Abstract
Immune checkpoint inhibitors (ICIs) are a major class of immuno-oncology therapeutics that have significantly improved the prognosis of various cancers, both in (neo)adjuvant and metastatic settings. Unlike other conventional therapies, ICIs elicit antitumor effects by enhancing host immune systems to eliminate cancer cells. There are 3 approved ICI classes by the U.S. Food and Drug Administration: inhibitors targeting cytotoxic T lymphocyte associated antigen 4, programmed death 1/programmed death-ligand 1, and lymphocyte-activation gene 3, with many more in development. ICIs are commonly associated with distinct toxicities, known as immune-related adverse events, which can arise during treatment or less frequently be of late onset, usually relating to excessive activation of the immune system. Acute cardiovascular immune-related adverse events such as myocarditis are rare; however, data suggesting chronic cardiovascular sequelae are emerging. This review presents the current landscape of ICIs in oncology, with a focus on important aspects relevant to cardiology.
Key Words: biomarkers, cardio-oncology, cardiotoxicity, immune checkpoint inhibitors, immune related adverse events, immunotherapy, medical oncology
Abbreviations and Acronyms: CTLA-4, cytotoxic T lymphocyte associated antigen 4; ICI, immune checkpoint inhibitors; irAE, immune-related adverse event; LAG-3, lymphocyte-activation gene 3; MSI, microsatellite instability; PD-L1, programmed death-ligand 1; TMB, tumor mutational burden; TME, tumor microenvironment
Central Illustration
Highlights
-
•
Immune checkpoint inhibitors (ICIs) stimulate and enhance host immunity to eliminate cancer cells.
-
•
Indications are rapidly broadening across various cancer types and settings (cure, palliation, adjuvant).
-
•
Although generally well tolerated, ICIs cause immune-related adverse events (irAEs), related to excessive immune activation.
-
•
Attention should be directed toward the detection and management of chronic irAEs.
The development of immune checkpoint inhibitors (ICIs) culminated decades of searching for the elusive keys to activating host immunity to regulate cancer growth. These agents have dramatically changed the landscape of cancer treatment in the past decade. The success of ICIs was recognized by the awarding of the 2018 Nobel Prize in Medicine or Physiology to 2 immunologists who developed the concept of ICIs, James Allison and Tasuku Honjo.1 As of April 2022, therapies targeting 3 immune checkpoints: cytotoxic T lymphocyte associated antigen 4 (CTLA-4), programmed death-1 (PD-1) and its ligand (PD-L1), and lymphocyte-activation gene 3 (LAG-3), are approved by the U.S. Food and Drug Administration (FDA) for use as anticancer agents, either as monotherapy or in combination with other ICIs or with chemotherapy and/or molecularly targeted agents. Currently, almost half of all patients with metastatic cancer in high-income countries are being treated with ICIs.2 However, there are a number of cancers in which ICIs are minimally effective; understanding primary and secondary resistance is a major focus of research. In this State-of-the-Art Review, we discuss the broad principles of ICI cancer treatment for solid tumors, including mechanisms of action, clinical indications, immune-related adverse effects (irAEs), predictive biomarkers, contemporary issues and future directions, and emerging cardiovascular issues (Central Illustration).
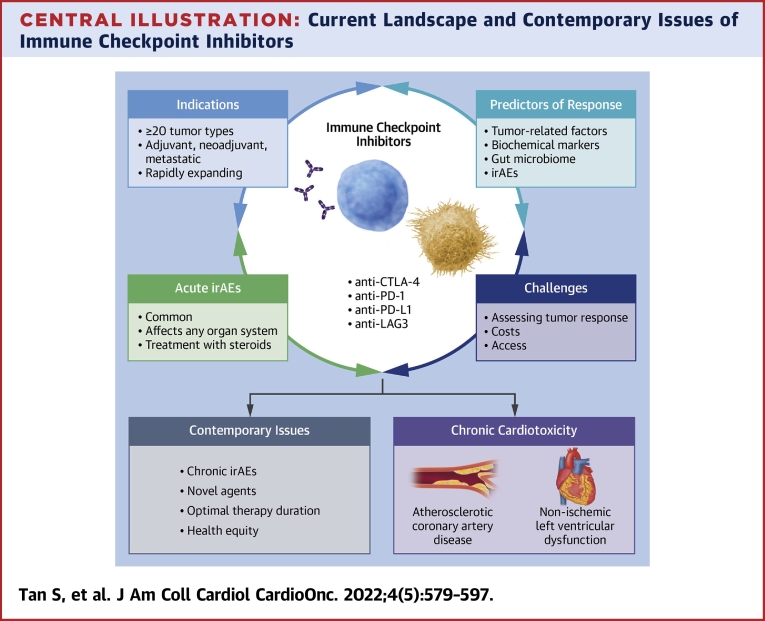
Central Illustration.
Current Landscape and Contemporary Issues of Immune Checkpoint Inhibitors
The use of immune checkpoint inhibitors in oncology has grown significantly in the past decade, with significant focus on expanding indications, determining predictors for response, treating immune-related adverse events (irAEs), and developing methods to assess tumor response. Further efforts are required to address emerging contemporary issues surrounding potential chronic cardiotoxicities, optimal length of therapy, and health equity.
CTLA-4 = cytotoxic T lymphocyte associated antigen 4; LAG-3 = lymphocyte-activation gene 3; PD-1 = programmed death 1; PD-L1 = programmed death ligand 1.
Mechanism of Action
Immune response to cancer
Current ICIs were developed based on the cancer immunosurveillance hypothesis, which stipulated that the immune system is capable of detecting and eliminating nascent cancer cells.3 Harnessing the host’s own immunity to recognize and eliminate cancer cells as “foreign” has been a goal for decades, although the clinical utility of earlier cytokine-based immunotherapies such as interleukin-2 and interferon alpha were hampered by lack of specificity, causing low efficacy and high toxicity.4
Success of immunotherapies is reliant on tumor immunogenicity (ie, the ability of a tumor to induce a host immune response). Tumors with poor immunogenicity, simplistically denoted as “cold” tumors, such as primary brain, pancreatic and most colorectal cancer have poor response rates to immunotherapy5,6; conversely, “hot” tumors such as melanoma and squamous cell carcinomas, can respond dramatically.5 Tumor immunogenicity is affected by intrinsic characteristics such as tumor antigen expression and tumor-infiltrating cytotoxic CD8 lymphocytes,5,6 as well as the tumor microenvironment (TME), which consists of anatomical and physiological properties extrinsic to cancer cells (Figure 1). The host’s gut microbiome is also proving to be an important determinant of response, with mechanisms still poorly understood.
