication. Diabetes mellitus was defined as glycated hemoglobin ≥6.5% or receipt of insulin therapy or oral medication for diabetes mellitus. Dyslipidemia was defined as a low-density lipoprotein cholesterol level >140 mg/dL or receipt of medication for dyslipidemia. Chronic kidney disease was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2.
Echocardiographic assessment including regional LS
Experienced sonographers performed standard echocardiographic examinations according to the American Society of Echocardiography guidelines.16 Vivid E95 ultrasound systems (GE Healthcare) were used and data were analyzed using GE EchoPAC software (v203, GE Healthcare). Left ventricular ejection fraction was measured using the biplane Simpson’s method of disks. GLS and regional LS measurements were acquired by an experienced sonographer and an echocardiographic physician in a blinded manner. Strain measurements were obtained from the apical long-axis, 4-chamber, and 2-chamber views using semi-automated image analysis. Regional LS was measured through the same process, using each mean peak strain value of 5 or 6 segments. Basal, mid, and apical LS values were calculated by using values in the basal (6 segments), mid (6 segments), and apical (5 segments) layers, respectively. LS are reported as absolute values in this study. We calculated percent changes in LS using the following equation: ([strain value on days 8 to 14 − strain value at baseline] · 100 /strain value at baseline). Percentage variation (relative change) in strain values meant the absolute value of strain parameters, with negative and positive variations indicating worsening and improving deformations, respectively. The position statements of the Heart Failure Association, the European Association of Cardiovascular Imaging, and the Cardio-Oncology Council of the European Society of Cardiology state that a relative LS reduction of 10% to 15% from baseline is considered a clinically significant change.7 Therefore, we analyzed the relative reduction in GLS and regional LS at cutoff values of 10%, 12.5%, and 15%.
Definitions of clinical outcomes
We evaluated 2 clinical outcomes: 1) myocarditis; and 2) high-sensitivity (hsTnI) elevation after the first follow-up echocardiogram (days 8-14). Myocarditis was diagnosed as a pathological finding based on the lymphocytic infiltration in the myocardium with myocyte loss or standard guideline-recommended diagnostic criteria for clinically suspected myocarditis among patients without a myocardial biopsy.17 hsTnI elevation was defined as >26.8 pg/mL (99th reference percentile, standard value of Abbott hsTnI assay) within 365 days. If the baseline hsTnI was above the reference value, we defined elevation as twice the baseline level.
Statistical analysis
Continuous variables are presented as mean ± SD for those with a normal distribution, as median (IQR) for those with a non-normal distribution, and as numbers (percentages) for categorical variables. The Mann-Whitney U test or Student's t test was used to compare continuous variables between the groups. The Wilcoxon signed rank test was used for comparing continuous variables pre- and post-ICI therapy. We also used the Fisher exact test to compare proportions of categorical variables between the groups. Sensitivity was calculated as the proportion of patients with relative decrease in LS among all patients with hsTnI elevation, and specificity was calculated as the proportion of patients without relative decrease in LS among all patients without hsTnI elevation. The positive predictive value was defined as the proportion of patients with hsTnI elevation among all patients with relative decrease in LS, and the negative predictive value was calculated as the proportion of patients without hsTnI elevation among all patients without relative decrease in LS. The HR for the association between LS decline and hsTnI elevation was analyzed using univariable and multivariable age- and sex-adjusted Cox proportional hazards models. Model 1 considered an early relative worsening in basal LS of ≥10% as the main predictor and model 2 considered an early relative worsening in GLS of ≥15% as the main predictor. Model results are presented as adjusted HR with 95% CIs. The proportionality assumption was tested using the Schoenfeld residuals method. The Kaplan-Meier curves generated for hsTnI elevation–free survival were compared using the log-rank test (early relative decrease in basal LS of ≥10% or not, mid LS of ≥10% or not, and GLS of ≥15% or not). Time to event was the time from first ICI administration to TnI elevation or myocarditis. The maximum observation period was 1 year after the first ICI administration. Patients were censored at time of death, and death was also considered as a competing risk in sensitivity analysis. A 2-sided P value <0.05 was considered significant for all statistical tests. Statistical analyses were performed using R version 4.1.2 (R Foundation for Statistical Computing).
