Corresponding Author
Key Words: abatacept, immunotherapy, lung cancer, myocarditis, pharmacology
Abbreviations and Acronyms: CD86RO, cluster of differentiation 86 receptor occupancy; CTLA-4, cytotoxic T-lymphocyte antigen; ICI, immune checkpoint inhibitor; JAK, Janus kinase; MACE, major adverse cardiac event; PD-1, programmed cell death 1 receptor
Immune checkpoint inhibitors (ICIs) have transformed cancer therapy, and indications have expanded quickly, including moving to earlier settings. These therapeutics activate the immune system and include monoclonal antibodies blocking cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed death 1 receptor (PD-1) and programmed death-1 ligand, or lymphocyte activating gene 3, “brakes” in T-cell and immune cell activation.1 Responses are often durable and are improved by combination therapy (eg, anti–PD-1 + anti–CTLA-4 and anti–PD-1 + anti–lymphocyte activating gene 3). However, toxicities from these agents are caused by the interruption of immunologic T-cell self-tolerance, manifesting as autoimmune-like events that may affect any organ system.1,2
ICI myocarditis is an infrequent but potentially fulminant inflammatory side effect with a variable presentation.3 The best studied are the most severe cases, which can present early after initial exposure to ICI (often after 1 or 2 doses) and often include electrocardiographic disturbances, concomitant skeletal myositis potentially mimicking myasthenia gravis, and high mortality.4, 5, 6 Although there have been many efforts to identify risk factors for developing ICI myocarditis, the most validated risk factor is combination ICI therapy (eg, anti–CTLA-4 + anti–PD-1).5,7 After initial descriptions of the syndrome that involved more severe cases, higher vigilance by clinicians as well as screening and surveillance strategies have resulted in a higher detection of cases. However, this has also resulted in the detection of more subacute or smoldering and potentially clinically insignificant cases in which patients may be asymptomatic but have abnormal cardiac biomarkers or imaging studies.8,9 An important, unanswered question is the proper care of these less severe cases. This is an important consideration, especially because the care will involve the cessation of further treatment with ICI, a therapy that may be potentially lifesaving from an oncologic perspective.
Treatment of myocarditis recommended by the various cardiology and oncology society guidelines has been largely extrapolated from therapies for noncardiac ICI-associated toxicities, including the cessation of ICI, supportive management, and corticosteroid therapy.10 Generally, immune-related adverse events are treated with prednisone ranging from 0.5 to 2.0 mg/kg (or equivalent) followed by a 4- to 6-week taper upon symptom and biomarker improvement. In the case of myocarditis, higher steroid doses (eg, intravenous methylprednisolone 1 g) have been advocated.11 This recommendation comes from a case series of clinically suspected ICI myocarditis (N = 126) in which patient outcomes were retrospectively assessed based on the timing of the initial treatment with corticosteroids (in relation to presentation) as well as the dose of therapy (with stratification of corticosteroid dose into low [<60 mg/d], intermediate [60-500 mg/d], and high-dose [501-1,000 mg/d] groups based on the initial methylprednisolone equivalent).11 The time of initiation was associated with major adverse cardiac events (MACEs) in this study whereby patients receiving corticosteroids within 24 hours regardless of dosage showed the best outcome, and patients receiving corticosteroids after 72 hours regardless of dosage showed the worst outcome.11 In addition, there was an inverse relationship between the initial dose of corticosteroids and the occurrence of MACEs, with high-dose corticosteroids associated with a 73% lower risk of MACEs. Because this study is hypothesis generating, caution is required when interpreting it. Limited data were provided regarding the severity of cases and the certainty of diagnosis. Confirming ICI myocarditis diagnosis in <24 hours is challenging, particularly because potentially greater than half of cardiac magnetic resonance images are not diagnostic at presentation and a third of cardiac magnetic resonance images can be abnormal in the cancer population eligible to receive ICI (before ICI start).3,12 Moreover, there can be significant delays between the time of biopsy and the final results being available from cardiac pathology.3
In our experience, among 60 patients consecutively admitted with suspicion of ICI myocarditis, myocarditis was subsequently excluded in approximately half of the cases.9 Multiple other case series showed poor cardiac prognosis despite prompt initiation of high-dose corticosteroid therapy. In these latter cases, deterioration of the clinical condition often occurred while tapering steroids from methylprednisolone intravenous boluses (>500 mg) to 1 to 2 mg/kg/d prednisone.6,13,14 For example, a recent retrospective case series on 24 patients from China with definite ICI myocarditis showed that 16 of 24 (67%) evolved toward corticosteroid resistance despite the prompt use of high-dose corticosteroids.13
Adding further complexity to the treatment decision has been the growing recognition that not all ICI myocarditis may be the same and that there may be a subset of patients who do not need aggressive treatment. Both Palaskas et al8 and Ederhy et al9 recently described cases in their prospective cohorts (5/18 [28%] at MD Anderson and 2/27 [7%] at Assistance Publique - Hôpitaux de Paris Sorbonne, respectively) of low-grade ICI myocarditis suspected based on asymptomatic troponin increases and confirmed on endomyocardial biopsies that did not receive or require initiation of immunosuppressive therapies. Four such patients were able to safely continue ICI treatment.8,9 These cases further highlight the clinical heterogeneity of ICI myocarditis and the critical importance of establishing criteria to guide treatment. In addition, the potential risk of fostering tumor growth upon aggressive and untargeted immunosuppression needs to be considered.1,8 These issues are critically important and suggest that a “one-size-fits-all” approach would not be an effective treatment strategy in ICI myocarditis.