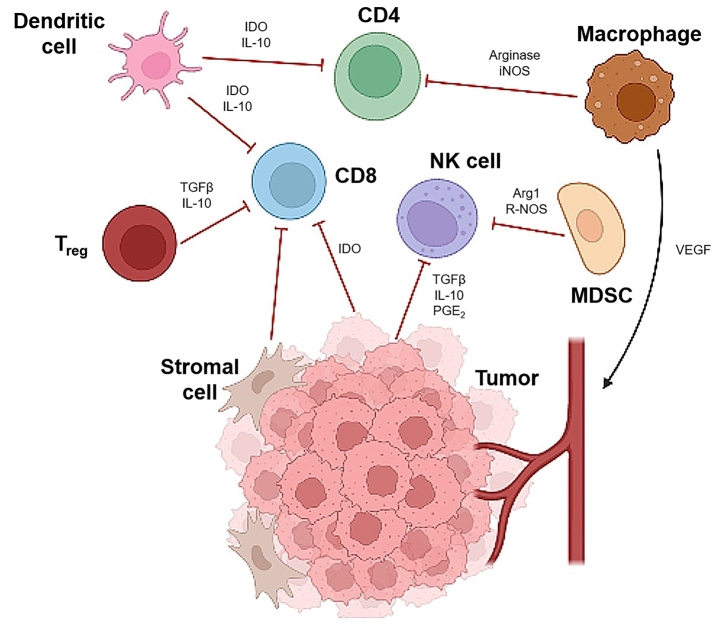
Figure 1.
Tumor Microenvironment
The tumor microenvironment consists of immune cells (CD8 T cells, CD4 T cells, regulatory T cells [Tregs], natural killer [NK] cells, dendritic cells, macrophages, myeloid-derived suppressor cells [MDSCs]) within the extracellular matrix, stromal cells, surrounding vascular supply, and numerous cytokines.6,113 Through expression of various cytokines (inhibitory cytokines shown by red lines, stimulatory cytokines shown by black arrows), the tumor microenvironment can promote tumor growth and immune evasion. Created with BioRender. Arg1 = arginase 1; IDO = indoleamine 2,3-dioxygenase; IL = interleukin; iNOS = inducible nitric oxide synthase; PGE2 = prostaglandin E2; R-NOS = reactive nitric oxide species; TGF = transforming growth factor; VEGF = vascular endothelial growth factor.
Immune checkpoint inhibitors
ICIs are monoclonal antibodies that achieve immune activation by inhibiting key regulatory mechanisms known as checkpoints. Immune checkpoints are physiologically designed to limit immune responses within the body, and thus their inhibition restores host T cell immunity toward cancer cells that have adapted toward immune evasion. The processes involved in cytotoxic CD8 T cell–mediated immunity are summarized in Figure 2.
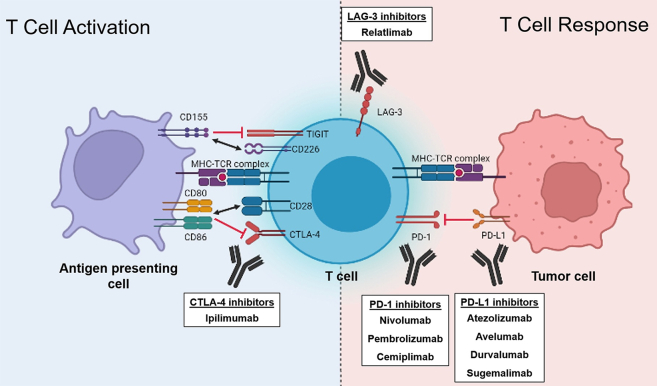
Figure 2.
Mechanisms of T Cell Activation, Response, and Immune Checkpoint Inhibitors
T cell activation is a multiple signal process that begins with tumor antigen presentation by antigen-presenting cells (APCs) from the innate immune system to T cells via the major histocompatibility complex (MHC). A second co-stimulatory signal from the binding of CD80 and CD86 on APCs to CD28 on T cells is also required.114,115 The intracellular cascade from these signals results in the differentiation of resting CD8 T cells into activated cytotoxic CD8 T cells, which have the ability to recognize tumor cells and promote tumor cell apoptosis through secretion of various cytotoxins. This process is further assisted by additional co-stimulatory signals (black 2-way arrows) or inhibited by co-inhibitory signals (red lines) that are both present in T cell activation and response. Cytotoxic T lymphocyte associated antigen 4 (CTLA-4), programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1), and lymphocyte-activation gene 3 (LAG-3) are co-inhibitory signal targets for immune checkpoint inhibition, allowing increased T cell activation and response toward tumor cells. Additional nonimmune mechanisms of immune checkpoint inhibitors (not depicted) may be involved in the treatment of lymphoma. Created with BioRender. TCR = T cell receptor; TIGIT = T cell Immunoreceptor with Ig and ITIM domains.
CTLA-4 inhibitors
CTLA-4 is an inhibitory receptor present on T cells that downregulates T cell activity and prevents unnecessary T cell activation (Figure 2).7, 8, 9 Inhibition of CTLA-4 receptors blocks these inhibitory signals and upregulates T cell activation and response toward cancer cells.10
Ipilimumab, a CTLA-4 inhibitor, was the first ICI to enter clinical use, approved in 2011 by the U.S. FDA following demonstration of an overall survival benefit in a randomized phase III trial in metastatic melanoma, a previously treatment-resistant, highly fatal malignancy with median survival <1 year11; with modern immunotherapy at least 50% of patients, even with widespread metastatic disease including brain secondaries, appear to be cured.12,13 This is the only CTLA-4 inhibitor commonly used in standard practice, either as monotherapy or in combination with PD-1/PD-L1 inhibitors. Ipilimumab is usually prescribed a total of 4 cycles (termed induction) and not continually like PD-1/PD-L1 inhibitors, mainly due to toxicity. An adjustment of dose from initial schedules has made this a far more tolerable treatment.14
PD-1 and PD-L1 inhibitors
PD-L1 is a protein on target cells that binds to PD-1 receptors on CD8 T cells to limit inflammation and prevent healthy tissue from damage (Figure 2).15 Tumor cells have the ability to express PD-L1 to cause T cell anergy and evade immune response. ICIs targeting PD-1 receptors or PD-L1 prevent tumor escape from overexpression of PD-L1, leading to cytotoxic CD8 T cell–mediated tumor cell destruction.