Results
Baseline characteristics
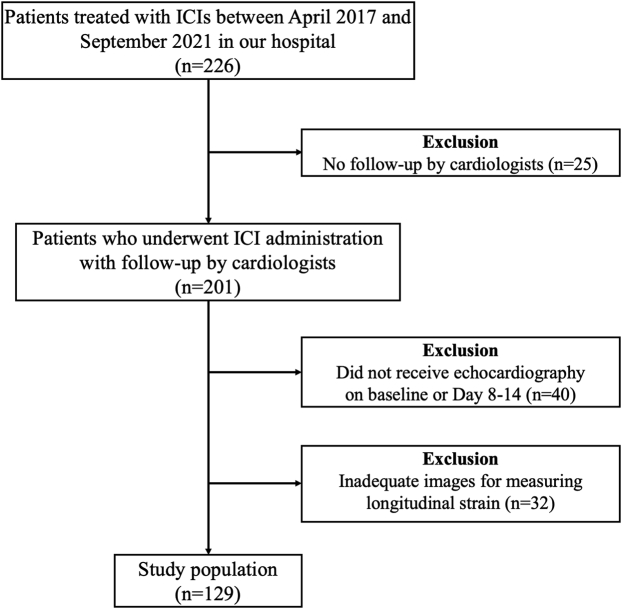
We enrolled 226 patients treated with ICIs in our hospital. We excluded patients without follow-up by cardiology, echocardiography at baseline or on days 8 to 14, or adequate images for measuring LS; this excluded 25, 40, and 32 patients, respectively (Figure 2). As a result, 129 patients (65.6 ± 11.2 years of age, 98 [76.0%] men) were analyzed in this study. The characteristics of patients included in this study analysis (n = 129) and those excluded (n = 97) were mostly similar (Supplemental Table 1).
Figure 2.
Flow Diagram Showing the Recruitment of ICI Therapy Patients
The CONSORT flow diagram showing the cases with immune checkpoint inhibitor (ICI) administration in our hospital.
Baseline characteristics of patients are shown in Table 1; few patients were treated with anthracyclines before ICI administration. The most common indications for ICI treatment were head and neck cancer, and nivolumab and pembrolizumab were common ICIs used (Table 1). Only 2 patients received combination ICI therapy.
Table 1.
Baseline Characteristics of Study Participants
| Total (N = 129) | TnI Elevation and/or Myocarditis (n = 19) | No TnI Elevation or Myocarditis (n = 110) | P Value | |
|---|---|---|---|---|
| Age, y | 65.6 ± 11.2 | 66.4 ± 15.5 | 65.5 ± 10.3 | 0.74 |
| Male | 98 (76.0) | 16 (84.2) | 82 (74.5) | 0.56 |
| Body mass index, kg/m2 | 20.3 (17.8-23.4) | 21.2 (17.8-23.7) | 20.2 (17.9-23.1) | 0.48 |
| Cardiovascular risk factor and disease | ||||
| Hypertension | 41 (31.8) | 6 (31.6) | 35 (31.8) | >0.99 |
| Diabetes mellitus | 14 (10.9) | 0 (0.0) | 14 (12.7) | 0.22 |
| Dyslipidemia | 16 (12.4) | 1 (5.3) | 15 (13.6) | 0.46 |
| Chronic kidney disease | 40 (31.0) | 8 (42.1) | 32 (29.1) | 0.29 |
| Current or prior smoking | 79 (61.2) | 13 (68.4) | 66 (60.0) | 0.61 |
| Baseline cardiac findings | ||||
| BNP, pg/mL | 20.1 (10.1-34.5) | 24.7 (10.7-38.6) | 19.9 (9.9-34.3) | 0.72 |
| TnI, pg/mL | 3.2 (1.9-6.0) | 10.1 (3.0-14.8) | 3.1 (1.9-5.3) | 0.004 |
| LVEF, % | 65.0 (62.4-67.7) | 64.9 (61.9-66.5) | 65.1 (62.5-67.8) | 0.47 |
| LVEF <40% | 0 (0.0) | 0 (0.0) | 0 (0.0) | >0.99 |
| GLS, % | 17.7 (16.2-19.2) | 17.3 (16.8-18.4) | 17.7 (16.2-19.4) | 0.74 |
| Pre-ICI home cardiac medications | ||||
| Renin-angiotensin system inhibitor | 21 (16.3) | 1 (5.3) | 20 (18.2) | 0.31 |
| Beta-blocker | 9 (7.0) | 2 (10.5) | 7 (6.4) | 0.62 |
| Mineralocorticoid receptor antagonist | 3 (2.3) | 1 (5.3) | 2 (1.8) | 0.38 |
| Loop diuretic | 4 (3.1) | 0 (0.0) | 4 (3.6) | >0.99 |
| Primary cancer type | ||||
| Head and neck cancer | 85 (65.9) | 9 (47.4) | 76 (69.1) | |
| Lung cancer | 23 (17.8) | 8 (42.1) | 15 (13.6) | |
| Gastrointestinal cancer | 8 (6.2) | 0 (0.0) | 8 (7.3) | |
| Renal cell carcinoma/urothelial cancer | 6 (4.7) | 1 (5.3) | 5 (4.5) | |
| Melanoma | 3 (2.3) | 1 (5.3) | 2 (1.8) | |
| Hepatocellular carcinoma | 3 (2.3) | 0 (0.0) | 3 (2.7) | |
| Breast cancer | 1 (0.8) | 0 (0.0) | 1 (0.9) | |
| Prior chemotherapy or radiation | ||||
| Anthracyclines | 4 (3.1) | 2 (10.5) | 2 (1.8) | 0.10 |
| Radiation | 85 (65.9) | 11 (57.9) | 74 (67.3) | 0.44 |
| Thoracic irradiation | 21 (16.3) | 4 (21.1) | 17 (15.5) | 0.51 |
| Anti-VEGFR TKIs | 7 (5.4) | 2 (10.5) | 5 (4.5) | 0.27 |
| ICIs type | ||||
| Nivolumab (anti-PD-1) monotherapy | 58 (45.0) | 4 (21.1) | 54 (49.1) | |
| Pembrolizumab (anti-PD-1) monotherapy | 53 (41.1) | 8 (42.1) | 43 (39.1) | |
| Atezolizumab (anti-PD-L1) monotherapy | 13 (10.1) | 5 (26.3) | 8 (7.3) | |
| Durvalumab (anti-PD-L1) monotherapy | 3 (2.3) | 1 (5.3) | 2 (1.8) | |
| Combination therapy (nivolumab + ipilimumab) | 2 (1.6) | 1 (5.3) | 1 (0.9) | |
Values are mean ± SD, n (%), or median (IQR).