Besides corticosteroids, there have been a number of other immunosuppressive therapies mainly targeting T lymphocytes that have been tested for ICI myocarditis in a small number of patients.10 Such therapies have included abatacept and Janus kinase (JAK) inhibitors (tofacitinib and ruxolitinib).1,6,13,14 Further studies are indeed required, and some are ongoing, particularly concerning the use of abatacept (NCT05195645 and NCT05335928). Abatacept is a CTLA-4 immunoglobulin fusion protein binding CD80/CD86 on antigen-presenting cells (eg, macrophages) and leads to global T-cell anergy by specifically reversing pathways activated by ICI (Figure 1).1 The rationale for the use of abatacept in ICI myocarditis has been confirmed in a preclinical mouse model of ICI myocarditis. These mice have monoallelic deletion of Ctla4 superimposed on homozygous loss of Pdcd1, which is of translational interest because they provide a preclinical model of combined anti–CTLA-4/anti–PD-1 therapy, an important risk factor for ICI myocarditis.7 Approximately half of these mice die by 3 months of age and manifest severe electrocardiographic abnormalities accompanied by myocardial infiltration by T cells and macrophages, closely recapitulating the clinical and pathological hallmarks of ICI-associated myocarditis observed in patients.7 This model suggests that Ctla4 and Pdcd1 functionally interact in a gene dosage–dependent manner, providing a mechanism by which myocarditis arises with increased frequency in the setting of combination ICI therapy. Concordantly, intervention with abatacept ameliorated disease progression and reversed mortality induced by myocarditis in this model, consistent with clinical case reports that abatacept attenuates fulminant ICI myocarditis.7
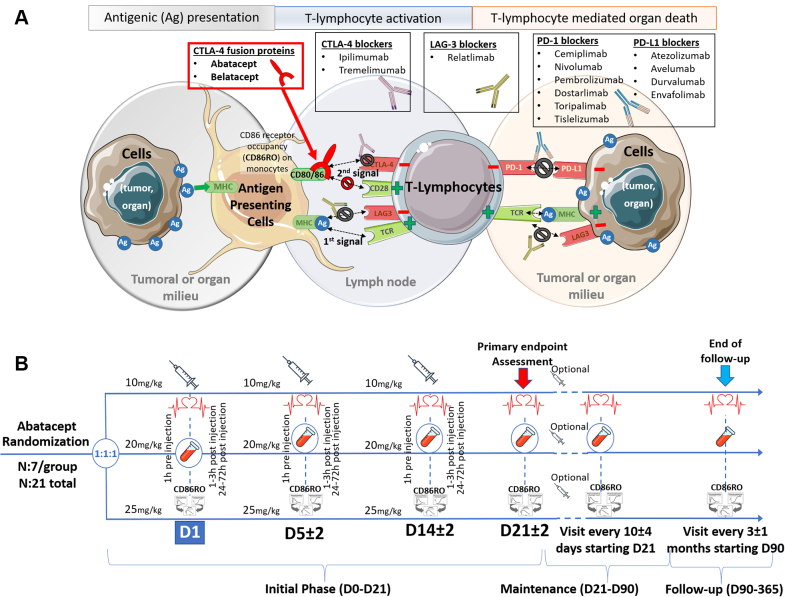
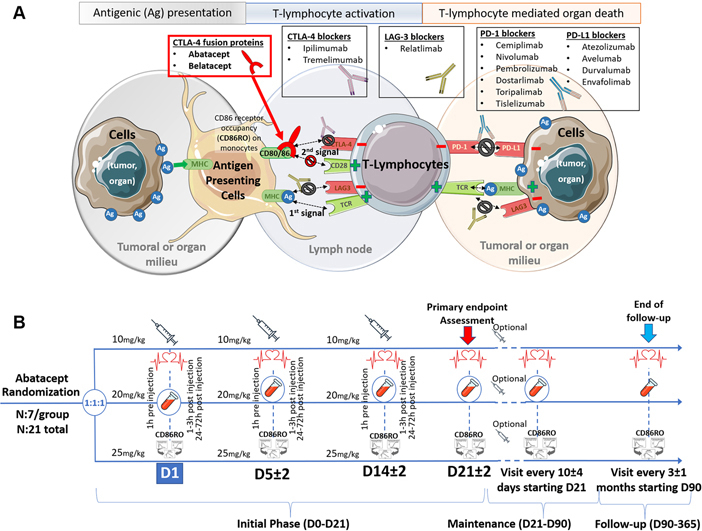
Figure 1.