U.S. FDA approval for this ICI subclass followed shortly after the success of ipilimumab. Widespread use of these agents as standard therapies has rapidly been adopted after reporting of large scale randomized controlled trials across a broad range of cancers. Generally this subclass of ICI has demonstrated less toxicity than CTLA-4 inhibitors.16 Commonly used PD-1 inhibitors include nivolumab, pembrolizumab, and cemiplimab; PD-L1 inhibitors include atezolizumab, avelumab, and durvalumab.
Lymphocyte-activation gene 3 inhibitors
LAG-3 is a co-inhibitory protein that is uniquely present on both cytotoxic CD8 T cells as well as immunosuppressive regulatory T cells. Inhibition of LAG-3 increases CD8 T cell response both directly and indirectly through reduced regulatory T cell mechanisms (Figure 2),17,18 with additive antitumor effects when combined with PD-1 inhibition.19
LAG-3 inhibitors are the latest class of ICIs to be granted approval by the U.S. FDA. Currently, there is 1 LAG-3 inhibitor in clinical use, relatlimab, which is only available in a fixed dose combination with nivolumab in the treatment of advanced melanoma.20
Key Points
-
•
ICIs utilize host immunity to eliminate cancer cells.
-
•
Response to ICIs is reliant on tumor immunogenicity.
-
•
ICIs in use include anti-CTLA-4, anti-PD-1/PD-L1, and anti-LAG-3 antibodies.
Practical Applications
Clinical indications
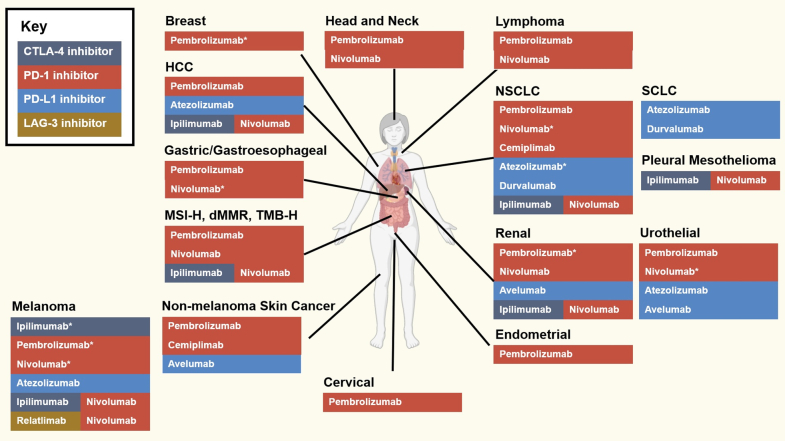
ICIs are routinely used in patients across approximately 20 tumor types, with rapidly broadening indications as numerous ongoing trials are reported (Figure 3). All ICIs in clinical use are only available as intravenous formulations. PD-1/PD-L1 inhibitors are the most widely used, commonly as monotherapy, or as the backbone of combination regimens with ipilimumab or relatlimab, cytotoxic chemotherapy, and/or molecularly targeted therapies. Current indications for patients with metastatic cancer are summarized in Table 1, while current indications in the adjuvant (defined as systemic therapy to eliminate micrometastases and reduce risk of recurrence after definitive cancer treatment, usually surgery or [chemo]radiotherapy) and neoadjuvant (defined as systemic therapy to downsize tumors prior to definitive therapy) settings are summarized in Table 2.21
Figure 3.
U.S. Food and Drug Administration–Approved Immune Checkpoint Inhibitors as of April 2022
There are ≥20 approved indications for immune checkpoint inhibitors at time of writing, although this is expected to expand significantly in the future. Created with BioRender. ∗Also approved for use in adjuvant or neoadjuvant settings. dMMR = deficient mismatch repair; HCC = hepatocellular carcinoma; MSI-H = microsatellite instability-high; NSCLC = non-small cell lung cancer; SCLC = small cell lung cancer; TMB-H = tumor mutational burden-high; other abbreviations as in Figure 2.
Table 1.