CTL4 = cytotoxic T lymphocyte associated protein 4; BNP = B-type natriuretic peptide; GLS = global longitudinal strain; ICI = immune checkpoint inhibitor; LVEF = left ventricular ejection fraction; PD-1 = programmed cell death protein-1; PD-L1 = programmed death-ligand 1; TKI = tyrosine kinase inhibitor; TNI = troponin I; VEGFR = vascular endothelial growth factor receptor.
Myocarditis and hsTnI elevation after ICI therapy
The median observation period from the start of ICI administration to the last follow-up by the cardiologist was 170 (IQR: 62-365) days. Death occurred in 26 patients. Among patients treated with ICIs, 6 were diagnosed with myocarditis and 18 had hsTnI elevation following ICI therapy. The median time to TnI elevation and myocarditis was 62 (IQR: 34-134) days and 98 (IQR: 26-206) days, respectively. The number of patients and timing of TnI elevation are shown in Figure 3. The types of cancer and ICIs in patients with myocarditis and hsTnI elevation are shown in Supplemental Table 2. All patients diagnosed with myocarditis had received pembrolizumab prior to the diagnosis of myocarditis. Of the 6 myocarditis patients, 1 had cardiogenic shock, while another patient made the decision to pursue palliative care and passed away. In contrast, patients without myocarditis had no major adverse cardiovascular events (cardiovascular death, cardiac arrest, cardiogenic shock, or complete heart block requiring pacemaker).
Figure 3.
Number of Patients and Time to Troponin I Elevation or Myocarditis
Time from first immune checkpoint inhibitor (ICI) administration to troponin I elevation or myocarditis. Most clinical events occurred early in the course of ICI administration, and less frequently after 6 months. The first administration of ICI therapy was on day 0.
Five of the 6 patients with myocarditis had elevated TnI at diagnosis. Three patients underwent magnetic resonance imaging (MRI) to confirm the diagnosis, while 2 underwent myocardial biopsy. One of the patients who underwent MRI had no myocarditis-related findings on imaging but did have pathologic confirmation. Blood tests, electrocardiography, and echocardiography were repeated early for patients with elevated TnI to identify the cause of TnI elevation. Table 2 shows the results of these tests and other clinical characteristics for the 6 patients with myocarditis.
Table 2.
Laboratory and Imaging Findings in Patients With Myocarditis at Baseline and Diagnosis
|
Case |
Sex | Age at ICI Start (y) | TnI at Baseline (pg/mL) | Symptoms/Presentation | TnI at Diagnosis (pg/mL) | ECG | Echocardiography | MRI | ESC Criteria of Hermann et al33 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 36 | 1.4 | Dyspnea Fatigue |
380 | Reduced R-wave height ST-segment elevation in V1-V3 |
Preserved LVEF LV wall thickening |
Definite findings | Pathological diagnosis |
| 2 | Female | 49 | 0.5 | Dyspnea Fatigue Cardiogenic shock |
441 | Reduced R-wave height | Preserved LVEF LV wall thickening |
No signs of myocarditis | Pathological diagnosis |
| 3 | Male | 88 | 89.7 | Dyspnea Fatigue Muscle weakness (Myositis) |
1,374 | Ectopy T-wave inversion |
Reduced LVEF Diffuse hypokinesis LV wall thickening |
Not performed | Troponin elevation 3 minor criteria |
| 4 | Male | 51 | 5.3 | Dizziness | 16.7 | Ectopy | Reduced LVEF Diffuse hypokinesis |
Definite findings | Not applicable (1 major/2 minor) |
| 5 | Male | 65 | 10.1 | Dizziness Fatigue Diplopia (Myositis) |
200 | No change | Preserved LVEF | Not performed | Troponin elevation 2 minor criteria |
| 6 | Male | 73 | 15.5 | Fatigue Muscle weakness (Myositis) |
285 | Reduced R-wave height | Preserved LVEF | Not performed | Troponin elevation 2 minor criteria |
CMR = cardiac magnetic resonance; ECG = electrocardiogram; ESC = European Society of Cardiology; MRI = magnetic resonance imaging; other abbreviations as in Table 1.
