Abstract
As the clinical applications of immune checkpoint inhibitors (ICIs) expand, our knowledge of the potential adverse effects of these drugs continues to broaden. Emerging evidence supports the association between ICI therapy with accelerated atherosclerosis and atherosclerotic cardiovascular (CV) events. We discuss the biological plausibility and the clinical evidence supporting an effect of inhibition of these immune checkpoints on atherosclerotic CV disease. Further, we provide a perspective on potential diagnostic and pharmacological strategies to reduce atherosclerotic risk in ICI-treated patients. Our understanding of the pathophysiology of ICI-related atherosclerosis is in its early stages. Further research is needed to identify the mechanisms linking ICI therapy to atherosclerosis, leverage the insight that ICI therapy provides into CV biology, and develop robust approaches to manage the expanding cohort of patients who may be at risk for atherosclerotic CV disease.
Key Words: atherosclerosis, cardio-oncology, cardiovascular disease, immune checkpoint inhibitor, inflammation
Abbreviations and Acronyms: ASCVD, atherosclerotic cardiovascular disease; CTLA-4, cytotoxic T-lymphocyte associated protein 4; ICI, immune checkpoint inhibitor; IFN, interferon; IL, interleukin; irAE, immune-related adverse event; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; TNF, tumor necrosis
Central Illustration
Highlights
-
•
Immune checkpoint inhibitor (ICI) therapy is associated with an increased risk of atherosclerotic CV events likely mediated by accelerated atherosclerosis.
-
•
The pathophysiological mechanism of ICI-related atherosclerosis is incompletely understood but may be linked to inflammation and immune dysregulation.
-
•
There is a need for heightened awareness of potential atherosclerotic CV events during and after ICI treatment.
-
•
Optimization of CV risk factors and pharmacological interventions may help mitigate atherosclerotic CV risk and improve the prognosis of ICI-treated patients.
Cancer immunotherapy has changed the landscape of oncology, amplified by the emergence of immune checkpoint inhibitors (ICIs).1, 2, 3, 4 Immune checkpoints are molecules expressed by multiple immune regulators to activate or inactivate the immune system. However, cancer cells can also express these molecules to avoid detection by the immune system. Current Food and Drug Administration (FDA)–approved ICIs are monoclonal antibodies that block these immune checkpoints to reduce the negative regulatory signals and enhance the positive co-stimulatory signals that modulate cytotoxic T cell recognition against tumor neoantigens.2 ICIs blocking the cytotoxic T-lymphocyte associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed cell death ligand 1 (PD-L1) pathways have become pillars in the treatment of numerous malignancies when used either alone or in combination regimens.5
Beyond these, multiple other immune checkpoints are being targeted for cancer therapy. Some of these are more recently FDA-approved (lymphocyte-activation gene 3 [LAG-3]), and many others are in the later stages of clinical development such as those targeting CD47, T cell immunoglobulin and mucin-domain containing-3 (TIM-3), inducible T cell costimulatory (ICOS), T cell immunoglobulin and ITIM domain (TIGIT), B7 homolog 3 protein (B7-H3), V-domain immunoglobulin suppressor of T cell activation (VISTA), and B and T lymphocyte attenuator (BTLA). There are 10 ICIs approved by the U.S. FDA: 2 CTLA-4–blocking antibodies (ipilimumab, tremelimumab), 4 PD-1–blocking antibodies (nivolumab, cemiplimab, dostarlimab, and pembrolizumab), 3 PD-L1–blocking antibodies (atezolizumab, avelumab, durvalumab), and 1 targeting LAG-3 (relatlimab).1,2 Given the effectiveness of these therapies, the number of approvals for ICIs has rapidly increased. As of June 2022, there were approximately 125 indications for ICIs in adjuvant and neoadjuvant settings, including some as first-line therapy, in more than 20 distinct cancer types.6 This number will increase as multiple ICIs targeting over 300 different proteins are being tested in more than 5600 clinical trials. It is estimated that approximately 36% of U.S. cancer patients are currently eligible for ICI therapy.7,8 Because immune checkpoints also regulate autoreactivity, it is not surprising that ICIs have been associated with disinhibited cytotoxic T cells off-targeting healthy tissue in multiple organs, known as immune-related adverse events (irAEs). These irAEs are clinically diverse, may arise in up to 70% of patients, and are typically mild and easy-to-manage events.9 However, disabling or life-threatening high-grade events, such as ICI-associated cardiotoxicities, can also occur and significantly impact both cancer therapy and overall outcomes. Our understanding of the range and incidence of cardiotoxicity associated with ICI use has improved. For example, the frequency of cardiac events in patients treated with an ICI has, until recently, been underestimated but studies now show an incidence of major adverse cardiac events up to 10.3%.10, 11, 12 Additionally, the understanding of the breadth of ICI-related toxicities and their potential cardiovascular (CV) effects have increased. Initially, ICI-related cardiac events focused on myocarditis; however, recent data have expanded this association to include heart failure, cardiomyopathy, conduction abnormalities, venous thrombosis, pericardial disease, vasculitis, and the focus of this review, atherosclerotic-related events.10,11,13, 14, 15, 16 Given the success of ICIs across many cancers, the increased risk of aggravated atherosclerosis and atherosclerotic cardiovascular disease (ASCVD) in ICI-treated patients, particularly in adjuvant and neoadjuvant settings, is potentially important.
Key Points
-
•
ICIs have revolutionized the field of oncology, becoming the standard of care for numerous malignancies.
-
•
The rapidly expanding use of ICIs has revealed many possible irAEs, including several ICI-related cardiac events beyond the rare incidences of myocarditis.
ICI-Associated ASCVD: An Overview
Several established cancer therapies such as radiation and targeted therapies have been shown to increase atherosclerosis and related events.17,18 Evolving data suggest that current FDA-approved ICIs may accelerate atherosclerosis and lead to an increase in atherosclerosis-related CV events such as acute myocardial infarction (MI), stroke, and peripheral arterial disease.19 Heart disease is a leading cause of death in cancer survivors, and cancer and cardiovascular disease (CVD) share risk factors such as aging, diabetes, hypertension, cardiometabolic dysfunction, physical inactivity, tobacco use, and chronic low-grade inflammation.20,21 Additionally, cancer, beyond shared risk factors and cancer therapies, is being studied as a potential independent risk factor for the development of heart disease.22,23 Accordingly, as the ICI approvals expand, especially to adjuvant and neoadjuvant care, and the overall survival time of patients receiving ICIs improves, the potential risk of CV events in this population needs to be considered. This risk is supported by a deep understanding of the role of these ICIs in critical atherosclerosis pathways, as illustrated in Central Illustration.6,8 Specifically, robust basic cellular and animal data demonstrate that the immune checkpoint proteins, which are currently targeted in FDA-approved ICI-approved therapies (CTLA-4, PD-1, LAG-3, and PD-L1), are also critical negative regulators of atherosclerosis.24, 25, 26 Thus, their blockade may lead to accelerated atherosclerosis by enhancing effector T cell responses, limiting regulatory T (Treg) cell function, and infiltrating the vascular endothelium.25, 26, 27, 28, 29 Furthermore, a growing body of clinical evidence supports these preclinical findings by showing that ICI therapy leads to the accelerated progression of atherosclerotic plaque, thereby increasing the risk of ASCVD.30, 31, 32 In this review, we highlight the existing evidence on the impact of ICIs on coronary atherosclerosis and describe the molecular mechanisms linking inflammation and cell-mediated immunity to atherogenesis in the context of ICI therapy. We discuss potential diagnostic and therapeutic approaches to reduce the impact of ASCVD on ICI-treated patients with cancer—and the clinical implications for optimal CV risk management and cardio-oncology care in ICI-treated patients during and after immunotherapy.
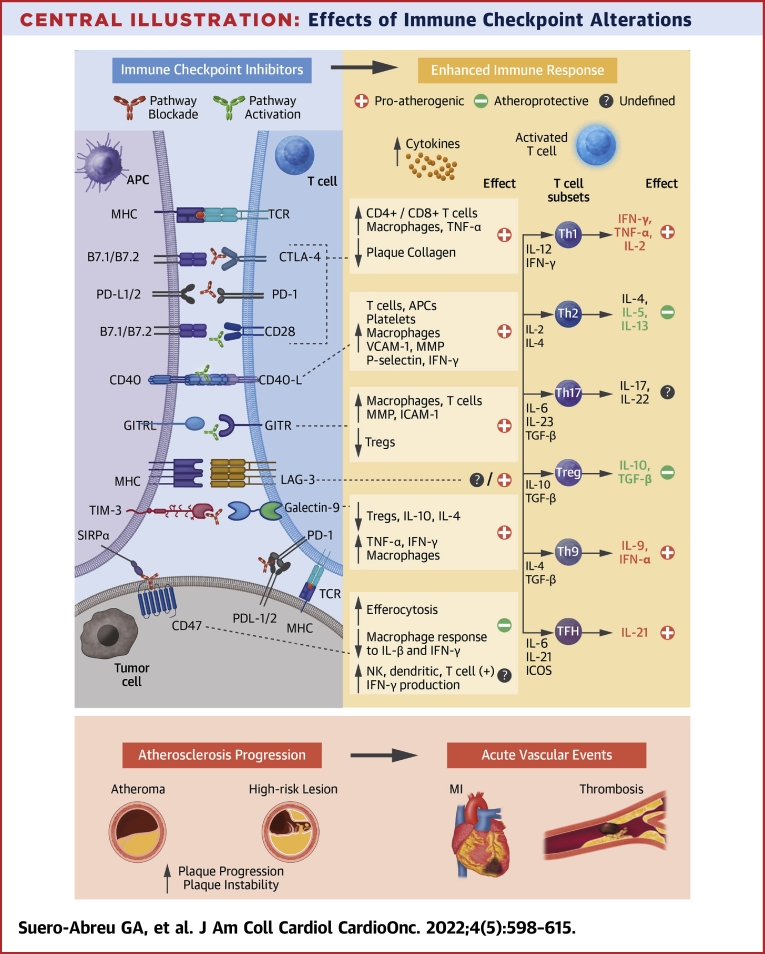
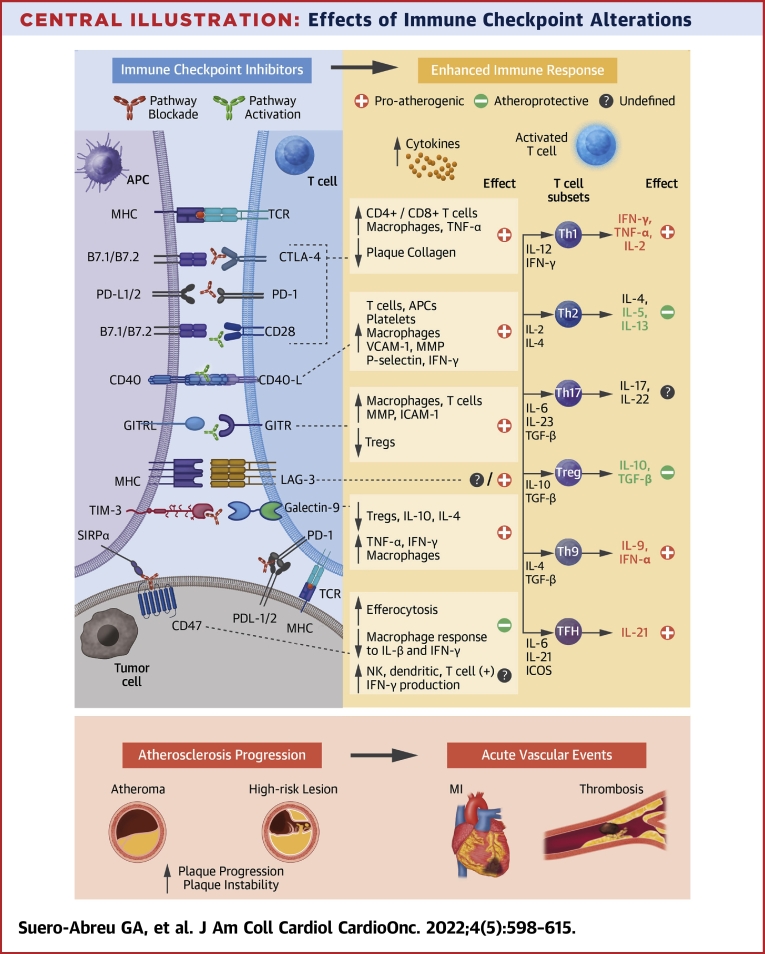
Central Illustration.