U.S. Food and Drug Administration Approved Immune Checkpoint Inhibitors as of April 2022: Advanced or Metastatic Cancers
| Organ | Cancer | Immune Checkpoint Inhibitor | Line of Therapy | Schedule |
|---|---|---|---|---|
| Skin | Melanoma | Ipilimumab116 | First | Every 3 wk |
| Pembrolizumab16 | First | Every 3 or 6 wk | ||
| Nivolumab117 | First or second | Every 2 or 4 wk | ||
| Atezolizumab118 | First | Every 2-4 wk | ||
| Ipilimumab and Nivolumab13 | First | Every 3 wk | ||
| Relatlimab and Nivolumab20 | First | Every 4 wk | ||
| Cutaneous squamous cell carcinoma | Pembrolizumab119 | First | Every 3 or 6 wk | |
| Cemiplimab120 | First | Every 3 wk | ||
| Basal cell carcinoma | Cemiplimab121 | Second | Every 3 wk | |
| Merkel cell carcinoma | Pembrolizumab122 | First | Every 3 or 6 wk | |
| Avelumab123 | First | Every 2 wk | ||
| Lung | Non-small cell lung cancer | Pembrolizumab31,32,124 | First | Every 3 or 6 wk |
| Nivolumab125,126 | First or second | Every 2 or 4 wk | ||
| Cemiplimab127 | First | Every 3 wk | ||
| Atezolizumab33,128 | First | Every 2-4 wk | ||
| Ipilimumab and Nivolumab129 | First | Every 3 wk | ||
| Small cell lung cancer | Atezolizumab34 | First | Every 2-4 wk | |
| Durvalumab35 | First | Every 3-4 wk | ||
| Pleural mesothelioma | Ipilimumab and Nivolumab130 | First | Every 6 wk | |
| Urological | Renal cell cancer | Pembrolizumab44,45 | First | Every 3 or 6 wk |
| Nivolumab46 | First or second | Every 2 or 4 wk | ||
| Avelumab47 | First | Every 2 wk | ||
| Ipilimumab and Nivolumab131 | First | Every 3 wk | ||
| Urothelial carcinoma | Pembrolizumab132,133 | Second | Every 3 or 6 wk | |
| Nivolumab134 | Second | Every 2 or 4 wk | ||
| Atezolizumab135, 136, 137 | First or second | Every 2-4 wk | ||
| Avelumab138 | Second | Every 2 wk | ||
| Head and neck | Squamous cell carcinoma | Pembrolizumab36 | First | Every 3 or 6 wk |
| Nivolumab139 | Second | Every 2 or 4 wk | ||
| Gastrointestinal | Hepatocellular carcinoma | Pembrolizumab140 | Second | Every 3 or 6 wk |
| Atezolizumab48 | First | Every 2-4 wk | ||
| Ipilimumab and Nivolumab141 | Second | Every 3 wk | ||
| MSI-H or dMMR colorectal cancer | Pembrolizumab80 | First | Every 3 or 6 wk | |
| Nivolumab81 | Second | Every 2 or 4 wk | ||
| Ipilimumab and Nivolumab142 | Second | Every 3 wk | ||
| Gastric and gastroesophageal cancer | Pembrolizumab39,41 | First | Every 3 or 6 wk | |
| Nivolumab40,42 | First or second | Every 2-4 wk | ||
| Gynecological | Cervical cancer | Pembrolizumab143 | First or second | Every 3 or 6 wk |
| Endometrial cancer | Pembrolizumab49,144 | Second | Every 3 or 6 wk | |
| Breast | Triple negative breast cancer | Pembrolizumab37 | First | Every 3 or 6 wk |
| Lymphoma | Classical Hodgkin’s lymphoma | Pembrolizumab145 | Third | Every 3 or 6 wk |
| Nivolumab146 | Fourth | Every 2 or 4 wk | ||
| Mediastinal B cell lymphoma | Pembrolizumab147 | Third | Every 3 or 6 wk | |
| Others | MSI-H, dMMR, or TMB-H solid organ tumors | Pembrolizumab82,148 | Salvage therapy | Every 3 or 6 wk |
Source: U.S. FDA.21
dMMR = deficient mismatch repair; MSI-H = microsatellite instability-high; TMB-H = tumor mutational burden-high.
Table 2.
U.S. Food and Drug Administration Approved Immune Checkpoint Inhibitors as of April 2022: Adjuvant and Neoadjuvant Therapies
| Organ | Cancer | Immune Checkpoint Inhibitor | Scenario | Cancer Stage | Schedule |
|---|---|---|---|---|---|
| Skin | Melanoma | Ipilimumab149 | Adjuvant | III | Every 3 wk |
| Pembrolizumab150 | Adjuvant | II-III | Every 3 or 6 wk | ||
| Nivolumab151 | Adjuvant | III | Every 2 or 4 wk | ||
| Lung | Non-small cell lung cancer | Nivolumab152 | Neoadjuvant | I-III | Every 3 wk |
| Atezolizumab153 | Adjuvant | II-III | Every 2-4 wk | ||
| Durvalumab154 | Adjuvant | III | Every 2 or 4 wk | ||
| Urological | Renal cell cancer | Pembrolizumab155 | Adjuvant | II-IV | Every 3 or 6 wk |
| Urothelial carcinoma | Nivolumab156 | Adjuvant | III | Every 2 or 4 wk | |
| Gastrointestinal | Gastric and gastroesophageal cancer | Nivolumab157 | Adjuvant | II-III | Every 2 or 4 wk |
| Breast | Triple negative breast cancer | Pembrolizumab38 | Adjuvant and neoadjuvant | II-III | Every 3 or 6 wk |
Abbreviations as in Table 1.
Measuring tumor response
Response to standard anticancer treatment has been defined in clinical trials using serial measurements of tumor size and/or volume by conventional radiological imaging, predominantly computed tomography, positron emission tomography–computed tomography, or magnetic resonance imaging. Compared with conventional systemic treatments, lesions may sometimes increase in size early after commencement of ICIs (usually at first tumor assessment at 6-8 weeks). This phenomenon, known as pseudoprogression, is postulated to be due to initial massive recruitment of immune cells/inflammatory response.12 Although uncommon, pseudoprogression can be difficult to distinguish from early tumor progression. Standardized radiological response criteria, RECIST (Response Evaluation Criteria in Solid Tumors),22 used in early clinical trials failed to account for this phenomenon, leading to the development of modified versions in current use, denoted iRECIST (immune RECIST).23 Current guidelines recommend considering radiological assessment in context with clinical status (improvement in symptoms, weight), however evaluation of early tumor response can remain challenging.23
Optimal therapy duration
One remarkable feature of treatment with ICIs is the possibility of durable stable disease, or response (including complete response) and “clinical cure” for patients with metastatic disease.12 Sixteen percent of 655 patients with metastatic melanoma treated with PD-1 inhibitors with or without anti-CTLA-4 had complete response and after cessation of ICI, <10% relapsed over 5-year follow-up.24 Even better results were demonstrated with PD-1 inhibition in a pooled cohort of patients with advanced melanoma, renal cell cancer, and non-small cell lung cancers, with 5 year survival rates of 34%, 28% and 16%, respectively, after achieving either complete or partial response.25 This has led to substantial hope for a possible cure for metastatic cancers in some patients treated with ICIs, although it is important to acknowledge that durable response has only been reported in a minority of patients (more frequently seen in patients who achieve complete rather than partial response), with many still succumbing to cancer.12 Observations of durable response have led to current studies to define optimal duration of ICI therapy, particularly in complete or partial responders. In practice, a 2-year treatment duration is commonly used based on consensus opinion.26 The small proportion of patients with disease progression after ICI discontinuation may be retreated, although observational studies have reported variable response rates on rechallenge.24,27
Duration of ICI in adjuvant settings is also of interest due to balancing survival benefit with ICI-related morbidity from acute and chronic irAEs, especially in low-risk patients in which surgery alone could be curative. Further research is currently directed at evaluating this challenging paradigm, with postulation that shorter regimens could reduce the occurrence of irAEs without compromising cancer-related survival.28 Costs and use of health care resources are also factors to be considered in this regard.