Association between longitudinal strain hsTnI elevation after ICI therapy
Table 3 shows the baseline and LS measures stratified according to the presence or absence of hsTnI elevation after ICI therapy. More patients with hsTnI elevation showed a relative change of ≥15% in GLS than those without hsTnI elevation (22.2% vs 4.5%; P = 0.022), and the absolute value of GLS was worse in patients with hsTnI elevation (median 15.7% [IQR: 14.8%-17.4%] vs 17.7% [IQR: 16.3%-18.7%]; P = 0.005). Patients with hsTnI elevation were significantly more likely to show a decline in regional LS than patients without hsTnI elevation. Specifically, the relative change of ≥12.5% in basal LS occurred in 44.4% with hsTnI elevation vs 18.9% without (P = 0.029), ≥10% in basal LS occurred in 55.6% vs 25.2% (P = 0.013), and ≥10% in mid LS in 44.4% vs 18.9% (P = 0.029). The sensitivity, specificity, and positive and negative predictive values of basal LS for relative decrease ≥10% in hsTnI elevation were 55.6%, 74.8%, 26.3%, and 91.2%, respectively. The relative decrease in GLS by ≥15% for elevated hsTnI had a sensitivity, specificity, positive predictive value, and negative predictive value of 22.2%, 95.5%, 44.4%, and 88.3%, respectively.
Table 3.
Description of Patients With/Without hsTnI Elevation After ICI Administration
| hsTnI Elevation (+) (n = 18) | hsTnI Elevation (–) (n = 111) | P Value | |
|---|---|---|---|
| Age, y | 67.3 ± 15.5 | 65.4 ± 10.4 | 0.50 |
| Male | 15 (83.3) | 83 (74.8) | 0.56 |
| Hypertension | 6 (33.3) | 35 (31.5) | >0.99 |
| Diabetes mellitus | 0 (0.0) | 14 (12.6) | 0.22 |
| Dyslipidemia | 1 (5.6) | 15 (13.5) | 0.47 |
| Chronic kidney disease | 8 (44.4) | 32 (28.8) | 0.27 |
| Current or prior smoking | 12 (66.7) | 67 (60.4) | 0.80 |
| Baseline | |||
| BNP, pg/mL | 22.0 (10.3-40.0) | 20.1 (10.1-34.1) | 0.88 |
| Elevated TnI | 1 (5.6) | 2 (1.8) | 0.38 |
| GLS, % | 17.3 (16.7-18.5) | 17.7 (16.2-19.3) | 0.69 |
| Basal LS, % | 16.0 (13.2-17.5) | 15.8 (14.3-17.9) | 0.65 |
| Mid LS, % | 18.3 (16.2-19.7) | 18.0 (16.3-19.7) | 0.96 |
| Apical LS, % | 20.4 (18.7-22.7) | 20.0 (18.3-22.3) | 0.77 |
| LVEF, % | 65.2 (62.2-66.7) | 65.0 (62.5-67.8) | 0.73 |
| Days 8-14 after ICI | |||
| GLS | |||
| Absolute value, % | 15.7 (14.8-17.4) | 17.7 (16.3-18.7) | 0.005 |
| Relative change ≥15% | 4 (22.2) | 5 (4.5) | 0.022 |
| Relative change ≥12.5% | 5 (27.8) | 14 (12.6) | 0.14 |
| Relative change ≥10% | 7 (38.9) | 21 (18.9) | 0.069 |
| Basal LS | |||
| Absolute value, % | 14.6 (13.5-15.6) | 15.8 (13.8-17.3) | 0.026 |
| Relative change ≥15% | 5 (27.8) | 17 (15.3) | 0.19 |
| Relative change ≥12.5% | 8 (44.4) | 21 (18.9) | 0.029 |
| Relative change ≥10% | 10 (55.6) | 28 (25.2) | 0.013 |
| Mid LS | |||
| Absolute value, % | 16.2 (15.1-18.1) | 17.8 (16.5-19.0) | 0.023 |
| Relative change ≥15% | 4 (22.2) | 12 (10.8) | 0.24 |
| Relative change ≥12.5% | 5 (27.8) | 15 (13.5) | 0.16 |
| Relative change ≥10% | 8 (44.4) | 21 (18.9) | 0.029 |
| Apical LS | |||
| Absolute value, % | 18.5 (16.3-21.3) | 20.0 (17.9-21.9) | 0.14 |
| Relative change ≥15% | 4 (22.2) | 20 (18.0) | 0.74 |
| Relative change ≥12.5% | 5 (27.8) | 28 (25.2) | 0.78 |
| Relative change ≥10% | 6 (33.3) | 34 (30.6) | 0.79 |
| LVEF, % | 64.9 (62.1-67.3) | 65.1 (62.6-66.7) | 0.98 |
Values are mean ± SD, n (%), or median (IQR).