Effects of Immune Checkpoint Alterations
Immune checkpoint inhibitors (ICIs) cause an enhanced immune response and persistent inflammation. Several pathways are being implicated in post-ICI aggravated atherosclerosis, with the strongest evidence for the co-inhibitory blockade via the cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) and the co-stimulatory agonism of CD40/CD40L and glucocorticoid-induced tumor necrosis factor family-related protein (GITR) pathways. Costimulatory molecules were B7.1= CD80 and B7.2 = CD86. APC = antigen-presenting cell; GITRL = glucocorticoid-induced tumor necrosis factor family-related protein ligand; ICAM= intracellular adhesion molecule; ICOS = inducible costimulatory; IFN = interferon; IL = interleukin; LAG-3 = lymphocyte activation gene 3; MHC = major histocompatibility complex; MI = myocardial infarction; MMP = matrix metalloproteinase; NK = natural killer; SIRP = signal-regulatory protein; TCR = T cell receptor; TGF = transforming growth factor; Th1 = T helper 1; TIM-3 = T cell immunoglobulin and mucin containing domain-3; TNF = tumor necrosis factor; Treg = regulatory T; VCAM = vascular cell adhesion molecule.
Key Points
-
•
Cancer and CVD share many risk factors, and heart disease is a leading cause of morbidity and mortality in cancer survivors.
-
•
Emerging robust preclinical and clinical evidence associates ICIs with progression of atherosclerosis and increased ASCVD.
The Role of Inflammation in Atherosclerosis
Decades of insightful basic, translational, and clinical research have established atherosclerosis as a chronic inflammatory disease driven by an imbalance in lipid metabolism, vascular function, and a maladaptive immune response.33 At the broadest level, activated M1 macrophages initiate and sustain inflammation, whereas activated M2 macrophages are linked to inflammation resolution. M1 macrophages promote the accumulation of intracellular lipids and the secretion of proinflammatory factors such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6. Conversely, M2 macrophages (associated with IL-4 and IL-13) promote the clearance of lipids and the secretion of anti-inflammatory factors such as IL-10 and collagen.34,35 These M1/M2 macrophage phenotypes are present in human and mouse atherosclerotic lesions, and their balance is a dynamic process recognized as an essential driver in plaque formation, progression, and vulnerability. ICIs may accelerate atherosclerosis and increase ASCVD through inflammation and immune activation. This is similar to other established disease models of ASCVD such as HIV. Specifically, studies have shown that patients with HIV with undetectable viral loads have a heightened CVD risk that is not fully accounted for by traditional risk factors, with a persistent immune activation and inflammation, leading to the progression of atherosclerosis.36, 37, 38 Moreover, studies using coronary computed tomography (CT) angiography have described an association between arterial inflammation and increased noncalcified plaque (NCP) and high-risk plaque (HRP) morphology among treated HIV patients with low Framingham Risk Score and without known CVD as compared with control subjects.39,40 The overlap here is not insignificant as the same immune checkpoints targeted in cancer likely also play a key role in HIV, and these patients have increased CTLA-4 and PD-1 on T cells.41 Further, HIV is known to evade the immune system by promoting exhaustion, similar to how cancers elude immune eradication.2,41
Studies such as CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study), COLCOT (Colchicine Cardiovascular Outcomes Trial), and LoDoCo (Low-dose Colchicine) have suggested that therapeutic targeting of some inflammatory and immune-related pathways may reduce atherosclerosis-related CV events.42 Therefore, understanding this mechanistic diversity and immune dysregulation at the plaque site is crucial in identifying immunotherapeutic targets for CVD beyond the standard of care management.
Key Points
-
•
Atherosclerosis is a complex process of persistent inflammation and immune activation.
-
•
An imbalance between proinflammatory and anti-inflammatory factors is an essential driver of atherosclerotic plaque formation, progression, and rupture, which is potentially influenced by the effect of ICIs.
The Role of Immunity in Atherosclerosis
While B and T lymphocytes both contribute to plaque development and progression, atherosclerosis is mainly considered a T cell-driven disease.43 Innovative single-cell proteomic and transcriptomic analyses of murine and human atherosclerotic lesions have helped characterize the immune cell repertoire and the distinct molecular features of innate and adaptive immune cells associated with stable and vulnerable lesions.44, 45, 46, 47 For example, single-cell RNA studies in human atherosclerotic carotid lesions from patients with clinically symptomatic disease identified activated T cells such as CD8+ cytotoxic T lymphocytes and CD4+ lymphocytes (T helper 1 [Th1] cells, Th2 cells, Th17 cells, and Treg cells).26,48 Th1 cells are the predominant cell type found in plaques and have been associated with proatherogenic cytokines such as interferon gamma (IFN-γ) and TNF-α.49 In mouse studies, TNF-α is linked to advanced necrotic plaques, and Th1 inhibition is atheroprotective through reduced IFN-γ levels in plaques. Another well-defined atheroprotective role is related to Treg cells through secretion of transforming growth factor β and IL-10 thus promoting the anti-inflammatory macrophage phenotype.50,51 Studies also showed that Treg cells constitutively express CTLA-4, and their quantity is inversely correlated with human plaque size and vulnerability.52 Additionally, plaques of ICI-treated patients contain T cell subsets with markers of cell exhaustion (high levels of PD-1 and perforin as well as transcriptional signatures for PDCD1 and LAG-3) and macrophages with activated phenotypes associated with plaque vulnerability.26 Because exhausted T cells expressing PD-1 exist in atherosclerotic plaques, it suggests that PD-1 inhibitors may activate T cells in plaques and aggravate atherosclerosis.53
Key Points
-
•
Immune checkpoint proteins have a role in the crosstalk between and within immune and nonimmune cells that modulate atherogenesis.
-
•
The immune checkpoint proteins that are inhibited to treat cancer (CTLA-4, PD-1, PD-L1, and LAG-3) are also critical negative regulators of atherosclerosis.
Immune Checkpoints and the Pathophysiology of ICI-Related Atherosclerotic CVD
Numerous studies have explored immune checkpoints as potential therapeutic targets for atherosclerosis and the first 2 therapeutic strategies translated from bench to bedside are the antibody-mediated blockade of CTLA-4 and the PD-1–PD-L1 dyad.54 Genetic knockout models and pharmacological modulation of the ICI target proteins PD-1, PD-L1, and CTLA-4 have been used to elucidate the role of these co-inhibitory proteins in experimental atherogenesis (Table 1).
Table 1.
Preclinical Studies on the Role of CTLA-4, PD-1, and PD-L1/2 in Atherosclerosis
| Model | Atherosclerosis | Effect on plaque |
|---|---|---|
| Pdl1/2-/-Ldlr-/- mice25 | ↑ | ↑ CD4+/CD8+ T ↑ Macrophages |
| Pd1-/-Ldlr-/- mice27 | ↑ | ↑ CD4+/CD8+ T ↑ Macrophages ↑ Apoptotic cells |
| Antibody-mediated PD-1 inhibition in Ldlr-/- mice27 | ↑ | ↑ CD4+/CD8+ T |
| Abatacept in ApoE3 Leiden mice55 | ↓ | ↓ Intimal thickening ↓ Intimal leukocytes |
| Anti-CTLA-4 antibody in ApoE3 Leiden mice55 | ↑ | ↑ Intimal thickening = intimal leukocytes |
| CTLA-4 transgenic T cell–specific constitutive expression (CTLA-4-Tg)/Apoe(-/-) mice29 | ↓ | ↓ CD4+ T cells ↓ Macrophages |
| Anti-CTLA-4 antibody in Ldlr-/- mice56 | ↑ | ↑ Advanced lesions ↑ Necrotic core ↑ CD3+ T cells |
| Combined anti-CTLA-4 and anti-PD-1 antibodies in Ldlr-/- mice57 | ↑ | ↑ CD3+/CD8+ T↑ Advanced lesions ↑ Necrotic core ↑ Apoptotic macrophages |
Up arrows indicate an increase in atherosclerotic burden; down arrows indicate a decrease in atherosclerotic burden.
CTLA-4 = cytotoxic T-lymphocyte associated protein 4; PD-1 = programmed cell death protein 1; PD-L1 = programmed cell death protein ligand 1.
Mechanistically, lower levels of the PD-1–PD-L1 dyad are linked to higher coronary atherosclerotic plaque burden, in which both PD-1 and PD-L1 suppress T cell–driven inflammation in plaques and plaque progression.28 Gotsman et al25 studied the role of the PD-L1/2 pathway in regulating proatherogenic T cell responses by comparing atherosclerotic lesion burden and phenotype in hypercholesterolemic PD-L1/2-/-LDLR-/- mice and LDLR-/- control subjects. They showed that PD-L1/2 deficiency correlated with increased atherosclerotic burden throughout the aorta and increased numbers of CD4+ and CD8+ T cells in the lesions.25 Similarly, a study by Bu et al27 showed that PD-L1/2-/-Ldlr-/- mice developed larger lesions with abundant CD8+ T cells and macrophages. Additionally, these studies showed that PD-L1/2-/-Ldlr-/- cells were more susceptible to antigen-presenting cell–induced proliferation, had an activated phenotype, and expressed higher levels of proatherosclerotic cytokines (IFN-γ and TNF-α).25,27 Further, expression of PD-1 and PD-L1 on human peripheral blood mononuclear cells by flow cytometry was significantly down-regulated on T cells and myeloid dendritic cells in 76 patients with coronary artery disease when compared with healthy control subjects.28 These findings indicated the critical role of the PD-1/PD-L pathway in down-regulating proatherogenic T cell response and atherosclerosis by limiting antigen-presenting cell–dependent T cell activation.
The effects of the CTLA-4 pathway in atherosclerosis were also studied in a transgenic (CTLA-4-Tg)/Apoe(-/-) mouse model in which constitutive overexpression of CTLA-4 in T cells reduced atherosclerotic lesion formation and intraplaque accumulation of macrophages and CD4+ T cells.29 In addition, systemic treatment with abatacept, a soluble CTLA-4Ig fusion protein that prevents CD28-CD80/86 co-stimulatory T cell activation, reduced accelerated atherosclerosis development and prevented CD4 T cell activation in hypercholesterolemic ApoE3∗Leiden mice.55 Poels et al56 showed that antibody-mediated inhibition of CTLA-4 Ldlr-/- mice accelerated the progression of atherosclerosis by inducing a predominantly T cell-driven endothelial inflammation and resulted in a 2-fold increase in plaque area with an advanced, clinically unfavorable phenotype. Similarly, T cell–mediated inflammation, vascular dysfunction, and plaque progression were seen with dual antibody-mediated inhibition of CTLA-4 and PD-1 on atherosclerosis in hyperlipidemic mice (Ldlr-/- and Apoe-/- mice) using a combination of immunological and 2-deoxy-2-[fluorine-18] fluoro-D-glucose (18F-FDG) positron emission tomography–CT (PET–CT) techniques.57
In summary, these experimental models of atherosclerosis have elucidated that the PD-1–PD-L1 dyad and CTLA-4 reduce T cell–driven inflammation, thereby mitigating plaque development and progression. Therefore, their inhibition in current oncological immunotherapies may activate T cells in plaques and aggravate atherosclerosis in these patients. While the molecular pathways of ICI-associated atherosclerosis beyond PD-1, PD-L1, and CTLA-4 are incompletely understood, approaches targeting novel co-stimulatory and co-inhibitory immune checkpoints, are currently under investigation (Table 2).58, 59, 60
Table 2.