Interaction with other therapies
Corticosteroids
High-dose and chronic low-dose corticosteroids are relatively contraindicated during ICI therapy, particularly early in the treatment course due to potential to negate antitumor efficacy, although definitive evidence is lacking.29 Steroid use for antiemesis particularly for concurrent chemotherapy is tolerated but should be minimized by use of alternate agents where practical.
Cytotoxic chemotherapy
Cytotoxic chemotherapy can synergistically enhance ICI activity by triggering release of tumor antigen from dying cancer cells for immune cell recognition.30 Combination ICI and cytotoxic chemotherapy, in particular platinum or taxane-based regimens, are employed in the treatment of non-small cell cancer31, 32, 33 and small cell lung cancer,34,35 head and neck cancer,36 triple negative breast cancer,37,38 and esophageal cancer.39, 40, 41, 42 Further trials in other cancer types are ongoing.
Targeted therapies
Vascular endothelial growth factor (VEGF) inhibitors are targeted therapies which inhibit angiogenesis signatures within the TME and improve tumor response to ICI therapy.43 VEGF inhibitors are commonly prescribed with ICIs in the treatment of renal cell carcinoma,44, 45, 46, 47 with clinical benefit also demonstrated in hepatocellular carcinoma48 and endometrial cancer.49
Radiotherapy
Radiotherapy has been shown to enhance tumor immunogenicity by increasing tumor antigen release and inflammatory cytokine signaling within the TME.50 Several preclinical studies have shown radiotherapy to enhance T cell activation and response, suggesting a synergistic potential if used in combination with ICIs.50 Clinical studies investigating ICI treatment with radiotherapy are underway.
Key Points
-
•
Three ICI classes (9 agents) are currently approved for use across 20 tumor types as monotherapy and in various combinations, with anti-PD-1/PD-L1 antibodies being the most commonly used.
-
•
Durable response can be observed in patients with metastatic disease.
-
•
The optimal duration of treatment with ICIs in both metastatic and adjuvant settings is under investigation.
Immune-Related Adverse Events
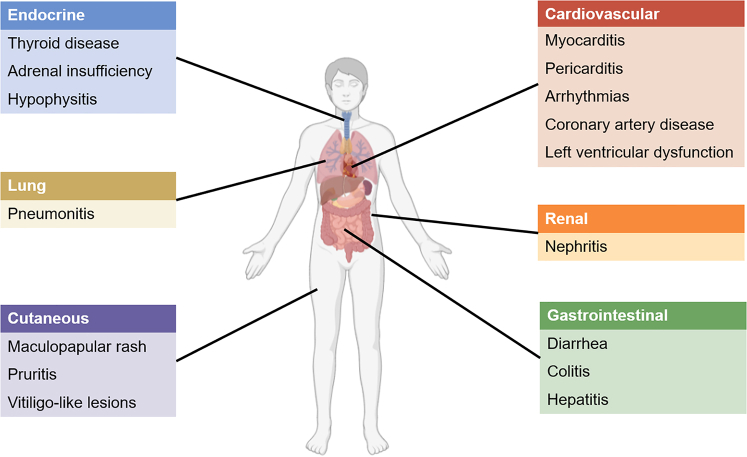
IrAEs refer to autoimmune-like adverse reactions that can occur in any organ, particularly those with extensive environmental exposure (eg, skin, gastrointestinal tract, lungs) or predisposition to autoimmunity (eg, thyroid) (Figure 4, Tables 3 and 4).51 Although irAEs mirror de novo autoimmune disease, the timing in relation to treatment is usually diagnostic, with manifestation usually within the first 3 months of ICI initiation, although more rarely at later time points, including after ICI discontinuation.52 Severity is graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (Table 5).53
Figure 4.
Main Organs Affected by Immune-Related Adverse Events
Any organ system can be affected. Most common are skin, endocrine, gastrointestinal tract, kidney and lung. Cardiovascular immune-related adverse events are rare but carry significant mortality and morbidity. Created with BioRender.
Table 3.
Cardiovascular Immune-Related Adverse Events
| Cardiac Immune-Related Adverse Events60,108 | Incidencea | Grading | Management |
|---|---|---|---|
| Myocarditis | 0.3%-0.5% | Grade 1: Abnormal cardiac biomarker without symptoms or ECG abnormalities Grade 2: Abnormal cardiac biomarker with mild symptoms or new ECG abnormalities without conduction delay Grade 3: Abnormal cardiac biomarker with moderate symptoms or new conduction delay Grade 4: Life-threatening conditions with moderate-severe decompensation requiring intervention or intravenous medications |
Grade 1:
|
| Pericarditis | 0.8% | ||
| Arrhythmias | 1% | ||
| Heart failure | 0.9% | ||
| Myocardial infarction | 0.7% | ||
| Ischemic stroke | 0.9% | ||
| Venous thromboembolism | 12.9% | Grade 1: Superficial thrombosis Grade 2: Uncomplicated deep vein thrombosis Grade 3: Uncomplicated pulmonary embolism Grade 4: Life-threatening conditions |
Grade 1:
|
| Dyslipidemia | 2% | Not applicable | Treat as per standard guideline-directed recommendations |
ECG = electrocardiography; ICI = immune checkpoint inhibitor.
Incidences primarily derived from a meta-analysis (108) of ICI clinical trials of patients treated during a follow-up ranging from 3.2 to 32.8 months.
Table 4.