Mechanism of Action of Approved Immune Checkpoint Agents
(A) T-cell activation or anergy is mediated by a complex interaction between antigen-presenting cells (APCs) (eg, macrophages) and organ or tumor cells. The first step (1st signal) of T-cell immune activation against liable cells relies on the presentation of a culprit antigen by the APCs (major histocompatibility complex [MHC] class II) to T cells through the T-cell receptor (TCR). For complete activation (2nd signal), CD28 (on T cells) needs to bind with CD80/86 on the APC side. Notably, cytotoxic T-lymphocyte antigen 4 (CTLA-4) (on T cells) has a higher affinity for CD80/86 than for CD28, impeding CD28-CD80/86 interaction and toning down T-cell activation. When exposed to CTLA-4 blockers, CD28-CD80/86 binding and T-cell activation are facilitated. CTLA-4 fusion protein (eg, abatacept) has opposite effects. (B) A scheme of the ongoing phase II ACHLYS trial (NCT05195645), seeking to define the optimal abatacept dose to be used in ICI myocarditis to achieve a prompt and strong blockade (receptor occupancy, RO) of its target (CD86) on circulating monocytes (ie, CD86RO ≥ 80%). Ag, antigen; CTLA-4, cytotoxic T-lymphocyte antigen 4; LAG-3, lymphocyte-activation gene 3; PD-1, programmed cell death 1 receptor; PD-L1, programmed death 1 ligand.
A few considerations are important in future studies with abatacept. Perhaps most importantly, with its standard dosing (10 mg/kg every 2 weeks), abatacept has a slow time to onset and would not be optimal for the management of rapidly evolving and life-threatening ICI myocarditis cases.1,7 A pharmacodynamic biomarker tracking abatacept’s clinical efficacy has been proposed, which relies on the assessment of the receptor occupancy of its target (cluster of differentiation 86 receptor occupancy [CD86RO]) on circulating monocytes. Levels of CD86RO ≥ 80% have been proposed as satisfactory.14 We recently successfully used such immune-monitoring tools and personalized abatacept dose administration combined with ruxolitinib in a young thymoma patient who developed fulminant ICI myocarditis resistant to 1-g methylprednisolone intravenous boluses instituted within 24 hours of presentation. In this case, the abatacept dose used in the first 2 weeks was 4 to 6 times higher compared with those expected to be used in already approved indications. To further assess the optimal starting dose of abatacept to be used to achieve promptly CD86RO ≥80% in ICI myocarditis, we are leading a phase II trial. Three groups of 7 patients by dose strategy (10 vs 20 vs 25 mg/kg intravenous on days 1, 5, and 14) will be included in the clinical trial (NCT05195645) (Figure 1).
To mitigate the expected delayed full efficacy of abatacept, the use of other synergistic immunosuppressants with faster time to onset in combination may be needed. JAK inhibitors (eg, ruxolitinib), which impair T-cell activation via the blockade of proinflammatory cytokines and are used to treat graft versus host disease, are potential candidates and have shown promising results in near-lethal ICI myocarditis patients.14 Among JAK inhibitors, ruxolitinib has the additional benefit of decreasing CD86 expression on macrophages, synergizing CD86 blockade by abatacept, while also acting downstream in the immunologic synapse and inactivating T cells.1,15,16
To conclude, further research will be required to evaluate the optimal drugs, including the dosage and duration to be used, to preserve ICI therapeutic effects while treating severe ICI-related adverse events. A combination of multiple lower-dose synergistic immunosuppressants rather than boluses of high-dose corticosteroids as initial therapy followed by second-line immunosuppressants upon corticosteroid failure are 2 strategies to be compared. The emergence of an active surveillance strategy in patients on ICI may also lead to the identification of low-grade asymptomatic ICI myocarditis, for which the natural history (and opportunities for ICI challenge) is unknown. International efforts are ongoing (NCT04294771) to help better stratify the risks, grade the severity, evaluate best treatment strategies, and determine the full clinical spectrum of ICI myocarditis.4
Funding Support and Author Disclosures
Dr Moslehi is supported by National Institutes of Health grants (R01HL141466, R01HL155990, R01HL156021, and R01HL160688); and has served on advisory boards for Pfizer, Novartis, Bristol-Myers Squibb, Deciphera, Audentes Pharmaceuticals, Takeda, AstraZeneca, Heat Biologics, Incyte, Vertex, Verastem Oncology, Acceleron Pharmaceuticals, GlaxoSmithKline, TripleGene LLC, Regeneron, Nektar Therapeutics, Boston Biomedical, ImmunoCore, Janssen, Myovant, Silverback Therapeutics, Amgen, Kurome Therapeutics, Kiniksa Pharmaceuticals, Daiichi Sankyo, CRC Oncology, BeiGene, Star Therapeutics, ProteinQure, Pharmacyclics, Kiniksa, Prelude Therapeutics Mallinckrodt Pharmaceuticals, Boehringer, TransThera Sciences, Antev Ltd, IQVIA, AskBio, Lapcorp, Paladin, Quell Therapeutics, Voyager Therapeutics, CRC Oncology, and Cytokinetics. Dr Moslehi and Salem have patents pending related to the treatment of immune-related adverse events including ICI myocarditis.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Contributor Information
Javid Moslehi, Email: javid.moslehi@ucsf.edu.