Immune Checkpoint Targets Associated With Atherosclerosis
| Immune Checkpoint Class | ICI Target | Role on Immunitya | Role on Atherosclerosisa | Effect of ICIb | Therapies |
|---|---|---|---|---|---|
| Co-inhibitory | PD-1/PD-L1122, 123, 124, 125, 126 | ↓ Immunity ↑ Self-tolerance ↓ T cell activation ↑ APC apoptosis ↓ Treg apoptosis |
Atheroprotective25, 26, 27, 28,47,127 | ↑ Plaque size ↑ Plaque progression ↑ CD4+/CD8+ activation ↑ Macrophage activation ↑ TNF-α ↑ Intracellular cholesterol |
Anti-PD-1: Nivolumabc Pembrolizumabc Cemiplimabd Spartalizumabd Camrelixumabd Tislelizumabd Anti-PD-L1: Atezolizumabc Avelumabc Durvalumabc Cosebelimabd Sugemalimabd CX-072d |
| Co-inhibitory | CTLA-4128,129 | Atheroprotective25,29,55,56,130,131 | ↑ Plaque size ↑ Plaque progression ↑ Endothelial activation ↓ Collagen ↑ Necrotic core |
Ipilimumabc Tremelimumabd Zalifrelimabd |
|
| Co-inhibitory | TIM-360,132,133 | ↓ Immunity ↓ Th1 apoptosis ↓ CD8+ apoptosis ↓ NK cells |
Atheroprotective134,135 | ↑ Plaque size ↓ Treg intraplaque ↑ Macrophage activation ↑ IFN-γ ↑ TNF-α |
Cobolimabd MBG453d BMS-986258d BGB-A425d TSR-022d Sym023d |
| Co-inhibitory | LAG-360,136 | ↓ Immunity ↓ T cell suppression ↑ Treg |
Likely atheroprotective137 | ↓ CD4+/CD8+ activation ↓ NK cells ↓ DC cells Synergistic with PD-1 |
Relatlimabd Eftilagimod alphad Favezelimabd Tebotelimabd LAG525d REGN 3767d |
| Co-stimulatory | GITR/GITRL138,139 | ↑ Macrophage ↑ CD4+/CD8+ ↓ Treg ↑ IFN-γ ↑ IL-2 ↑ ICAM-1 ↑MMP |
Proatherogenic140,141 | ↓ Plaque size ↑ Plaque stability ↓ Macrophages activation ↓ T cell recruitment ↓ Necrotic core |
AMG228d MEDI-1873d BMS-986156ad |
| Co-stimulatory | CD40/CD40L142,143 | ↑ B cells ↑ APCs ↑ IFN-γ ↑ TNF-α ↑ IL-6 ↑ IL-1β ↑ VCAM-1 ↑ MMP ↑ Selectin |
Proatherogenic93,144,145 | ↑ Plaque stability Inconsistent plaque size ↓ Atherothrombosis ↓ T cells ↓ Th1 ↓ IFN-γ |
Selicrelumabd Dazetuzumabd CP-870893d JNJ-107d APX005Md |
| Co-stimulatory | CD27/CD70146,147 | ↑ T cell expansion ↑ Treg survival ↑ IFN-γ ↑ Efferocytosis |
Atheroprotective148,149 | ↑ Plaque size ↑ Plaque progression ↑ Plaque inflammation ↓ oxLDL removal |
Anti-CD27: Varlilumabd AMG-172d Anti-CD70: Cusatuzumabd BMS-936561163d |
| Co-stimulatory | ICOS/ICOSL 150,151 | ↑ Cytotoxic T cell ↑ Effector T cell ↑ Treg activity ↑ IL-4, IL-5 ↑ TNF-α, IL-10 |
Atheroprotective152, 153, 154 | ↑ Plaque size ↓ Treg intraplaque ↑ IFN-γ ↓ IL-10 |
ICOS agonists: Feladilimabd Vopratelimabd ICOS antagonists: MEDI-570d KY1044100 |
| Macrophage signal | CD4761,155 | ↑ Efferocytosis ↑ Phagocytosis ↓ Inflammation SIRP-α blockade |
Likely atheroprotective62,64, 65, 66 | ↓ Macrophage response to IL-β and IFN-γ ↓ Vascular inflammation ↑ Clearance of VSMCs |
Magrolimabd SRF231d AO-176d CC-900021d |
AGEN = Agenus; AMG = Amgen; AO = Arch Oncology; APX = Apexigen; APC = antigen-presenting cell; BGB-A = BeiGene; BMS = Bristol Myers Squibb; CC = Celgene; CTLA-4 = cytotoxic T-lymphocyte associated protein 4; CX = CytomX; DC = dendritic cell; ICAM = intracellular adhesion molecule; ICI = immune checkpoint inhibitor; IFN = interferon; IL = interleukin; JNJ = Johnson & Johnson; KY = Kymab; MMP = matrix metalloproteinase; NK = natural killer; oxLDL = oxidized low-density lipoprotein; PD-1 = programmed cell death protein 1; PD-L1 = programmed cell death ligand 1; REGN = Regeneron Pharmaceuticals; SIRP = signal regulatory protein; SRF = Surface Oncology; Sym = Symphogen; Th1 = T helper 1; TNF = tumor necrosis factor; Treg = regulatory T; VCAM = vascular cell adhesion molecule; VSMC = vascular smooth muscle cell.
IC/pathway effect under normal conditions.
Effect for the immune checkpoint target from preclinical/clinical data as of June 2022.
Food and Drug Administration approved.
Investigational.
Importantly, not all ICIs that are targeted for cancer are likely to aggravate atherosclerosis, and some may prevent the progression of atherosclerosis. There are significant data on the role of CD47, a critical macrophage-mediated immune checkpoint associated with efferocytosis, in atherosclerosis regression. Specifically, CD47 is increased in cancer and is being targeted in several cancer trials.61, 62, 63 CD47 is also increased in the atherosclerotic plaque and treatment with CD47 inhibitory antibodies in mouse models reduced atherosclerosis by restoring efferocytosis and removing diseased vascular smooth muscles and macrophages.32,64,65 In addition, CD47 inhibition down-regulated genes implicated in the macrophage response to IL-1 and IFN-γ, leading to a reduction in atherosclerotic inflammation in PET–CT imaging.64 However, in contrast, a study by Engelbertsen et al66 observed increased plaque formation in CD47-deficient mice compared with wild-type control mice via activation of dendritic cells, T cells, and natural killer cells. Clinically, there are some early supportive data toward an atheroprotective role via CD47 inhibition.66 In a small, single-institution retrospective analysis of data from a phase 1b-2 trial of a CD47-targeting macrophage checkpoint inhibitor (magrolimab) there was a reduction in arterial 18F-FDG uptake suggestive of suppressed vascular inflammation. However, further work is warranted, as this study was limited by the inclusion of only a small number of patients at a single institution, and the study was neither randomized nor placebo controlled.61
Key Points
-
•
Robust preclinical data show that ICI therapies modulate T cell activation—via either co-inhibitory signal blockade or co-stimulatory signal agonism—and promote atherosclerosis.
-
•
Future translational and clinical studies are warranted to elucidate the inflammatory and immune biomarkers linked to ICI-associated atherosclerosis.
-
•
Not all ICIs will accelerate atherosclerosis and some may reduce atherosclerosis.
Clinical Implications of ICI Therapy and Atherosclerotic CV Events
There are limited clinical data on the effects of FDA-approved ICI therapies on atherosclerotic lesions and on the incidence of atherosclerosis-related CV events. Initially, data were from case reports and small cohort observational studies.67, 68, 69, 70, 71, 72, 73 In 2017, Tomita et al67 reported the first case of acute coronary syndrome as a possible ICI-related irAEs in a lung cancer patient receiving nivolumab who was concomitantly diagnosed with acute occlusion of the right coronary artery and ICI-induced interstitial pneumonia.67 In contrast to this finding, Gelsomino et al68 published an observational series of 11 patients also receiving nivolumab over 8 weeks and showed that 3 (27.3%) patients had a significant improvement in atherosclerotic plaque burden and 7 (63.6%) had no significant changes, while only 1 (9.1%) patient showed a modest worsening of atherosclerotic lesions.68 A follow-up report was published on 1 of the 3 patients who showed improvement but subsequently had a cancer relapse with new complicated aortic plaques seen in diagnostic imaging. After rechallenging with the anti-PD-L1 antibody atezolizumab, the patient had a further reduction and nearly complete resolution of the aortic plaques seen at relapse.74 However, Kwan et al69 reported accelerated atherosclerosis in a patient who developed 2 non–ST-segment elevation MIs during pembrolizumab treatment with repeat coronary angiography showing a rapid progression of the left circumflex stenosis (from 50% to 95%) within 2 months.69 Additionally, an autopsy study of 11 cancer patients treated with an ICI compared with 11 control subjects showed no significant differences in the atherosclerotic burden between the 2 groups, but immune checkpoint inhibition was associated with a markedly increased ratio of CD3+ T cells to CD68+ macrophages.75
Recently, several studies have investigated the association between ICI therapy, atherosclerosis, and rates of ASCVD, as summarized in Table 3. Initial small retrospective studies with shorter follow-ups showed no increase in ASCVD with ICI therapy.68,71,75 Additionally, a large pharmacovigilance study using VigiBase, the World Health Organization's global database of suspected novel adverse drug effects, reported the incidence of MI to be 0.53% and cerebral arterial ischemia to be 0.62% in ICI-treated patients. These rates were not higher than those reported in the full World Health Organization global health data collections.11 Subsequently, a few larger retrospective studies and meta-analyses also found low rates of ASCVD post-ICI.76, 77, 78
Table 3.