LS = longitudinal strain; other abbreviations as in Table 1.
In univariable analysis, relative changes of ≥12.5% and ≥10% in basal LS were associated with hsTnI elevation, and relative changes of ≥15% in GLS and ≥10% in mid LS were also associated with hsTnI elevation (Table 4). An age- and sex-adjusted multivariable Cox model showed that relative changes of ≥10% in basal LS (HR: 3.22; 95% CI: 1.26-8.23; P = 0.015), ≥10% in mid LS (HR: 2.77; 95% CI: 1.09-7.02; P = 0.032), and ≥15% in GLS (HR: 4.75; 95% CI: 1.53-14.80; P = 0.007) were significantly associated with hsTnI elevation (Table 5). In Kaplan-Meier curves, there was a significant difference between relative change ≥10% and <10% in basal LS in terms of hsTnI elevation (log-rank P = 0.011) (Figure 4A). The Kaplan-Meier curves for mid LS (relative change ≥10% and <10%) and GLS (relative change ≥15% and <15%) are shown in Figures 4B and 4C.
Table 4.
Univariable Cox Regression Analysis of the Association of LS and TnI Elevation
| Univariable |
|||
|---|---|---|---|
| HR | 95% CI | P Value | |
| Age | 1.02 | 0.98-1.07 | 0.32 |
| Male | 1.21 | 0.35-4.19 | 0.76 |
| GLS | |||
| Relative change ≥15% | 4.90 | 1.61-14.97 | 0.005 |
| Relative change ≥12.5% | 2.47 | 0.88-6.93 | 0.086 |
| Relative change ≥10% | 2.26 | 0.87-5.82 | 0.093 |
| Basal LS | |||
| Relative change ≥15% | 2.13 | 0.76-5.97 | 0.15 |
| Relative change ≥12.5% | 3.07 | 1.21-7.80 | 0.019 |
| Relative change ≥10% | 3.13 | 1.23-7.94 | 0.016 |
| Mid LS | |||
| Relative change ≥15% | 2.04 | 0.67-6.21 | 0.21 |
| Relative change ≥12.5% | 2.02 | 0.72-5.66 | 0.18 |
| Relative change ≥10% | 2.74 | 1.08-6.95 | 0.034 |
| Apical LS | |||
| Relative change ≥15% | 1.37 | 0.45-4.16 | 0.58 |
| Relative change ≥12.5% | 1.16 | 0.41-3.24 | 0.78 |
| Relative change ≥10% | 1.16 | 0.44-3.10 | 0.76 |
Table 5.
Age- and Sex-Adjusted Associations Between LS and TnI Elevation
| Predictor | HR | 95% CI | P Value |
|---|---|---|---|
| Basal LS relative change ≥10% | 3.22 | 1.26-8.23 | 0.015 |
| Mid LS relative change ≥10% | 2.77 | 1.09-7.02 | 0.032 |
| GLS relative change ≥15% | 4.75 | 1.53-14.80 | 0.007 |
Figure 4.
Kaplan-Meier Curves of Event-Free Survival With Change in LS
Kaplan-Meier estimates of event-free (troponin I [TnI] elevation) survival with relative change in longitudinal strain (LS): (A) ≥10% in basal LS, (B) ≥10% in mid LS, (C) ≥15% in global longitudinal strain (GLS). The first immune checkpoint inhibitor administration was on day 0.
Comparisons of clinical characteristics, as well as the absolute values and relative changes in LS between those with and without myocarditis, are presented in Table 6. Although there were no significant differences between the 2 groups, relative changes of ≥10% in basal LS were common in patients with myocarditis (4 of 6 patients).
Table 6.