Joe-Elie Salem, Email: joe-elie.salem@aphp.fr.
References
- 1.Geraud A., Gougis P., Vozy A., et al. Clinical pharmacology and interplay of immune checkpoint agents: a yin-yang balance. Annu Rev Pharmacol Toxicol. 2021;61:85–112. doi: 10.1146/annurev-pharmtox-022820-093805. [DOI] [PubMed] [Google Scholar]
- 2.Wang D.Y., Salem J.E., Cohen J.V., et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehmann L.H., Cautela J., Palaskas N., et al. Clinical strategy for the diagnosis and treatment of immune checkpoint inhibitor-associated myocarditis: a narrative review. JAMA Cardiol. 2021;6:1329–1337. doi: 10.1001/jamacardio.2021.2241. [DOI] [PubMed] [Google Scholar]
- 4.Power J.R., Alexandre J., Choudhary A., et al. Electrocardiographic manifestations of immune checkpoint inhibitor myocarditis. Circulation. 2021;144:1521–1523. doi: 10.1161/CIRCULATIONAHA.121.055816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salem J.E., Manouchehri A., Moey M., et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salem J.E., Allenbach Y., Vozy A., et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. 2019;380:2377–2379. doi: 10.1056/NEJMc1901677. [DOI] [PubMed] [Google Scholar]
- 7.Wei S.C., Meijers W.C., Axelrod M.L., et al. A genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discov. 2021;11:614–625. doi: 10.1158/2159-8290.CD-20-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palaskas N.L., Segura A., Lelenwa L., et al. Immune checkpoint inhibitor myocarditis: elucidating the spectrum of disease through endomyocardial biopsy. Eur J Heart Fail. 2021;23:1725–1735. doi: 10.1002/ejhf.2265. [DOI] [PubMed] [Google Scholar]
- 9.Ederhy S., Devos P., Pinna B., et al. (18)F-fluorodeoxyglucose positron emission tomography/computed tomography imaging for the diagnosis of immune checkpoint inhibitor-associated myocarditis. Arch Cardiovasc Dis. 2022;115:114–116. doi: 10.1016/j.acvd.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Schneider B.J., Naidoo J., Santomasso B.D., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39:4073–4126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L., Zlotoff D.A., Awadalla M., et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation. 2020;141:2031–2034. doi: 10.1161/CIRCULATIONAHA.119.044703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faron A., Isaak A., Mesropyan N., et al. Cardiac MRI depicts immune checkpoint inhibitor-induced myocarditis: a prospective study. Radiology. 2021;301:602–609. doi: 10.1148/radiol.2021210814. [DOI] [PubMed] [Google Scholar]
- 13.Wang C., Lin J., Wang Y., et al. Case series of steroid-resistant immune checkpoint inhibitor associated myocarditis: a comparative analysis of corticosteroid and tofacitinib treatment. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.770631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen L.S., Bretagne M., Arrondeau J., et al. Reversal of immune-checkpoint inhibitor fulminant myocarditis using personalized-dose-adjusted abatacept and ruxolitinib: proof of concept. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2022-004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lescoat A., Lelong M., Jeljeli M., et al. Combined anti-fibrotic and anti-inflammatory properties of JAK-inhibitors on macrophages in vitro and in vivo: perspectives for scleroderma-associated interstitial lung disease. Biochem Pharmacol. 2020;178 doi: 10.1016/j.bcp.2020.114103. [DOI] [PubMed] [Google Scholar]
- 16.Deszo E.L., Brake D.K., Kelley K.W., Freund G.G. IL-4-dependent CD86 expression requires JAK/STAT6 activation and is negatively regulated by PKCδ. Cell Signal. 2004;16:271–280. doi: 10.1016/s0898-6568(03)00137-2. [DOI] [PubMed] [Google Scholar]