Summary of Clinical Studies of ICI Therapy and Atherosclerotic CV Events
| First Author (Year) | Study | Sample | Design/Findings | Main ICI Effect |
|---|---|---|---|---|
| Hu et al (2017)76 | Meta-analysis (22 studies) | 4,828 | 1.0% incidence of MI (95% CI: 0%-3.8%) and 2.0% of stroke (95% CI: 0%-13.0%) | ↓ ASCVD rate |
| Gelsomino et al (2018)68 | Single-center observational | 38 | 29% (n = 11) patients with plaques; 27.3% decreased, 63.6% had no changes, 9.1% had plaque progression post-ICIs | ↓ Plaque burden |
| Salem et al (2018)11 | Multicenter registry (Vigibase) | 31,321 irAEs | Incidence of MI was 0.53%, and cerebral arterial ischemia was 0.62%, similar to non-ICI patients. | ↓ ASCVD rate |
| Newman et al (2019)75 | Single center Comparative |
22 | Autopsy-based: no difference in plaque burden between matched control subjects and post-ICI patients but higher ratio of CD3+ T cells to CD68+ macrophages in post-ICI plaques | = Plaque burden ↑ CD3+/CD68+ |
| Nichetti et al (2020)156 | Post hoc prospective observational | 217 | 6.5% incidence of acute vascular events (2 ACS, 9 strokes, 3 visceral arterial thromboses) within 16 mo | ↑ ASCVD rate |
| Chitturi et al (2019)71 | Single center Retrospective |
252 | 135 post-ICI: 37.8% incidence of CV irAEs; no increase in MACE (CV death, MI, stroke, or HF hospitalization) post-ICIs within 6 mo (HR: 1.2; 95% CI: 0.6-2.4; P = 0.66); pretreatment or combined ICI and VEGFIs or TKIs had an increased risk of MACE (HR: 2.15; 95% CI: 1.05-4.37; P = 0.04) | No increase ASCVD |
| Wang et al (2019)32 | Meta-analysis (125 studies) | 20,128 | 9.8% incidence of treatment-related deaths due to CV irAEs, including MI, HF, and CM | ↑ ASCVD rate |
| Bar et al (2019)72 | Single center Retrospective |
1,215 | Single cohort: acute vascular events within 6 mo post-ICIs: 2.6% (95% CI: 1.8%-3.6%); event rate was 5.2% (95% CI: 2.8%-9.2%); acute vascular events incidence higher within 6 mo (OR: 3.49; 95% CI: 1.45-8.41; P = 0.002). 1% of cases were MI or ischemic stroke. OS worse in post-ICI patients with acute vascular events (3 mo vs 14 mo; HR: 3.01; 95% CI: 2.07-4.39; P < 0.0001). |
↑ ASCVD |
| Drobni et al (2020)31 | Single center Retrospective |
5,684 | Matched cohort, case crossover, and imaging study: higher risk of acute vascular events in ICIs (HR: 3.3; 95% CI: 2.0-5.5; P < 0.001) Case crossover: higher incidence of acute vascular events at 2 years after ICIs vs 2 years before ICIs (adjusted HR: 4.8; 95% CI: 3.6-6.5; P < 0.001) Imaging: Higher aortic plaque progression rate (2.1%/y before ICIs to 6.7%/y after ICIs) |
↑ Plaque ↑ ASCVD |
| Oren et al (2020)79 | Single center Retrospective |
3,326 | Rate of MI was 213 (7%) and stroke was 227 (7%) patients | ↑ ASCVD rate |
| Nso et al (2020)77 | Meta-analysis (26 studies) | 4,622 | 0.4% incidence of MI (95% CI: 0.1%-0.8%) | ↓ ASCVD rate |
| Solinas et al (2020)78 | Meta-analysis (68 studies) | 20,273 | Incidence of arterial thromboembolism 1.1% (95% CI: 0.5%-2.1%), stroke 1.1% (95% CI: 0.65%-1.45%), and MI 0.7% (95% CI: 0.15%-1.15%) | ↓ ASCVD rate |
| Dolladille et al (2021)81 | Meta-analysis (63 studies) | 32,528 | Incidence of MI was 7.4 (95% CI: 6.0-9.1) and cerebral ischemia was 8.8 (95% CI: 7.2-10.7) per 1,000 patients; ICIs significantly increased the risk of MI (OR: 1.51) and cerebral arterial ischemia (OR: 1.56) compared with control subjects | ↑ ASCVD rate |
| D'Souza et al (2021)80 | Multicenter registry (Danish) | 1,100 | 9.7% increased risk of CV irAEs (myocarditis, HF, arrhythmias, pericarditis, or CV death in 6 y | ↑ ASCVD rate |
| Laenens et al (2022)12 | Single center Retrospective |
672 | Matched cohort: 10.3% incidence of CV irAEs (MI, HF, and cerebral ischemia) with an overall mortality of 54.9% and a CV death rate of 1.9% over 13 mo | ↑ ASCVD rate |
ACS = acute coronary syndrome; ASCVD = atherosclerotic cardiovascular disease; CM = cardiomyopathy; CV = cardiovascular; HF = heart failure; ICI = immune checkpoint inhibitor; irAE = immune-related adverse event; MACE = major cardiovascular events; MI = myocardial infarction; OS = overall survival; TIA = transient ischemic attack; TKI = tyrosine kinase inhibitor; VEGFI = vascular endothelial growth factor inhibitor.
Conversely, other larger single-center retrospective studies and meta-analyses have increasingly shown a higher incidence of atherosclerosis-related CV events in patients receiving ICI therapy.12,30, 31, 32,72,79, 80, 81 For example, a meta-analysis of 63 randomized trials including 32,518 ICI-treated patients showed an incidence of MI and cerebral ischemia per 1,000 patients of 7.4 (95% CI: 6.0-9.1) and 8.8 (95% CI: 7.2-10.7), respectively. The study also found that ICIs significantly increased the risk of MI (OR: 1.51) and cerebral arterial ischemia (OR: 1.56) compared with control subjects.81 Laenens et al12 also studied 672 ICI-treated patients over a median follow-up of 13 months and found a 10.3% incidence of CV events (including MI, heart failure, and cerebral ischemia) with an overall mortality of 54.9% and a CV death rate of 1.9%.
In a retrospective matched cohort study, Drobni et al31 reported an incidence of 4.2% of a combined endpoint of MI, coronary revascularization, and ischemic stroke in 2,842 patients. The incidence of atherosclerotic CV events (including MI, coronary revascularization, and ischemic stroke) was 3 times higher after starting an ICI (HR: 3.3; 95 CI: 2.0-5.5; P < 0.001). In a case crossover study, atherosclerotic CV events increased from 1.37 to 6.55 per 100 person-years at 2 years (adjusted HR: 4.8; 95 CI: 3.5-6.5; P < 0.001). In this study, combination ICI use was not associated with a higher rate of atherosclerotic CV events; however, only 6.9% of the patients were treated with dual ICI therapy. Interestingly, this study showed that the incidence of other irAEs or the presence of conventional risk factors such as age, sex, body mass index, baseline diabetes, prior CV events, or cancer had no significant impact on the risk for an atherosclerotic CV event.31 This suggests that other pathophysiological factors potentially related to inflammation and immune activation may mediate the effects of ICIs on plaque lesions and the development of ICI-related atherosclerotic CV events.
Key Points
-
•
Increasing clinical data suggest the association between ICI therapy and ASCVD, potentially mediated by an accelerated progression of atherosclerosis.
-
•
Large cohort prospective studies and clinical trials are warranted to further study the clinical implications of ICI-associated ASCVD.
Imaging Insights Into Atherosclerosis With ICIs
The progression of atherosclerotic plaque is a robust predictor of CV events and an established outcome measure for randomized clinical trials.82, 83, 84 The first description of the potential effect of ICI therapy on human atherosclerotic plaques using imaging was based on contrast-enhanced CT scans at baseline and a minimum of 8 weeks, with mainly no change or resolution of the atherosclerotic lesions post-ICI.68,74 In contrast, an imaging study cohort by Drobni et al31 followed 40 patients with melanoma and used conventional CT imaging at 3 time points to assess the effect of ICIs on atherosclerotic plaque burden. This study demonstrated a greater progression rate of total and NCP volume after ICI therapy. Specifically, thoracic plaque burden increased from 2.1% per year to 6.7% per year (P = 0.02) before and after the initiation of ICI therapy, providing biological support to the clinical observations of increased atherosclerotic events in the study.31
In addition, functional imaging studies can provide valuable insights into molecular and metabolic processes underlying the pathophysiology of atherosclerotic plaque progression during ICI therapy. For instance, increased systemic low-grade arterial wall inflammation is critical in developing coronary atherosclerotic lesions and can relate to high-risk plaque morphology.37,39 Studies implementing 18F-FDG PET–CT provide the opportunity to evaluate the effects of ICI therapy on vascular inflammation and plaque progression in these patients. Calabretta et al85 used 18F-FDG PET–CT to quantify atherosclerotic inflammatory activity in twenty patients with melanoma undergoing ICI treatment over a 4-month period. FDG uptake significantly increased in noncalcified and mildly calcified segments, indicating increased atherosclerotic inflammatory activity in these early lesions after ICI therapy. These findings suggest that ICI therapy may influence local innate immune cells and aggravate inflammation, particularly in early noncalcified and mildly calcified atherosclerotic lesions and not in advanced calcified ones.85 In contrast, Poels et al57 investigated the effects of short-term ICI therapy on vascular inflammation in the thoracic aorta and carotid arteries using a combination of 18F-FDG PET–CT and immunological techniques in 10 melanoma patients post-ICI. In this prospective study, combination ICI therapy with anti-CTLA-4 and anti-PD-1 antibodies did not affect systemic or myeloid-driven vascular inflammation in patients, measured 6 weeks after initiation, or in a complementary hyperlipidemic mouse model. Interestingly, in hyperlipidemic mice, there was a progression toward a T cell–mediated inflammatory phenotype with an increased presence of CD4+ and CD8+ T cells. Although plaque size was unaffected, plaques were characterized by a 2.7-fold increase of CD8+ T cells and a 3.9-fold increase in necrotic core size. Also, there was evidence of endothelial activation with a 2.2-fold and 1.6-fold increase, respectively, in vascular cell adhesion molecule-1 and intercellular adhesion molecule-1.57
Together, these studies demonstrate that ICI therapy may trigger low-grade inflammation of the arterial wall and may mainly affect vulnerable NCP, which is traditionally related to a higher risk for rupture. Consequently, this progression of atherosclerosis in ICI-treated patients may contribute to the increased occurrence of acute CV events in these patients.
Novel Directions for Imaging ICI-Associated Atherosclerosis
As studies continue to demonstrate significant atherosclerotic risk in patients receiving ICIs, effort must be taken to integrate conventional and novel imaging approaches to identify and monitor atherosclerosis in this high-risk population. At a basic level, conventional CT imaging that is part of standard cancer care can be instrumental in longitudinally evaluating atherosclerotic plaque progression in ICI-treated patients.86 In addition, coronary CT angiography could be implemented in future studies to assess high-risk plaque features.
Furthermore, recent molecular imaging studies have used labeled antibodies as immune-PET tracers to detect additional features of vulnerable atherosclerotic lesions.87,88 Immune checkpoints are attractive imaging targets to detect inflammatory and immune markers within the plaque microenvironment, and translational and human studies have tested immune-PET tracers with molecules such as CD40-CD40L, CD80/86-CD28, and CD47.61,89, 90, 91, 92 For example, Poels et al showed the feasibility of imaging plaques in a mouse model of atherosclerosis using [89Zr]Zr-labeled monoclonal antibody against CD40, a co-stimulatory molecule highly expressed on activated macrophages as well as on B cells and activated endothelial and smooth muscle cells.93 Interestingly, this approach can be valuable in human studies by labeling the anti-human CD40 monoclonal antibody, iscalimab, which is currently being tested in clinical trials for other chronic autoimmune conditions (NCT03905525, NCT03656562, NCT04129528).94
Similarly, the feasibility of imaging the dyad CD80/CD86, a co-stimulatory checkpoint molecule in macrophages inside vulnerable plaques, was initially tested in ApoE-/- mice using the PET tracer [11C]AM7. While there was signal uptake in atherosclerotic lesions, its applicability was limited due to high background signals.91 In a subsequent study, belatacept, a CTLA-4 fusion protein that binds to CD80/86, was radiolabeled with indium-111 and showed increased signal uptake in ApoE-/- mice and human carotid specimens, which correlated with the infiltration by immune cells into the plaques.92 Additionally, Flores et al65 conducted a study using immune-PET tracers for CD47 and showed that its inhibition down-regulated genes implicated in the macrophage response to IL-1 and IFN-γ, which led to a significant reduction in atherosclerotic inflammation in a mouse model of atherosclerosis.65 As detailed previously, CD47 inhibitor therapies have been used in various clinical trials to increase tumor cell recognition and phagocytosis by macrophages. For instance, magrolimab, the first-in-class anti-CD47 antibody, is being tested in ongoing clinical trials of hematological and solid malignancies.61,63 Interestingly, an analysis of 13 non-Hodgkin’s lymphoma trial patients using 18F-FDG PET–CT after 9 weeks of magrolimab treatment showed a reduction of FDG signal uptake in the most diseased carotid artery segments, implying that CD47 inhibition may reduce vascular inflammation.61
Key Points
-
•
ICI therapy is associated with the progression of total and noncalcified atherosclerotic lesions in studies using conventional CT imaging.
-
•
18F-FDG PET–CT studies showed increased inflammatory activity in noncalcified and mildly calcified atherosclerotic lesions in ICI-treated patients.
-
•
Studies using novel molecular imaging studies using labeled antibodies as immune-PET tracers may help detect vulnerable atherosclerotic lesions in the context of ICI therapy.