Noncardiovascular Immune-Related Adverse Events
| Organ System | Immune-Related Adverse Events60,158, 159, 160 | Estimated Incidence |
|---|---|---|
| Skin | Maculopapular rash, pruritis, vitiligo-like lesions, erythema multiforme, lichenoid, eczematous, psoriasiform, morbilliform, palmar-plantar erythrodysesthesia, bullous dermatoses, hand-foot syndrome | Overall: 71.5% Maculopapular rash: 14%-24% |
| Gastrointestinal | Immune-related diarrhea, colitis, hepatitis, gastritis, enterocolitis | Diarrhea: 25%-54% Colitis: 3% Hepatitis: 10%-30% |
| Endocrine | Hypothyroidism, hyperthyroidism, primary adrenal insufficiency, hypophysitis, diabetes | Hypothyroidism: 6.6% Hyperthyroidism: 2.9% Primary adrenal insufficiency: 0.7% Hypophysitis: 1.3% |
| Lung | Pneumonitis | 2.7%-10% |
| Musculoskeletal | Arthralgia, myalgia, inflammatory arthritis, myositis, polymyalgia-like syndrome | Arthralgia or myalgia: 40% |
| Renal | Nephritis, acute kidney injury | Acute kidney injury: 1%-5% |
| Neurological | Headache, myasthenia gravis or myasthenic syndrome, aseptic meningitis, encephalitis, Guillain-Barré syndrome, peripheral neuropathy, demyelinating disorders | Overall: 3%-12% High-grade: <1% |
| Hematologic | Hemolytic anemia, acquired thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, aplastic anemia, lymphopenia, immune thrombocytopenia | Anemia: 9.8% Thrombocytopenia: 2.8% |
| Ocular | Uveitis, iritis, episcleritis | Uveitis: 1% Episcleritis: <1% |
Incidences provided are for both monotherapy and combination immune checkpoint inhibitor regimens.
Table 5.
National Cancer Institute Common Terminology Criteria for Adverse Events Grading for Immune-Related Adverse Events
| Grade | Severity | Definition53 |
|---|---|---|
| 1 | Mild | Asymptomatic or mild symptoms Clinical or diagnostic observation only Intervention not indicated |
| 2 | Moderate | Limiting age-appropriate instrumental activities of daily living Minimal, local, or noninvasive intervention indicated |
| 3 | Severe | Disabling or limiting self-care activities of daily living Medically significant but not immediately life-threatening Hospitalization or prolongation of hospitalization indicated |
| 4 | Life-threatening | Urgent intervention indicated |
| 5 | Fatal | Death related to adverse event |
Each subclass of ICIs has subtly different patterns but often quite different incidence of individual irAEs.54 In general, high grade (grade ≥3) irAEs occur most frequently in patients treated with combination anti-CTLA-4 and anti-PD-1/PD-L1, followed by anti-CTLA-4 monotherapy, combination anti-LAG-3 and anti-PD-1, and PD-1/PD-L1 monotherapy. Combination anti-CTLA-4 with anti-PD-1/PD-L1 is associated with increased incidence and severity of irAEs, with an estimated 55% incidence of high-grade irAEs, leading to significantly higher rates treatment discontinuation.55,56 In a meta-analysis of 22 trials including 1,265 patients, CTLA-4 inhibition was associated with a 72% overall incidence of irAEs, of which 24% were high grade and 0.9% were fatal.57 In contrast, a meta-analysis of 46 studies and 12,808 patients treated with PD-1/PD-L1 inhibitors reported an overall irAE incidence of 27%, of which 6% were high grade and mortality was 0.2%.58 The risk of CTLA-4 inhibitor-associated toxicities increases with dose, which is not the case with PD-1/PD-L1 inhibitors, which are used at fixed dosing schedules.57,58
Management of immune-related adverse events
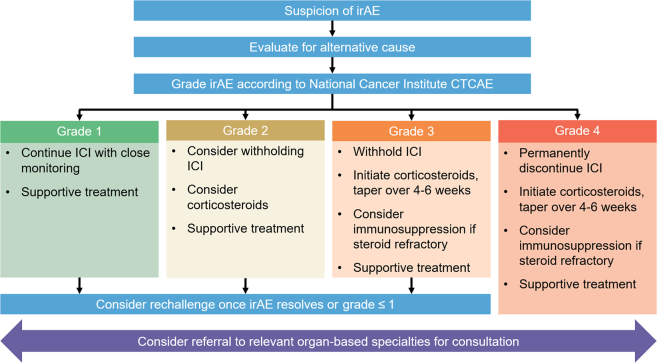
Guideline-directed recommendations for managing irAEs have been developed by oncological societies such as the American Society of Clinical Oncology, National Comprehensive Cancer Network, and European Society of Medical Oncology (Figure 5).59, 60, 61, 62 These are categorized into affected organ systems (Table 4) and specific recommendations are available for each individual irAE. Mild-to-moderate (grade 1 or 2) irAEs may be observed or managed symptomatically while continuing therapy; however, intolerable low grade irAEs may require treatment interruption or cessation. Corticosteroids form the mainstay of treatment for high-grade irAEs, including high-dose methylprednisolone, with rapid escalation to other immunosuppressive therapies, such as infliximab, mycophenolate mofetil, or cyclophosphamide, if severe or recalcitrant.60 Consultation with organ-based specialties is frequently required for high grade toxicities, particularly if diagnostic investigations are required (eg, colonoscopy in setting of colitis).
Figure 5.
General Management Principles of irAEs
The management of immune-related adverse events (irAEs) depends on severity and involves consideration for suspension of therapy, corticosteroids, and/or immunosuppression.59, 60, 61 Management of some organ-specific irAEs may differ from the provided flow chart. CTCAE = Common Terminology Criteria for Adverse Events; ICI = immune checkpoint inhibitor.
Association with clinical response
The occurrence of irAEs has been postulated to reflect a robust immune reaction with ICIs and has been demonstrated to modestly correlate with increased antitumor efficacy characterized by greater response rate, progression-free survival, and overall survival.52,63, 64, 65 This association has only been observed with PD-1/PD-L1 inhibitors63, 64, 65 but not CTLA-4 inhibitors.66 Although the association between antitumor benefits with irAE type, timing, treatment and course have yet to be described, the event of minor or treatable irAEs should provide clinicians with reassurance to persist with treatment.