Description of Patients With/Without Myocarditis After ICI Administration
| Myocarditis (+) (n = 6) | Myocarditis (–) (n = 123) | P Value | |
|---|---|---|---|
| Age, y | 60.3 ± 18.7 | 65.9 ± 10.7 | 0.24 |
| Male | 5 (83.3) | 93 (75.6) | >0.99 |
| Hypertension | 3 (50.0) | 38 (30.9) | 0.38 |
| Diabetes mellitus | 0 (0.0) | 14 (11.4) | >0.99 |
| Dyslipidemia | 0 (0.0) | 16 (13.0) | >0.99 |
| Chronic kidney disease | 2 (33.3) | 38 (30.9) | >0.99 |
| Current or prior smoking | 3 (50.0) | 76 (61.8) | 0.68 |
| Anthracycline | 0 (0.0) | 4 (3.3) | >0.99 |
| Trastuzumab | 0 (0.0) | 3 (2.4) | >0.99 |
| Thoracic irradiation | 1 (16.7) | 20 (16.3) | >0.99 |
| Baseline | |||
| BNP, pg/mL | 8.6 (5.8-28.2) | 20.3 (10.4-35.1) | 0.22 |
| Elevated TnI | 1 (16.7) | 2 (1.6) | 0.14 |
| GLS, % | 18.2 (17.3-18.5) | 17.7 (16.2-19.3) | 0.97 |
| Basal LS, % | 16.7 (12.4-17.7) | 15.8 (14.3-17.8) | 0.84 |
| Mid LS, % | 19.7 (18.9-20.2) | 18.0 (16.3-19.7) | 0.21 |
| Apical LS, % | 20.5 (19.0-21.6) | 20.0 (18.2-22.5) | 0.84 |
| LVEF, % | 65.2 (57.8-66.6) | 65.0 (62.5-67.8) | 0.68 |
| Days 8-14 after ICI administration | |||
| GLS | |||
| Absolute value, % | 17.3 (15.4-18.7) | 17.6 (16.1-18.7) | 0.88 |
| Relative change ≥15% | 0 (0.0) | 9 (7.3) | >0.99 |
| Relative change ≥12.5% | 1 (16.7) | 18 (14.6) | >0.99 |
| Relative change ≥10% | 1 (16.7) | 27 (22.0) | >0.99 |
| Basal LS | |||
| Absolute value, % | 14.7 (11.1-15.7) | 15.7 (13.8-17.3) | 0.14 |
| Relative change ≥15% | 1 (16.7) | 21 (17.1) | >0.99 |
| Relative change ≥12.5% | 3 (50.0) | 26 (21.1) | 0.12 |
| Relative change ≥10% | 4 (66.7) | 34 (27.6) | 0.062 |
| Mid LS | |||
| Absolute value, % | 18.3 (17.1-19.4) | 17.7 (16.2-18.8) | 0.53 |
| Relative change ≥15% | 0 (0.0) | 16 (13.0) | >0.99 |
| Relative change ≥12.5% | 0 (0.0) | 20 (16.3) | 0.59 |
| Relative change ≥10% | 1 (16.7) | 28 (22.8) | >0.99 |
| Apical LS | |||
| Absolute value, % | 20.5 (19.0-22.0) | 19.8 (17.5-21.8) | 0.39 |
| Relative change ≥15% | 0 (0.0) | 24 (19.5) | 0.59 |
| Relative change ≥12.5% | 0 (0.0) | 33 (26.8) | 0.34 |
| Relative change ≥10% | 1 (16.7) | 39 (31.7) | 0.67 |
| LVEF, % | 64.4 (59.3-66.3) | 65.1 (62.6-67.1) | 0.42 |
We have presented the changes in echocardiographic parameters other than LS in Supplemental Table 3. Supplemental Table 4 showed left ventricular ejection fraction, LS, and TnI from baseline to day 60, respectively. Supplemental Figures 1 and 2 showed the changes in GLS, regional LS, and TnI from baseline to day 60.
Discussion
In this study, we presented the relationship between GLS or regional LS and hsTnI elevation and myocarditis after ICI therapy. Our results are as follows: 1) early worsening in GLS, basal LS, and mid LS occurs more commonly in patients with hsTnI elevation compared with those who do not have hsTnI elevations; 2) early relative changes in basal and mid LS and GLS were associated with hsTnI elevation in patients who received ICI therapy; and 3) although GLS was not associated with subsequent risk of myocarditis, basal LS was decreased in 4 of 6 patients with myocarditis (Central Illustration). Although our findings need to be validated in larger populations, early changes in LS should be further studied as a tool for the early detection of myocardial damage in ICI-treated patients.
Central illustration.
LS and Cardiac Events in Patients Treated With ICIs
Overview of the method and the key findings of this cohort study of immune checkpoint inhibitor (ICI)–treated patients. In ICI-treated patients, early relative decreases in basal and mid longitudinal strain (LS) of ≥ 10% and in global longitudinal strain (GLS) of ≥15% were associated with troponin I (TnI) elevation. In addition, 67% of patients with myocarditis had an early decline in basal LS of ≥10%. BNP = B-type natriuretic peptide; ECG = electrocardiogram; TTE = transthoracic echocardiography.
Early worsening in basal LS, mid LS, and GLS were predictors of hsTnI elevation and may provide insight into cardiovascular risk stratification of patients with ICI therapy. In the present study, early basal LS, mid LS, and GLS declines were significantly associated with hsTnI elevation. Reduction in GLS has been reported to be an early predictor of cardiac events in patients receiving cancer drug therapy in meta-analyses and systematic reviews.10,11 As for regional LS, a decrease in basal LS was reported earlier than the decrease in GLS in patients treated with anthracyclines.13 These are consistent with the results of the present study. On the other hand, few studies have examined whether regional LS is a useful predictor of clinical cardiac events in ICI-treated patients. This study demonstrates that a relative decrease in basal LS demonstrates a high specificity for hsTnI elevation in ICI-treated patients.