Management Considerations in ICI-Associated Atherosclerosis
The Role of CV Risk Stratification
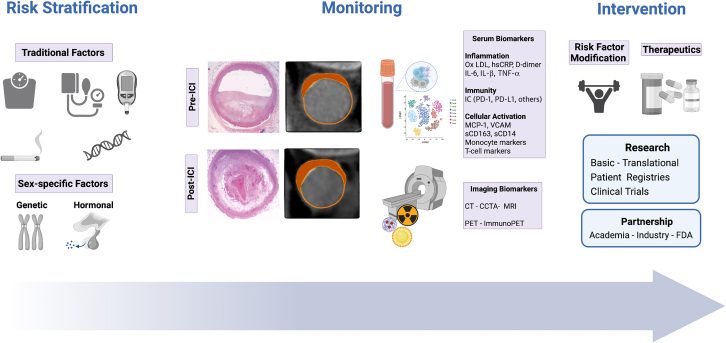
Increased awareness of ICI-associated CVD and risk stratification strategies is an important first step in identifying individuals that may benefit most from preventive measures (Figure 1). The effect of traditional CV risk factors on ICI-associated atherosclerotic CV events is unknown. Studies have shown conflicting data, with some describing a higher risk of CV events in patients with hypertension, dyslipidemia, prior CVD, or subclinical atherosclerosis.72 In contrast, other studies found no significant correlation between ICI-associated CV events and factors such as age, sex, diabetes, prior CVD, hyperlipidemia, or tobacco use.31,95
Figure 1.
Management Considerations in ICI-Associated Atherosclerosis
Management strategies for immune checkpoint inhibitors (ICI)–treated patients can include monitoring of atherosclerotic burden pre- and post-ICI therapy with serum and imaging biomarkers, and implementation of tailored interventions. Created with BioRender. CT = computed tomography; CCTA = coronary computer tomography angiography; FDA = Food and Drug Administration; hsCRP = high sensitivity C-reactive protein; IL = interleukin; MCP = monocyte chemoattractant protein; MRI = magnetic resonance imaging; Ox LDL = oxidized low density lipoprotein; PD-1 = programmed cell death protein 1; PD-L1 = programmed cell death ligand 1; sCD = serum cluster differentiation; PET = positron emission tomography; TNF = tumor necrosis factor; VCAM = vascular cell adhesion molecule.
Mechanistic studies on the effects of ICIs on atherosclerosis should evaluate traditional CV risk markers (such as lipid fractions, hemoglobin A1c) alongside prespecified biological factors potentially affected by ICI therapy such as markers of immune regulation (PD-1, PD-L1), immune cell activation (sCD163, sCD14), and inflammation (interleukins, C-reactive protein, vascular cell adhesion molecule). Multimodal sequencing approaches analyzing circulating immune cells and specific cell subsets on a single cell level will be valuable in phenotyping the immune microenvironment of atherosclerotic lesions.26,45,47 In turn, the characterization of proatherogenic immune responses may help to identify novel pharmacological targets, thereby reducing CV risk through preventive pharmacological strategies. Therefore, cardio-oncology registries combining data on CV risk factors (eg, age, diabetes, hypertension), ICI treatment characteristics (eg, type/combination of ICI, doses, cancer progression, development of irAEs), measurement of serum biomarkers, and morphological and functional imaging studies could aid in a comprehensive and longitudinal risk assessment of ICI-treated patients.
The Role of Preventive Pharmacological Strategies
A retrospective analysis from Drobni et al31 suggested that the effect of atherosclerotic plaque progression after ICI therapy may be modifiable by statin and corticosteroid use. Specifically, compared with those not on statins, those on statins after ICI therapy had a reduced annual rate of the progression of total atherosclerotic plaque volume (5.2% vs 8.3%; P = 0.04) and noncalcified plaque volume (3.1% vs 7.0%; P = 0.04).31 Notably, there was no association between statin use and CV events in the study, but this could be confounded as patients on a statin had a higher baseline CV risk profile. However, in a subsequent large study among 2,757 ICI-treated patients, 35% were on a statin at the time of starting ICI therapy, and statin therapy was associated with a more than 2-fold higher risk for myopathy, another potentially serious ICI toxicity.96 Given that most patients are prescribed a statin for dyslipidemia, and there is an increased risk of atherosclerosis associated with ICI therapy, large clinical studies are needed to further evaluate the efficacy and, importantly, the safety of statins in this context. In addition, the indication for a statin and the possibility of alternative nonstatin therapies such as proprotein convertase subtilisin/kexine type 9 (PCSK9) inhibitors, icosapent ethyl, bempedoic acid, and ezetimibe should also be considered in ICI-treated patients. PCSK9 inhibitors are a class of monoclonal antibodies that reduce serum low-density lipoprotein and atherosclerotic events in higher-risk patients. While less is known about the additional effects of this drug class, recent data suggest that PCSK9 inhibition does not affect cancer incidence in clinical trials.97,98 Furthermore, a study by Liu et al99 showed that PCSK9 inhibition potentiated ICI therapy through a mechanism independent of its function as a critical cholesterol regulator. Specifically, its effect caused a significant increase in the expression of major histocompatibility protein class I, which enhanced intratumoral cytotoxic T cell infiltration.99 In summary, recent studies have indicated a synergistic effect when ICI therapies are concomitantly used with statins and PCSK9 inhibitors through enhanced antigen presentation, promoting anti-tumor cytotoxic T cells, and the expression of co-inhibitory molecules, such as PD-1, LAG-3, and TIM-3.99, 100, 101, 102, 103, 104, 105, 106 Therefore, additional studies are needed to understand the potential of using lipid-lowering therapies such as statins and PCSK9 inhibitors to enhance ICI efficacy and treat ICI-related atherosclerosis.
Last, among patients receiving ICI therapy, there was a lower annual rate of plaque progression among those on corticosteroids compared with those not on corticosteroids (3.5% vs 6.9%, P = 0.04).31 While these findings could be related to the anti-inflammatory effect of corticosteroids, the results may be confounded by the indication for corticosteroid use and subsequent discontinuation of the ICI therapy. Moreover, the potential adverse effects of steroids and other anti-inflammatory or immunomodulatory drugs on cancer treatment efficacy may limit their use as an intervention in ICI-treated patients. These findings highlight the complex interplay between immune activation, inflammation, and cholesterol metabolism linked to ICI efficacy and ICI-associated atherosclerosis. Future studies are needed to understand the effect of conventional and novel CV therapies for atherosclerotic risk reduction in ICI-treated patients.
Key Points
-
•
The effect of ICIs on atherosclerosis may be heterogeneous, and careful consideration is needed regarding the risk stratification, surveillance, and treatment strategies in ICI-treated patients.
-
•
A comprehensive mechanistic understanding of the effect of ICIs on atherosclerosis is needed to determine the timing and need for CV preventive and treatment interventions.
-
•
The effectiveness and safety of statins, PCSK9 inhibitors, and other therapies for ICI-associated atherosclerosis should be explored in translational and clinical studies specific to this complex patient population.
Sex-Specific Considerations in ICI-Associated Atherosclerosis
There are data to suggest that the effect of ICI on atherosclerosis may be sex-specific. Broadly, there is known sexual dimorphism in the prevalence, risk profile, and prognosis of atherosclerosis. However, relatively limited data address sex-specific determinants as independent variables in inflammation and immune dysregulation related to atherosclerosis.107 Studies have shown sex differences in circulating CVD protein biomarkers associated with pathways implicated in inflammation, adiposity, fibrosis, and platelet homeostasis.108 In addition, evidence from cardiac imaging studies demonstrates unique differences in atherosclerotic plaques between men and women. A study by Kim et al109 showed that, despite similar luminal narrowing and plaque burden between women and men, the composition and frequencies of high-risk plaque characteristics were lower in women.
There are also sex-based differences in the onset, outcomes, and risk of cardiotoxicity associated with several cancer therapies.110 However, sex-specific differences in ICI efficacy and the development of irAEs are still not completely understood.110, 111, 112 Few studies have shown an increased risk in women or no difference between the sexes.113,114 Similarly, data on cardiac-related irAEs are based on retrospective studies limited by an imbalance in the number of female participants. For example, Zamami et al115 analyzed 107 ICI-treated patients registered in the U.S. Food and Drug Administration Adverse Event Reporting System and found that female patients were 1.92 times more likely to develop ICI myocarditis compared with men (OR: 1.92; 95 CI: 1.24-2.97; P = 0.004). In contrast, other retrospective studies found a 60% to 70% occurrence of ICI-related myocarditis in men.11,14,116 While these descriptive clinical studies do not necessarily indicate an association between sex and a higher risk of ICI myocarditis, a recent study with the Ctla4+/− Pdcd1-/-mouse model, which simulates dual anti-CTLA-4 and anti-PD-1 immunotherapy, showed that 50% of these mice died by 3 months with features of myocarditis and female mice were affected at a higher frequency.117
Sex-based differences specific to ICI-related atherosclerosis have not been elucidated. Of the few studies of ICI-associated atherosclerotic-CV events, Bar et al72 found that the male sex conveyed a 2.43-fold increase in the odds of developing acute vascular events when compared with the female sex (95 CI: 1.04-5.68), but the sample size was small (n = 31) and only 23% were women. In larger retrospective studies, these ICI-related atherosclerotic events were reported with higher frequency in men; however, the under-representation of women and the lack of stratified analysis by sex were common limitations.32,71,73,76,78,79. The study by Drobni et al31 consisted of 43% women and reported HRs with respect to ICI therapy for the composite CV outcome within the matched cohort study. Stratified by subgroups, an increased HR in women of 6.0 compared with an HR in men of 3.93 was observed; however, the difference was of borderline significance (P = 0.087).31
Sex differences in ICI therapy, atherosclerosis, and CV adverse events could be related to genetic and hormonal factors. There are differences in immunity and inflammation between the male and female sex, and women are known to have a higher incidence of autoimmune disorders. Genetically, the X chromosome encodes many immune function genes related to cytokine signaling pathways and immune cell activation such as ILR-2G, ILR-3, toll-like receptor 7, and CD40L. Furthermore, genes related to B and cytotoxic T cell responses are overexpressed in women, which may result in a stronger immunity but may also lead to an increased risk of irAEs in women.118 Hormones such as estradiol and progesterone are known to influence immune responses through increased expression of PD-1, IL-4, and the production of B and T cells CD4, while male androgens have an anti-inflammatory effect. Furthermore, preclinical studies suggested estrogen-mediated immunomodulation of the PD-1/PD-L1 pathway, thus regulating immune responses.119,120
Knowledge gaps remain in our understanding of the sex-specific effects of ICI therapy on atherosclerosis, and additional studies on its underlying mechanistic basis are needed. There are ongoing efforts to investigate gender differences related to ICI irAEs such as the prospective observational studies G-DEFINER (Gender Difference in sidE eFfects of immuNotherapy: a possible clue to optimize cancEr Treatment) and JOCARDITE (Joint Use of Database to Identify Risk Factors of Cardiovascular Toxicity Induced by Immune Checkpoint Inhibitors) (NCT04294771). Focusing on sex-based differences in ICI-associated cardiotoxicities including atherosclerosis can help develop tailored preventive and therapeutic cardioprotective strategies in women.
Key Points
-
•
Sex-specific differences in the effect of ICI therapy and irAEs such as atherosclerosis may be related to genetic, hormonal, inflammatory, and immune-related factors that remain to be studied.