Rechallenge
Most acute irAEs resolve with discontinuation of ICI. Current guidelines recommend permanent cessation only in patients with grade 4 irAEs,59, 60, 61 with the majority eligible for rechallenge after irAE resolution, particularly if anticancer efficacy has been demonstrated and/or in which few alternative treatments are available. In patients who experience limiting irAEs with combination ICI therapy, treatment can often be continued with a single-agent PD-1 inhibitor. ICI rechallenge following irAE resolution was reported to have a recurrence rate of 28.8% in an observational pharmacovigilance cohort study of 24,079 irAE cases.67 Colitis, hepatitis, and pneumonitis were associated with a higher recurrence with rechallenge.67 Similar results were demonstrated in a meta-analysis of 77 studies, with all-grade and high-grade recurrent irAEs being 34.2% and 11.7% respectively.68
Strategies for rechallenge include switching ICI classes, rechallenging with the same ICI as monotherapy, and prophylaxis with antihistamines and steroids.69 Importantly, no loss of antitumor efficacy has been reported in the specific scenario for ICIs used with concurrent immunosuppression for toxicity.69
Cardiovascular irAEs
Cardiovascular irAEs are rare but carry significant morbidity and mortality (Table 3).51,60 Acute fulminant myocarditis represents the most concerning irAE, with reported estimated incidence of up to 1% and mortality rates of up to 50%.70 Patients often present within 6 weeks of ICI commencement, with presentations ranging from abnormal results on cardiovascular surveillance (usually being performed for other reasons such as concurrent cardiotoxic therapy) to fulminant heart failure with cardiogenic shock.51,60 Diagnosis may not be straightforward and requires clinical correlation with 12-lead electrocardiography, biomarkers such as troponin, echocardiography, negative coronary angiography, magnetic resonance imaging, and in some cases endomyocardial biopsy.70,71 Depending on the combination of these features, patients may be classified into 3 groups—definite, probable and possible myocarditis.71 Positively adjudicated cases of myocarditis may be subclassified into fulminant, clinically significant nonfulminant, and subclinical disease.71
Current guidelines recommend treatment for all cases of myocarditis, including cessation of ICI, cardiology consultation, high-dose corticosteroids, and supportive treatments such as diuretics and inotropes where required.60 In cases of steroid-refractory myocarditis, other immunosuppressive agents such as mycophenolate, infliximab, antithymocyte globulin, abatacept, and alemtuzumab have been used, although evidence is limited.60,70 With increasing awareness, there is growing identification of milder and subclinical cases, with management in the context of ongoing need for ICI therapy the subject of ongoing investigation.
Additional acute cardiovascular irAEs during treatment include pericarditis, arrhythmias, heart failure, coronary artery disease, venous thromboembolism, and dyslipidemia, which are managed similarly to non–immune-mediated cases, with additional considerations to suspend or cease treatment depending on severity.60
Key Points
-
•
ICIs can cause irAEs which may mimic autoimmune disease.
-
•
Systemic corticosteroids are essential in the management of high-grade irAEs.
-
•
Acute myocarditis is a rare but potentially fatal cardiovascular irAE.
Predictive Biomarkers
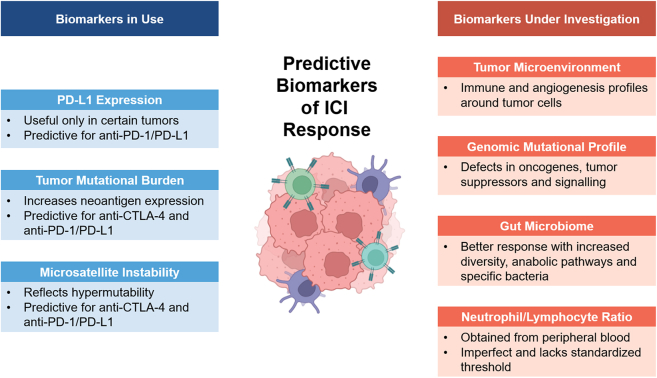
Predictive biomarkers of ICI activity is an area of intensive research with 3 in clinical use: PD-L1 expression, tumor mutational burden (TMB), and microsatellite instability (MSI) (Figure 6); under investigation are markers of TME, genomic mutational profile, gut microbiome, and neutrophil-to-lymphocyte ratio (NLR). Conceptually, these biomarkers evaluate elements of tumor and host immunogenicity, although none have been validated to be completely clinically reliable. Characterization of host immunogenicity may likely require composite biomarkers in future.
Figure 6.
Predictive Biomarkers for Response to ICI Therapy
Biomarkers evaluating tumor and host immunogenicity have the potential to predict response to ICIs. Created with BioRender.
PD-L1 expression
The degree of PD-L1 expression on tumor cells and antigen-presenting cells measured using immunohistochemistry has been shown to enrich for responders to treatment with PD-1/PD-L1 inhibitors.72 Many clinical trials evaluating PD-1/PD-L1 inhibitors utilized PD-L1 expression for target population selection, stratification, or to correlate with clinical efficacy, which led to PD-L1 expression being included by the FDA as an eligibility criterion for PD-1/PD-L1 inhibitor use in certain cancers.73 However, its predictive utility and the threshold level for clinical benefit seem to be tumor-specific; while high expression predicts for treatment efficacy in certain tumor types, lack of PD-L1 expression may not preclude clinical response entirely, and in other tumor types, it lacks predictive ability altogether. In addition, the available commercial immunohistochemistry assays are not interchangeable, tissue staining can be heterogeneous, and there can be significant interobserver variability.74
Tumor mutational burden and microsatellite instability
TMB, defined as the total number of mutations per coding area of tumor DNA,75 is predictive of response to CTLA-4 inhibitors76, 77, 78 and PD-1/PD-L1 inhibitors.75 It has been hypothesized that increased TMB leads to increased expression of different tumor neoantigens (peptides unique to cancer cells), thus enhanced recognition by cytotoxic CD8 T cells and increased tumor immunogenicity.77 However, the correlation is imperfect, postulated to be due to expression of less immunogenic tumor-specific antigens and genetic heterogeneity within individual tumors.79 Although TMB alone may not predict treatment response in all patients,73 the use of PD-1 inhibitors in patients with high-TMB advanced solid tumors, as determined by companion diagnostic tests, has been approved by the FDA in patients who have exhausted all other treatment options.
One critical determinant of TMB is whether there are defects in DNA damage repair pathways, which are innate cellular mechanisms responsible for repairing genetic aberrations during replication. Defects such as deficient mismatch repair (from germline mutations or somatic epigenetic changes in DNA mismatch repair genes) lead to increased genetic hypermutability in short repeated sequences of DNA called microsatellites. Tumors with deficient mismatch repair or its characteristic genetic signature of high MSI are highly sensitive to ICIs,80, 81, 82 resulting in the first biomarker-driven tumor-agnostic cancer treatments approved by the FDA.