We believe that there may be several reasons for the early decrease in basal LS. Because the onset of ICI-induced myocarditis is often observed early after ICI administration,5,14,15 it is assumed that patients with clinical events have myocardial damage due to inflammation at an early stage. As a result of the failure of the entire myocardium, the wall stress in the basal area, where the left ventricular lumen is larger than that in other areas, is increased by Laplace's law.18,19 Interestingly, patients with ICI-associated myocarditis presented with more late gadolinium enhancement in basal and mid segments than in apical segments in MRI.20 From a histopathological point of view, Michel et al21 reported increased myocardial lymphocytes and decreased strain in echocardiography in mice treated with ICI therapy, even in the absence of active myocarditis. In the largest pathological series on ICI myocarditis, intermediate-grade myocarditis without myocardial cell necrosis was seen in patients with laboratory or imaging abnormalities, including elevated TnI.22 These studies indicates that inflammatory cell infiltration without myocardial cell death might result in early decrease in LS without TnI elevations.
We believe that even an elevation in TnI alone is an important finding in patients with ICI therapy. Elevated cardiac TnI has been reported to predict cardiac events in patients receiving cancer drug therapy.23, 24, 25 Additionally, 94% of patients with ICI myocarditis have elevated troponin levels.14 The importance of TnI evaluation is also emphasized in the diagnostic criteria for ICI myocarditis in the new European guidelines.26 In this study, the number of patients with elevated hsTnI was 14.0%, similar to that in previous studies that measured hsTnI routinely in ICI-treated patients (11.2%).27 In our study, 5 were diagnosed with myocarditis among 18 patients with hsTnI elevation. Regarding the remaining 13 patients who did not develop clinical myocarditis despite elevated TnI, they might also present myocarditis even if not evident. There are some reports about the existence of clinically silent myocarditis, one is from myocardial biopsy findings,28 and another report described autopsy findings suggestive of myocarditis without any clinical presentations.29 Therefore, patients with elevated TnI would be candidates for closer observation.
In this context, early prediction of hsTnI elevation in patients receiving ICI may be of clinical relevance in the follow-up of such patients after ICI administration. The major adverse cardiac event–free survival rate was reported to be higher in a group of patients with ICI myocarditis who received early steroids,30 suggesting the need for early diagnosis, ICI discontinuation, and steroid therapy. Additionally, mortality rates for ICI myocarditis used to be very high5,15; however, recent reports indicate that these mortality rates are declining (14%-18%),31,32 partly due to increased awareness of ICI myocarditis. This may be related to earlier and more accurate diagnosis and appropriate management. Therefore, with further study, troponin measures could be used as a strategy to identify and stratify high-risk patients with ICI therapy.
Study limitations
First, this was a single-center study, and the results may not be generalizable. Second, the sample size was small and the myocarditis event rate was small, resulting in low power. Third, selection bias is a consideration, as some patients were unable to undergo echocardiography at baseline or the first follow-up. Finally, few patients in this study received ICI-combination therapy, which is a risk factor for myocarditis. Larger multicenter prospective studies are needed to define the external validity of this study.
Conclusions
In patients treated with ICI therapy, early relative worsening in basal and mid LS of ≥10% and in GLS of ≥15% were associated with hsTnI elevation. Early basal LS reduction was observed in the majority of cases of myocarditis in patients receiving ICIs.
Perspectives.
COMPETENCY IN PATIENT CARE: In patients receiving ICIs, early decreases in GLS, basal LS, and mid LS are associated with hsTnI elevation. An early worsening in basal and mid LS and GLS was associated with hsTnI elevation, and basal LS was commonly abnormal in myocarditis patients. With further study, LS analysis may be applied as a potential marker for the early detection of myocardial damage in ICI-treated patients.
TRANSLATIONAL OUTLOOK: Larger multicenter prospective studies are needed to define the external validity of this study. The role of LS to predict early cardiac injury and guide cardioprotective strategies with ICI therapy needs to be further clarified.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors express their gratitude to all the participants and staff involved in this study, especially Rika Takeyasu and Yui Shiga for data management.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Mahmood S.S., Fradley M.G., Cohen J.V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson D.B., Balko J.M., Compton M.L., et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agostinetto E., Eiger D., Lambertini M., et al. Cardiotoxicity of immune checkpoint inhibitors: a systematic review and meta-analysis of randomised clinical trials. Eur J Cancer. 2021;148:76–91. doi: 10.1016/j.ejca.2021.01.043. [DOI] [PubMed] [Google Scholar]
- 4.Brumbaugh A.D., Narurkar R., Parikh K., Fanucchi M., Frishman W.H. Cardiac immune-related adverse events in immune checkpoint inhibition therapy. Cardiol Rev. 2019;27:97–107. doi: 10.1097/CRD.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 5.Salem J.E., Manouchehri A., Moey M., et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pudil R., Mueller C., Čelutkienė J., et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail. 2020;22:1966–1983. doi: 10.1002/ejhf.2017. [DOI] [PubMed] [Google Scholar]