Conclusions
ICIs have changed oncology, and their implementation has provided an expanded window into CV biology and contributed to the exponential growth of the field of cardio-oncology in the last decade.121 The application of ICIs has also created clinical challenges for those patients who develop ICI-related cardiotoxicities. However, we are just beginning to understand the spectrum of atherosclerotic-related events and aggravated atherosclerosis due to ICI therapy. On a clinical level, more extensive longitudinal studies, and the inclusion of patients in systematic cardio-oncology registries are crucial to better understanding the link between ICI use and atherosclerosis. This will also help inform optimal atherosclerotic risk management, CVD surveillance, and therapeutic interventions in ICI-treated patients and long-term cancer survivors. On a basic and translational level, many questions remain about the mechanistic basis of ICI-associated atherosclerosis and how to define predictive biomarkers, improve diagnostic strategies, and develop effective treatments. Tools from immunology, genomics, bioinformatics, and imaging can help us better characterize the effects of ICI therapies on atherogenesis and the mechanisms involved in the progression of atherosclerotic lesions because of these therapies.
To date, evidence on the effects of ICI therapy on atherosclerotic CVD from clinical trials are scarce. In the future, routine assessment and systematic reporting of cardiac adverse events during and after novel cancer therapies should be implemented in oncology trials. Cardio-oncologists will continue to play an essential role in managing acute cardiotoxicity and reducing the risk of long-term sequelae in ICI-treated patients. This unique patient population can benefit from integrating current and novel imaging and pharmacotherapeutic strategies to mitigate risk and improve overall outcomes. Longer-term steps include broadening collaborations among cardio-oncologists, imagers, oncologists, and pharmaceutical partners to expand clinical research efforts based on innovative basic and translational experimental insights. These and other steps are needed to improve CV outcomes among cancer patients treated with ICI therapy.
Funding Support and Author Disclosures
Dr Neilan holds the Michael and Kathryn Park Endowed Chair in Cardiology and was also supported, in part, through a kind gift from A. Curtis Greer and Pamela Kohlberg and Christina and Paul Kazilionis, a Hassenfeld Scholar Award, and grants from the National Institutes of Health/National Heart, Lung, and Blood Institute (R01HL130539, R01HL159187, R01HL137562, K24HL150238). Dr Zanni was supported, in part, through grants from the National Institutes of Health/National Heart, Lung, and Blood Institute (R01HL146267 and R01HL137562) and from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (K24AI157882); and has served as the principal investigator on an investigator-initiated research grant from Gilead to her institution (Massachusetts General Hospital). Dr Neilan has served as a consultant for and received fees from Amgen, Genentech, Roche, BMS, Sanofi, CRC Oncology, and AbbVie outside of the current work; and received grant funding from AstraZeneca and Bristol Myers Squibb related to immune checkpoint inhibitors. Dr Suero-Abreu has reported that she has no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Zhang L., Reynolds K.L., Lyon A.R., Palaskas N., Neilan T.G. The evolving immunotherapy landscape and the epidemiology, diagnosis, and management of cardiotoxicity. J Am Coll Cardiol CardioOnc. 2021;3(1):35–47. doi: 10.1016/j.jaccao.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haslam A., Gill J., Prasad V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open. 2020;3(3) doi: 10.1001/jamanetworkopen.2020.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D.S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 5.Tang J., Shalabi A., Hubbard-Lucey V.M. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. 2018;29(1):84–91. doi: 10.1093/annonc/mdx755. [DOI] [PubMed] [Google Scholar]
- 6.Upadhaya S., Neftelinov S.T., Hodge J., Campbell J. Challenges and opportunities in the PD1/PDL1 inhibitor clinical trial landscape. Nat Rev Drug Discov. 2022;21(7):482–483. doi: 10.1038/d41573-022-00030-4. [DOI] [PubMed] [Google Scholar]
- 7.Tang J., Yu J.X., Hubbard-Lucey V.M., Neftelinov S.T., Hodge J.P., Lin Y. Trial watch: The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov. 2018;17(12):854–855. doi: 10.1038/nrd.2018.210. [DOI] [PubMed] [Google Scholar]
- 8.Haslam A., Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5) doi: 10.1001/jamanetworkopen.2019.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 10.Moslehi J.J., Salem J.E., Sosman J.A., Lebrun-Vignes B., Johnson D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391(10124):933. doi: 10.1016/s0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salem J.E., Manouchehri A., Moey M., et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laenens D., Yu Y., Santens B., et al. Incidence of cardiovascular events in patients treated with immune checkpoint inhibitors. J Clin Oncol. 2022;40(29):3430–3438. doi: 10.1200/jco.21.01808. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L., Zlotoff D.A., Awadalla M., et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation. 2020;141(24):2031–2034. doi: 10.1161/CIRCULATIONAHA.119.044703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahmood S.S., Fradley M.G., Cohen J.V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong J., Drobni Z.D., Zafar A., et al. Pericardial disease in patients treated with immune checkpoint inhibitors. J Immunother Cancer. 2021;9(6) doi: 10.1136/jitc-2021-002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong J., Drobni Z.D., Alvi R.M., et al. Immune checkpoint inhibitors for cancer and venous thromboembolic events. Eur J Cancer. 2021;158:99–110. doi: 10.1016/j.ejca.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang E.H., Marmagkiolis K., Balanescu D.V., et al. Radiation-induced vascular disease-a state-of-the-art review. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.652761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell J.D., Cehic D.A., Morgia M., et al. Cardiovascular manifestations from therapeutic radiation. J Am Coll Cardiol CardioOnc. 2021;3(3):360–380. doi: 10.1016/j.jaccao.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seijkens T.T.P., Lutgens E. Cardiovascular oncology: exploring the effects of targeted cancer therapies on atherosclerosis. Curr Opin Lipidol. 2018;29(5):381–388. doi: 10.1097/mol.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 20.Sturgeon K.M., Deng L., Bluethmann S.M., et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–3897. doi: 10.1093/eurheartj/ehz766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutgens E., Seijkens T.T.P. Cancer patients receiving immune checkpoint inhibitor therapy are at an increased risk for atherosclerotic cardiovascular disease. J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Florido R., Daya N.R., Ndumele C.E., et al. Cardiovascular disease risk among cancer survivors. J Am Coll Cardiol. 2022;80(1):22–32. doi: 10.1016/j.jacc.2022.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshu C.E., Barber J.R., Coresh J., et al. Enhancing the infrastructure of the Atherosclerosis Risk in Communities (ARIC) study for cancer epidemiology research: ARIC cancer. Cancer Epidemiol Biomarkers Prev. 2018;27(3):295–305. doi: 10.1158/1055-9965.Epi-17-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strauss L., Mahmoud M.A.A., Weaver J.D., et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol. 2020;5(43) doi: 10.1126/sciimmunol.aay1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gotsman I., Grabie N., Dacosta R., Sukhova G., Sharpe A., Lichtman A.H. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest. 2007;117(10):2974–2982. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez D.M., Rahman A.H., Fernandez N.F., et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med. 2019;25(10):1576–1588. doi: 10.1038/s41591-019-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bu D.X., Tarrio M., Maganto-Garcia E., et al. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):1100–1107. doi: 10.1161/ATVBAHA.111.224709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J., Zhuang Y., Wei X., et al. Contributions of PD-1/PD-L1 pathway to interactions of myeloid DCs with T cells in atherosclerosis. J Mol Cell Cardiol. 2009;46(2):169–176. doi: 10.1016/j.yjmcc.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto T., Sasaki N., Yamashita T., et al. Overexpression of cytotoxic T-lymphocyte-associated antigen-4 prevents atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2016;36(6):1141–1151. doi: 10.1161/ATVBAHA.115.306848. [DOI] [PubMed] [Google Scholar]
- 30.Amiri-Kordestani L., Moslehi J., Cheng C., et al. Cardiovascular adverse events in immune checkpoint inhibitor clinical trials: a U.S. Food and Drug Administration pooled analysis. J Clin Oncol. 2018;36(15_suppl):3009. [Google Scholar]
- 31.Drobni Z.D., Alvi R.M., Taron J., et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142(24):2299–2311. doi: 10.1161/CIRCULATIONAHA.120.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y., Zhou S., Yang F., et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5(7):1008–1019. doi: 10.1001/jamaoncol.2019.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Libby P. The changing landscape of atherosclerosis. Nature. 2021;592(7855):524–533. doi: 10.1038/s41586-021-03392-8. [DOI] [PubMed] [Google Scholar]
- 34.Moore K.J., Sheedy F.J., Fisher E.A. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13(10):709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett T.J. Macrophages in atherosclerosis regression. Arterioscler Thromb Vasc Biol. 2020;40(1):20–33. doi: 10.1161/atvbaha.119.312802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Triant V.A., Perez J., Regan S., et al. Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation. 2018;137(21):2203–2214. doi: 10.1161/CIRCULATIONAHA.117.028975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zanni M.V., Abbara S., Lo J., et al. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS. 2013;27(8):1263–1272. doi: 10.1097/QAD.0b013e32835eca9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tawakol A., Migrino R.Q., Hoffmann U., et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol. 2005;12(3):294–301. doi: 10.1016/j.nuclcard.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Tawakol A., Lo J., Zanni M.V., et al. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Defic Syndr. 2014;66(2):164–171. doi: 10.1097/QAI.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foldyna B., Lo J., Mayrhofer T., Grinspoon S.K., Hoffmann U., Lu M.T. Individual coronary plaque changes on serial CT angiography: within-patient heterogeneity, natural history, and statin effects in HIV. J Cardiovasc Comput Tomogr. 2020;14(2):144–148. doi: 10.1016/j.jcct.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Day C.L., Kaufmann D.E., Kiepiela P., et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 42.Wolf D., Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–327. doi: 10.1161/CIRCRESAHA.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winkels H., Wolf D. Heterogeneity of T cells in atherosclerosis defined by single-cell RNA-sequencing and cytometry by time of flight. Arterioscler Thromb Vasc Biol. 2021;41(2):549–563. doi: 10.1161/atvbaha.120.312137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winkels H., Ehinger E., Vassallo M., et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ Res. 2018;122(12):1675–1688. doi: 10.1161/circresaha.117.312513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin J.D., Nishi H., Poles J., et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 2019;4(4) doi: 10.1172/jci.insight.124574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cole J.E., Park I., Ahern D.J., et al. Immune cell census in murine atherosclerosis: cytometry by time of flight illuminates vascular myeloid cell diversity. Cardiovasc Res. 2018;114(10):1360–1371. doi: 10.1093/cvr/cvy109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cochain C., Vafadarnejad E., Arampatzi P., et al. Single-cell RNA-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res. 2018;122(12):1661–1674. doi: 10.1161/circresaha.117.312509. [DOI] [PubMed] [Google Scholar]
- 48.Seijkens T.T.P., van Tiel C.M., Kusters P.J.H., et al. Targeting CD40-induced TRAF6 signaling in macrophages reduces atherosclerosis. J Am Coll Cardiol. 2018;71(5):527–542. doi: 10.1016/j.jacc.2017.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brånén L., Hovgaard L., Nitulescu M., Bengtsson E., Nilsson J., Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24(11):2137–2142. doi: 10.1161/01.ATV.0000143933.20616. [DOI] [PubMed] [Google Scholar]
- 50.Laurat E., Poirier B., Tupin E., et al. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2001;104(2):197–202. doi: 10.1161/01.cir.104.2.197. [DOI] [PubMed] [Google Scholar]
- 51.Boesten L.S.M., Zadelaar A.S.M., van Nieuwkoop A., et al. Tumor necrosis factor-α promotes atherosclerotic lesion progression in APOE∗3-leiden transgenic mice. Cardiovasc Res. 2005;66(1):179–185. doi: 10.1016/j.cardiores.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Dietel B., Cicha I., Voskens C.J., Verhoeven E., Achenbach S., Garlichs C.D. Decreased numbers of regulatory T cells are associated with human atherosclerotic lesion vulnerability and inversely correlate with infiltrated mature dendritic cells. Atherosclerosis. 2013;230(1):92–99. doi: 10.1016/j.atherosclerosis.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Baitsch L., Baumgaertner P., Devêvre E., et al. Exhaustion of tumor-specific CD8⁺ T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350–2360. doi: 10.1172/jci46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vuong J.T., Stein-Merlob A.F., Nayeri A., Sallam T., Neilan T.G., Yang E.H. Immune checkpoint therapies and atherosclerosis: mechanisms and clinical implications: JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;79(6):577–593. doi: 10.1016/j.jacc.2021.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ewing M.M., Karper J.C., Abdul S., et al. T-cell co-stimulation by CD28-CD80/86 and its negative regulator CTLA-4 strongly influence accelerated atherosclerosis development. Int J Cardiol. 2013;168(3):1965–1974. doi: 10.1016/j.ijcard.2012.12.085. [DOI] [PubMed] [Google Scholar]
- 56.Poels K., van Leent M.M.T., Reiche M.E., et al. Antibody-mediated inhibition of CTLA4 aggravates atherosclerotic plaque inflammation and progression in hyperlipidemic mice. Cells. 2020;9(9):1987. doi: 10.3390/cells9091987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poels K., van Leent M.M.T., Boutros C., et al. Immune checkpoint inhibitor therapy aggravates T cell-driven plaque inflammation in atherosclerosis. J Am Coll Cardiol CardioOnc. 2020;2(4):599–610. doi: 10.1016/j.jaccao.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lutgens E., Atzler D., Doring Y., Duchene J., Steffens S., Weber C. Immunotherapy for cardiovascular disease. Eur Heart J. 2019;40(48):3937–3946. doi: 10.1093/eurheartj/ehz283. [DOI] [PubMed] [Google Scholar]
- 59.Lutgens E., Joffre J., van Os B., Ait-Oufella H. Targeting cytokines and immune checkpoints in atherosclerosis with monoclonal antibodies. Atherosclerosis. 2021;335:98–109. doi: 10.1016/j.atherosclerosis.2021.09.024. [DOI] [PubMed] [Google Scholar]
- 60.Qin S., Xu L., Yi M., Yu S., Wu K., Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18(1):155. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jarr K.U., Nakamoto R., Doan B.H., et al. Effect of CD47 blockade on vascular inflammation. N Engl J Med. 2021;384(4):382–383. doi: 10.1056/NEJMc2029834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Advani R., Flinn I., Popplewell L., et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N Engl J Med. 2018;379(18):1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang Z., Sun H., Yu J., Tian W., Song Y. Targeting CD47 for cancer immunotherapy. J Hematol Oncol. 2021;14(1):180. doi: 10.1186/s13045-021-01197-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kojima Y., Volkmer J.P., McKenna K., et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536(7614):86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flores A.M., Hosseini-Nassab N., Jarr K.U., et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat Nanotechnol. 2020;15(2):154–161. doi: 10.1038/s41565-019-0619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engelbertsen D., Autio A., Verwilligen R.A.F., et al. Increased lymphocyte activation and atherosclerosis in CD47-deficient mice. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-46942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tomita Y., Sueta D., Kakiuchi Y., et al. Acute coronary syndrome as a possible immune-related adverse event in a lung cancer patient achieving a complete response to anti-PD-1 immune checkpoint antibody. Ann Oncol. 2017;28(11):2893–2895. doi: 10.1093/annonc/mdx326. [DOI] [PubMed] [Google Scholar]
- 68.Gelsomino F., Fiorentino M., Zompatori M., et al. Programmed death-1 inhibition and atherosclerosis: can nivolumab vanish complicated atheromatous plaques? Ann Oncol. 2018;29(1):284–286. doi: 10.1093/annonc/mdx718. [DOI] [PubMed] [Google Scholar]
- 69.Kwan J.M., Cheng R., Feldman L.E. Hepatotoxicity and recurrent NSTEMI while on pembrolizumab for metastatic giant cell bone tumor. Am J Med Sci. 2019;357(4):343–347. doi: 10.1016/j.amjms.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 70.Vincent L., Leedy D., Masri S.C., Cheng R.K. Cardiovascular disease and cancer: is there increasing overlap? Curr Oncol Rep. 2019;21(6):47. doi: 10.1007/s11912-019-0796-0. [DOI] [PubMed] [Google Scholar]
- 71.Chitturi K.R., Xu J., Araujo-Gutierrez R., et al. Immune checkpoint inhibitor-related adverse cardiovascular events in patients with lung cancer. J Am Coll Cardiol CardioOnc. 2019;1(2):182–192. doi: 10.1016/j.jaccao.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bar J., Markel G., Gottfried T., et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122–131. doi: 10.1016/j.ejca.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 73.Cautela J., Rouby F., Salem J.E., et al. Acute coronary syndrome with immune checkpoint inhibitors: a proof-of-concept case and pharmacovigilance analysis of a life-threatening adverse event. Can J Cardiol. 2020;36(4):476–481. doi: 10.1016/j.cjca.2019.11.035. [DOI] [PubMed] [Google Scholar]
- 74.Lamberti G., Gelsomino F., Brocchi S., et al. New disappearance of complicated atheromatous plaques on rechallenge with PD-1/PD-L1 axis blockade in non-small cell lung cancer patient: follow up of an unexpected event. Ther Adv Med Oncol. 2020;12 doi: 10.1177/1758835920913801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Newman J.L., Stone J.R. Immune checkpoint inhibition alters the inflammatory cell composition of human coronary artery atherosclerosis. Cardiovasc Pathol. 2019;43 doi: 10.1016/j.carpath.2019.107148. [DOI] [PubMed] [Google Scholar]
- 76.Hu Y.-B., Zhang Q., Li H.-J., et al. Evaluation of rare but severe immune related adverse effects in PD-1 and PD-L1 inhibitors in non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res. 2017;6(Suppl 1):S8–S20. doi: 10.21037/tlcr.2017.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nso N., Antwi-Amoabeng D., et al. Cardiac adverse events of immune checkpoint inhibitors in oncology patients: a systematic review and meta-analysis. World J Cardiol. 2020;12(11):584–598. doi: 10.4330/wjc.v12.i11.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Solinas C., Saba L., Sganzerla P., Petrelli F. Venous and arterial thromboembolic events with immune checkpoint inhibitors: a systematic review. Thromb Res. 2020;196:444–453. doi: 10.1016/j.thromres.2020.09.038. [DOI] [PubMed] [Google Scholar]
- 79.Oren O., Yang E.H., Molina J.R., Bailey K.R., Blumenthal R.S., Kopecky S.L. Cardiovascular health and outcomes in cancer patients receiving immune checkpoint inhibitors. Am J Cardiol. 2020;125(12):1920–1926. doi: 10.1016/j.amjcard.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 80.D'Souza M., Nielsen D., Svane I.M., et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. Eur Heart J. 2021;42(16):1621–1631. doi: 10.1093/eurheartj/ehaa884. [DOI] [PubMed] [Google Scholar]
- 81.Dolladille C., Akroun J., Morice P.M., et al. Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: a safety meta-analysis. Eur Heart J. 2021;42(48):4964–4977. doi: 10.1093/eurheartj/ehab618. [DOI] [PubMed] [Google Scholar]
- 82.Nissen S.E., Tuzcu E.M., Libby P., et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA. 2004;292(18):2217–2225. doi: 10.1001/jama.292.18.2217. [DOI] [PubMed] [Google Scholar]
- 83.Nicholls S.J., Ballantyne C.M., Barter P.J., et al. Effect of 2 intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365(22):2078–2087. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 84.Azen S.P., Mack W.J., Cashin-Hemphill L., et al. Progression of coronary artery disease predicts clinical coronary events. Long-term follow-up from the Cholesterol Lowering Atherosclerosis Study. Circulation. 1996;93(1):34–41. doi: 10.1161/01.cir.93.1.34. [DOI] [PubMed] [Google Scholar]
- 85.Calabretta R., Hoeller C., Pichler V., et al. Immune checkpoint inhibitor therapy induces inflammatory activity in large arteries. Circulation. 2020;142(24):2396–2398. doi: 10.1161/CIRCULATIONAHA.120.048708. [DOI] [PubMed] [Google Scholar]
- 86.Lopez-Mattei J.C., Yang E.H., Ferencik M., Baldassarre L.A., Dent S., Budoff M.J. Cardiac computed tomography in cardio-oncology. J Am Coll Cardiol CardioOnc. 2021;3(5):635–649. doi: 10.1016/j.jaccao.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mani V., Woodward M., Samber D., et al. Predictors of change in carotid atherosclerotic plaque inflammation and burden as measured by 18-FDG-PET and MRI, respectively, in the dal-PLAQUE study. Int J Cardiovasc Imaging. 2014;30(3):571–582. doi: 10.1007/s10554-014-0370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pérez-Medina C., Fayad Z.A., Mulder W.J.M. Atherosclerosis immunoimaging by positron emission tomography. Arterioscler Thromb Vas Biol. 2020;40(4):865–873. doi: 10.1161/ATVBAHA.119.313455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kusters P.J.H., Lutgens E., Seijkens T.T.P. Exploring immune checkpoints as potential therapeutic targets in atherosclerosis. Cardiovasc Res. 2018;114(3):368–377. doi: 10.1093/cvr/cvx248. [DOI] [PubMed] [Google Scholar]
- 90.Poels K., Schreurs M., Jansen M., et al. Immuno-PET imaging of atherosclerotic plaques with [89Zr]Zr-anti-CD40 mAb—proof of concept. Biology (Basel) 2022;11(3):408. doi: 10.3390/biology11030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meletta R., Steier L., Borel N., et al. CD80 is upregulated in a mouse model with shear stress-induced atherosclerosis and allows for evaluating CD80-targeting PET tracers. Mol Imaging Biol. 2017;19(1):90–99. doi: 10.1007/s11307-016-0987-0. [DOI] [PubMed] [Google Scholar]
- 92.Meletta R., Müller Herde A., Dennler P., Fischer E., Schibli R., Krämer S.D. Preclinical imaging of the co-stimulatory molecules CD80 and CD86 with indium-111-labeled belatacept in atherosclerosis. EJNMMI Res. 2016;6(1):1. doi: 10.1186/s13550-015-0157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mach F., Schönbeck U., Sukhova G.K., Atkinson E., Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394(6689):200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 94.Kahaly G.J., Stan M.N., Frommer L., et al. A novel anti-CD40 monoclonal antibody, iscalimab, for Control of Graves hyperthyroidism-a proof-of-concept trial. J Clin Endocrinol Metab. 2020;105(3):dgz013. doi: 10.1210/clinem/dgz013. [DOI] [PubMed] [Google Scholar]
- 95.Schiffer W.B., Deych E., Lenihan D.J., Zhang K.W. Coronary and aortic calcification are associated with cardiovascular events on immune checkpoint inhibitor therapy. Int J Cardiol. 2021;322:177–182. doi: 10.1016/j.ijcard.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 96.Drobni Z.D., Murphy S.P., Alvi R.M., et al. Association between incidental statin use and skeletal myopathies in patients treated with immune checkpoint inhibitors. Immunother Adv. 2021;1(1):ltab014. doi: 10.1093/immadv/ltab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sabatine M.S., Giugliano R.P., Keech A.C., et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 98.Ray K.K., Colhoun H.M., Szarek M., et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(8):618–628. doi: 10.1016/s2213-8587(19)30158-5. [DOI] [PubMed] [Google Scholar]