- 7.Čelutkienė J., Pudil R., López-Fernández T., et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC) Eur J Heart Fail. 2020;22:1504–1524. doi: 10.1002/ejhf.1957. [DOI] [PubMed] [Google Scholar]
- 8.Armenian S.H., Lacchetti C., Barac A., et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 9.Curigliano G., Lenihan D., Fradley M., et al. ESMO Guidelines Committee. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31:171–190. doi: 10.1016/j.annonc.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oikonomou E.K., Kokkinidis D.G., Kampaktsis P.N., et al. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. 2019;4:1007–1018. doi: 10.1001/jamacardio.2019.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGregor P.C., Moura F.A., Banchs J., Aragam J.R. Role of myocardial strain imaging in surveillance and management of cancer therapeutics-related cardiac dysfunction: a systematic review. Echocardiography. 2021;38:314–328. doi: 10.1111/echo.14944. [DOI] [PubMed] [Google Scholar]
- 12.Thavendiranathan P., Negishi T., Somerset E., et al. SUCCOUR Investigators. Strain-guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol. 2021;77:392–401. doi: 10.1016/j.jacc.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Saijo Y., Kusunose K., Okushi Y., et al. Relationship between regional left ventricular dysfunction and cancer-therapy-related cardiac dysfunction. Heart. 2020;106:1752–1758. doi: 10.1136/heartjnl-2019-316339. [DOI] [PubMed] [Google Scholar]
- 14.Awadalla M., Mahmood S.S., Groarke J.D., et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol. 2020;75:467–478. doi: 10.1016/j.jacc.2019.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moslehi J.J., Salem J.E., Sosman J.A., Lebrun-Vignes B., Johnson D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. e14. [DOI] [PubMed] [Google Scholar]
- 17.Caforio A.L., Pankuweit S., Arbustini E., et al. European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. 2648a-2648d. [DOI] [PubMed] [Google Scholar]
- 18.Balzer P., Furber A., Delépine S., et al. Regional assessment of wall curvature and wall stress in left ventricle with magnetic resonance imaging. Am J Physiol. 1999;277:H901–H910. doi: 10.1152/ajpheart.1999.277.3.H901. [DOI] [PubMed] [Google Scholar]
- 19.Basford J.R. The law of Laplace and its relevance to contemporary medicine and rehabilitation. Arch Phys Med Rehabil. 2002;83:1165–1170. doi: 10.1053/apmr.2002.33985. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L., Awadalla M., Mahmood S.S., et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J. 2020;41:1733–1743. doi: 10.1093/eurheartj/ehaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michel L., Helfrich I., Hendgen-Cotta U.B., et al. Targeting early stages of cardiotoxicity from anti-PD-1 immune checkpoint inhibitor therapy. Eur Heart J. 2022;43:316–329. doi: 10.1093/eurheartj/ehab430. [DOI] [PubMed] [Google Scholar]
- 22.Palaskas N.L., Segura A., Lelenwa L., et al. Immune checkpoint inhibitor myocarditis: elucidating the spectrum of disease through endomyocardial biopsy. Eur J Heart Fail. 2021;23:1725–1735. doi: 10.1002/ejhf.2265. [DOI] [PubMed] [Google Scholar]
- 23.Michel L., Mincu R.I., Mahabadi A.A., et al. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail. 2020;22:350–361. doi: 10.1002/ejhf.1631. [DOI] [PubMed] [Google Scholar]
- 24.Pavo N., Raderer M., Hülsmann M., et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. 2015;101:1874–1880. doi: 10.1136/heartjnl-2015-307848. [DOI] [PubMed] [Google Scholar]
- 25.Ky B., Putt M., Sawaya H., et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyon A.R., López-Fernández T., Couch L.S., et al. ESC Scientific Document Group 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43:4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 27.Waliany S., Neal J.W., Reddy S., et al. Myocarditis surveillance with high-sensitivity troponin I during cancer treatment with immune checkpoint inhibitors. J Am Coll Cardiol CardioOnc. 2021;3:137–139. doi: 10.1016/j.jaccao.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giblin G.T., Dennehy C., Featherstone H., et al. Subclinical myocarditis after combination immune checkpoint inhibitor therapy. Circ Heart Fail. 2021;14 doi: 10.1161/CIRCHEARTFAILURE.120.007524. [DOI] [PubMed] [Google Scholar]
- 29.Koelzer V.H., Rothschild S.I., Zihler D., et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J Immunother Cancer. 2016;4:13. doi: 10.1186/s40425-016-0117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L., Zlotoff D.A., Awadalla M., et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation. 2020;141:2031–2034. doi: 10.1161/CIRCULATIONAHA.119.044703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zlotoff D.A., Hassan M.Z.O., Zafar A., et al. Electrocardiographic features of immune checkpoint inhibitor associated myocarditis. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2020-002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thavendiranathan P., Zhang L., Zafar A., et al. Myocardial T1 and T2 mapping by magnetic resonance in patients with immune checkpoint inhibitor-associated myocarditis. J Am Coll Cardiol. 2021;77:1503–1516. doi: 10.1016/j.jacc.2021.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herrmann J., Lenihan D., Armenian S., et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43:280–299. doi: 10.1093/eurheartj/ehab674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.