- 99.Liu X., Bao X., Hu M., et al. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature. 2020;588(7839):693–698. doi: 10.1038/s41586-020-2911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Momtazi-Borojeni A.A., Jaafari M.R., Badiee A., Sahebkar A. Long-term generation of antiPCSK9 antibody using a nanoliposome-based vaccine delivery system. Atherosclerosis. 2019;283:69–78. doi: 10.1016/j.atherosclerosis.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 101.Omori M., Okuma Y., Hakozaki T., Hosomi Y. Statins improve survival in patients previously treated with nivolumab for advanced non-small cell lung cancer: An observational study. Mol Clin Oncol. 2019;10(1):137–143. doi: 10.3892/mco.2018.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cantini L., Pecci F., Hurkmans D.P., et al. High-intensity statins are associated with improved clinical activity of PD-1 inhibitors in malignant pleural mesothelioma and advanced non-small cell lung cancer patients. Eur J Cancer. 2021;144:41–48. doi: 10.1016/j.ejca.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 103.Bird L. Statins as adjuvants. Nat Rev Immunol. 2018;18(11):669. doi: 10.1038/s41577-018-0076-5. [DOI] [PubMed] [Google Scholar]
- 104.Yang W., Bai Y., Xiong Y., et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531(7596):651–655. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ma X., Bi E., Lu Y., et al. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30(1):143–156.e5. doi: 10.1016/j.cmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xia Y., Xie Y., Yu Z., et al. The mevalonate pathway is a druggable target for vaccine adjuvant discovery. Cell. 2018;175(4):1059–1073.e21. doi: 10.1016/j.cell.2018.08.070. [DOI] [PubMed] [Google Scholar]
- 107.Lau E.S., Binek A., Parker S.J., et al. Sexual dimorphism in cardiovascular biomarkers: clinical and research implications. Circ Res. 2022;130(4):578–592. doi: 10.1161/circresaha.121.319916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lau E.S., Paniagua S.M., Guseh J.S., et al. Sex differences in circulating biomarkers of cardiovascular disease. J Am Coll Cardiol. 2019;74(12):1543–1553. doi: 10.1016/j.jacc.2019.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim C.H., Yang S., Zhang J., et al. Differences in plaque characteristics and myocardial mass. JACC: Asia. 2022;2(2):157–167. doi: 10.1016/j.jacasi.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilcox N.S., Rotz S.J., Mullen M., et al. Sex-specific cardiovascular risks of cancer and its therapies. Circ Res. 2022;130(4):632–651. doi: 10.1161/CIRCRESAHA.121.319901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wallis C.J.D., Butaney M., Satkunasivam R., et al. Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: a systematic review and meta-analysis. JAMA Oncol. 2019;5(4):529–536. doi: 10.1001/jamaoncol.2018.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Conforti F., Pala L., Bagnardi V., et al. Cancer immunotherapy efficacy and patients' sex: a systematic review and meta-analysis. Lancet Oncology. 2018;19(6):737–746. doi: 10.1016/s1470-2045(18)30261-4. [DOI] [PubMed] [Google Scholar]
- 113.Valpione S., Pasquali S., Campana L.G., et al. Sex and interleukin-6 are prognostic factors for autoimmune toxicity following treatment with anti-CTLA4 blockade. J Transl Med. 2018;16(1):94. doi: 10.1186/s12967-018-1467-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jing Y., Zhang Y., Wang J., et al. Association between sex and immune-related adverse events during immune checkpoint inhibitor therapy. J Natl Cancer Inst. 2021;113(10):1396–1404. doi: 10.1093/jnci/djab035. [DOI] [PubMed] [Google Scholar]
- 115.Zamami Y., Niimura T., Okada N., et al. Factors associated with immune checkpoint inhibitor-related myocarditis. JAMA Oncol. 2019;5(11):1635–1637. doi: 10.1001/jamaoncol.2019.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Escudier M., Cautela J., Malissen N., et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136(21):2085–2087. doi: 10.1161/circulationaha.117.030571. [DOI] [PubMed] [Google Scholar]
- 117.Wei S.C., Meijers W.C., Axelrod M.L., et al. A genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discov. 2021;11(3):614–625. doi: 10.1158/2159-8290.CD-20-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 119.Polanczyk M.J., Hopke C., Vandenbark A.A., Offner H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1) Int Immunol. 2007;19(3):337–343. doi: 10.1093/intimm/dxl151. [DOI] [PubMed] [Google Scholar]
- 120.Polanczyk M.J., Hopke C., Vandenbark A.A., Offner H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res. 2006;84(2):370–378. doi: 10.1002/jnr.20881. [DOI] [PubMed] [Google Scholar]
- 121.Suero-Abreu G.A., Barajas-Ochoa A., Berkowitz R. An analysis of global research trends and top-cited research articles in cardio-oncology. Cardiol Res. 2021;12(5):309–317. doi: 10.14740/cr1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo L., Wei R., Lin Y., Kwok H.F. Clinical and recent patents applications of PD-1/PD-L1 targeting immunotherapy in cancer treatment—current progress, strategy, and future perspective. Front Immunol. 2020;11:1508. doi: 10.3389/fimmu.2020.01508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Antonia S.J., Villegas A., Daniel D., et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 124.Herbst R.S., Giaccone G., de Marinis F., et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 125.Migden M.R., Rischin D., Schmults C.D., et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 126.Powles T., Park S.H., Voog E., et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 127.Cochain C., Chaudhari S.M., Koch M., Wiendl H., Eckstein H.H., Zernecke A. Programmed cell death-1 deficiency exacerbates T cell activation and atherogenesis despite expansion of regulatory T cells in atherosclerosis-prone mice. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0093280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hodi F.S., O'Day S.J., McDermott D.F., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ribas A., Kefford R., Marshall M.A., et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–622. doi: 10.1200/jco.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ma K., Lv S., Liu B., et al. CTLA4-IgG ameliorates homocysteine-accelerated atherosclerosis by inhibiting T-cell overactivation in apoE(-/-) mice. Cardiovasc Res. 2013;97(2):349–359. doi: 10.1093/cvr/cvs330. [DOI] [PubMed] [Google Scholar]
- 131.Li S.H., Chen W.J., Yan M., Shu Y.W., Liao Y.H. Expression of coinhibitory PD-L1 on CD4⁺CD25⁺FOXP3⁺ regulatory T cells is elevated in patients with acute coronary syndrome. Coron Artery Dis. 2015;26(7):598–603. doi: 10.1097/mca.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 132.Zang K., Hui L., Wang M., Huang Y., Zhu X., Yao B. TIM-3 as a prognostic marker and a potential immunotherapy target in human malignant tumors: a meta-analysis and bioinformatics validation. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.579351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Acharya N., Sabatos-Peyton C., Anderson A.C. Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2020-000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Foks A.C., Ran I.A., Wasserman L., et al. T-cell immunoglobulin and mucin domain 3 acts as a negative regulator of atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33(11):2558–2565. doi: 10.1161/atvbaha.113.301879. [DOI] [PubMed] [Google Scholar]
- 135.Qiu M.-K., Wang S.-C., Dai Y.-X., Wang S.-Q., Ou J.-M., Quan Z.-W. PD-1 and Tim-3 pathways regulate CD8+ T cells function in atherosclerosis. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0128523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lecocq Q., Keyaerts M., Devoogdt N., Breckpot K. The next-generation immune checkpoint LAG-3 and its therapeutic potential in oncology: third time's a charm. Int J Mol Sci. 2020;22(1):75. doi: 10.3390/ijms22010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Golden D., Kolmakova A., Sura S., et al. Lymphocyte activation gene 3 and coronary artery disease. JCI Insight. 2016;1(17) doi: 10.1172/jci.insight.88628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mahne A.E., Mauze S., Joyce-Shaikh B., et al. Dual roles for regulatory T-cell depletion and costimulatory signaling in agonistic GITR targeting for tumor immunotherapy. Cancer Res. 2017;77(5):1108–1118. doi: 10.1158/0008-5472.Can-16-0797. [DOI] [PubMed] [Google Scholar]
- 139.Zappasodi R., Sirard C., Li Y., et al. Rational design of anti-GITR-based combination immunotherapy. Nat Med. 2019;25(5):759–766. doi: 10.1038/s41591-019-0420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim W.-J., Bae E.-M., Kang Y.-J., et al. Glucocorticoid-induced tumour necrosis factor receptor family related protein (GITR) mediates inflammatory activation of macrophages that can destabilize atherosclerotic plaques. Immunology. 2006;119(3):421–429. doi: 10.1111/j.1365-2567.2006.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shami A., Atzler D., Bosmans L.A., et al. Glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) drives atherosclerosis in mice and is associated with an unstable plaque phenotype and cerebrovascular events in humans. Eur Heart J. 2020;41(31):2938–2948. doi: 10.1093/eurheartj/ehaa484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li D.K., Wang W. Characteristics and clinical trial results of agonistic anti-CD40 antibodies in the treatment of malignancies. Oncol Lett. 2020;20(5):176. doi: 10.3892/ol.2020.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Byrne K.T., Betts C.B., Mick R., et al. Neoadjuvant selicrelumab, an agonist CD40 antibody, induces changes in the tumor microenvironment in patients with resectable pancreatic cancer. Clin Cancer Res. 2021;27(16):4574–4586. doi: 10.1158/1078-0432.Ccr-21-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lacy M., Bürger C., Shami A., et al. Cell-specific and divergent roles of the CD40L-CD40 axis in atherosclerotic vascular disease. Nat Commun. 2021;12(1):3754. doi: 10.1038/s41467-021-23909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bosmans L.A., van Tiel C.M., Aarts S., et al. Myeloid CD40 deficiency reduces atherosclerosis by impairing macrophages' transition into a pro-inflammatory state. Cardiovasc Res. Published online May 19, 2022 doi: 10.1093/cvr/cvac084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ansell S.M., Flinn I., Taylor M.H., et al. Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, for hematologic malignancies. Blood Advances. 2020;4(9):1917–1926. doi: 10.1182/bloodadvances.2019001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ven Kvd, Borst J. Targeting the T-cell co-stimulatory CD27/CD70 pathway in cancer immunotherapy: rationale and potential. Immunotherapy. 2015;7(6):655–667. doi: 10.2217/imt.15.32. [DOI] [PubMed] [Google Scholar]
- 148.Winkels H., Meiler S., Lievens D., et al. CD27 co-stimulation increases the abundance of regulatory T cells and reduces atherosclerosis in hyperlipidaemic mice. Eur Heart J. 2017;38(48):3590–3599. doi: 10.1093/eurheartj/ehx517. [DOI] [PubMed] [Google Scholar]
- 149.Winkels H., Meiler S., Smeets E., et al. CD70 limits atherosclerosis and promotes macrophage function. Thromb Haemost. 2017;117(1):164–175. doi: 10.1160/th16-04-0318. [DOI] [PubMed] [Google Scholar]
- 150.Fan X., Quezada S.A., Sepulveda M.A., Sharma P., Allison J.P. Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J Exp Med. 2014;211(4):715–725. doi: 10.1084/jem.20130590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Solinas C., Gu-Trantien C., Willard-Gallo K. The rationale behind targeting the ICOS-ICOS ligand costimulatory pathway in cancer immunotherapy. ESMO Open. 2020;5(1) doi: 10.1136/esmoopen-2019-000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Afek A., Harats D., Roth A., Keren G., George J. A functional role for inducible costimulator (ICOS) in atherosclerosis. Atherosclerosis. 2005;183:57–63. doi: 10.1016/j.atherosclerosis.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 153.Gotsman I., Grabie N., Gupta R., et al. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114(19):2047–2055. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- 154.Zhong Z., Zhang Q., Tan L., Guo X., Gan C. T cell co-stimulator inducible co-stimulatory (ICOS) exerts potential anti-atherosclerotic roles through downregulation of vascular smooth muscle phagocytosis and proliferation. Ann Transl Med. 2020;8(23):1597. doi: 10.21037/atm-20-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang W., Huang Q., Xiao W., et al. Advances in anti-tumor treatments targeting the CD47/SIRPalpha Axis. Front Immunol. 2020;11:18. doi: 10.3389/fimmu.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nichetti F., Ligorio F., Zattarin E., et al. Is there an interplay between immune checkpoint inhibitors, thromboprophylactic treatments and thromboembolic events? Mechanisms and impact in non-small cell lung cancer patients. Cancers (Basel) 2019;12(1):67. doi: 10.3390/cancers12010067. [DOI] [PMC free article] [PubMed] [Google Scholar]