Tumor microenvironment
Potential novel biomarkers for TME employ transcriptomic analysis to profile messenger ribonucleic acids and identify immune and angiogenesis signatures within the TME.43 TME profiles can guide rational drug partners for ICI (eg, antiangiogenesis agents) to improve tumor response. Early studies evaluating these biomarkers are promising and further investigations are ongoing.83,84
Genomic mutational profile
Variations in oncogenes, tumor suppressors, and natural immune signaling pathways, such as MAPK, PI3K-AKT-mTOR, WNT-β, and IDO1, have been postulated as biomarkers to predict response to ICIs.73 Serial tumor biopsy studies to understand evolution of tumor mutational profile during ICI treatment are underway to outline mechanisms of resistance.
Gut microbiome
Human gut microbiomes with increased diversity, enriched anabolic pathways, and specific bacteria such as Ruminococcus, Firmicutes, and Akkermansia muciniphila have been associated with better response to ICIs.85,86 Conversely, medications that can alter microbiome composition such as antibiotics,86 proton pump inhibitors,87 histamine receptor antagonists,87 and cannabinoids88 have been associated with worse prognoses in observational studies and post hoc analyses of clinical trials. Trials to improve ICI efficacy using oral probiotics, various diets and supplements, and even fecal microbiota transplantation are ongoing.89
Neutrophil-to-lymphocyte ratio
NLR is a potential biochemical tool which reflects the balance between protumoral inflammation and antitumoral immune response.90 Elevated pretreatment NLR and ratios that decrease during treatment are associated with worse overall and progression-free survival in retrospective studies.90, 91, 92, 93 These observations are similar to that seen in cardiovascular disease, in which elevated NLR has been shown to predict mortality in patients with acute coronary syndrome and heart failure.94 However, NLR is not routinely used in clinical practice due to its limited predictive utility and lack of standardized thresholds.91
Key Points
-
•
PD-L1 expression, TMB, and MSI/mismatch repair status are biomarkers in use to predict ICI response.
-
•
Other genomic and immunological predictive biomarkers are under investigation.
Contemporary Issues and Future Directions
Management of chronic toxicities
As cancer survival improves as a result of ICIs, there is increasing focus on long-term sequelae in cancer survivorship care, which is defined as the health and well-being of a person with cancer from time of diagnosis to end of life.95 In a retrospective study of 387 patients treated with adjuvant PD-1 inhibitors, 43.2% of patients developed chronic irAEs, defined as persisting beyond 12 weeks after ICI discontinuation.96 Those involving nonvisceral systems such as endocrinopathies, neurotoxicities, and ocular toxicities were the commonest, and approximately one-third of patients required corticosteroids or persistent immunosuppression.96 Another retrospective study of 437 patients reported 35.7% of irAEs persisting for up to 24 months.97 Large registry and phase 4 trials are needed to provide more accurate estimates of chronic irAEs in real-world settings. Studies investigating long-term organ-specific treatments and/or immunosuppression are also needed to guide management. A multidisciplinary approach and development of innovative ICI-specific models of follow-up, posttreatment surveillance, and preventive care is required to address long-term issues that may affect many cancer survivors.
Novel agents
In 2019, there were more than 3,000 trials evaluating T cell modulators in oncology, representing two-thirds of all oncology trials.98 These include other ICIs targeting alternative co-inhibitory signals such as T cell Immunoreceptor with Ig and ITIM domains (TIGIT) (Figure 2).99 Although monotherapy anti-TIGIT has limited activity, preclinical and early efficacy results from combination TIGIT and PD-1/PD-L1 blockade look promising, including in tumors resistant to PD-1/PD-L1 inhibitors, with a more favorable safety profile compared with other ICI combinations.100
Agents that activate co-stimulatory signals such as GITR, CD40, CD40L, CD27, CD70, ICOS, and ICOSL, termed immune agonists, are being explored.99 Other targets are macrophage checkpoint inhibitors, newer-generation cytokines, vaccine approaches, agents targeting the TME, bispecific T cell engagers, and adoptive cell therapies.5,99
Health equity
The costs of ICI are substantial and are not expected to reduce for some years due to ongoing patents, a plethora of new agents, and challenges with generating biosimilars for monoclonal antibodies (which is much more complex than for most other drug classes), limiting access in low- and middle-income countries and contributing to ongoing disparities in global cancer care and outcomes. In high-income countries, ICIs are major contributors to the significant economic burden of cancer care, which is projected to approximate $246 billion in the United States by 2030.101 Global efforts are imperative to accelerate access to ICIs for cancer patients in low- and middle-income countries, as health access and outcome equity is increasingly recognized as a major sociopolitical issue.
Cost effectiveness studies of ICIs have demonstrated highly variable results depending on cancer and treatment type, setting, efficacy, sponsor of research, and study methodology.102,103 Hence, further research into patient selection and cost-effectiveness using standardized clinical benefit criteria are required to reduce economic burden on health care systems.
Key Points
-
•
Chronic irAEs are increasingly recognized in cancer survivorship.
-
•
There are over 3,000 ongoing trials evaluating immune-oncological therapies.
-
•
ICI cost is a barrier to broader uptake in low and middle income countries.
Emerging Cardiovascular Issues
Chronic cardiovascular toxicities from ICIs are of particular concern, as cardiovascular disease is the leading cause of non–cancer-related death in cancer survivors even in the pre-ICI era due to overlapping risk factors as well as treatment-related effects.104,105 There is growing appreciation of late onset cardiotoxicities such as atherosclerotic coronary artery disease99 and nonischemic left ventricular dysfunction.51,106
The risk of accelerated coronary artery disease with ICIs is biologically plausible due to its proinflammatory mechanisms.99 In a retrospective single-center analysis of 5,684 patients, ICI treatment resulted in a 7-fold increased risk of myocardial infarction compared with control subjects.107 Similar findings were reported in a meta-analysis of 48 cancer drug trials and 29,592 patients, with a 1.5 times increased risk of myocardial infarction over a median follow-up of 6.6 to 32.8 months.108 These estimates may underestimate the real-world risk, due to under-reporting of cardiovascular events,109 relatively short follow-up duration (limited use for >5 and particularly >10 years), and selection bias due to stringent inclusion criteria in cancer trials compared with real-world situations.110
Late onset left ventricular dysfunction could occur as a consequence of subclinical myocarditis during ICI treatment.51,106 In a prospective serial magnetic resonance imaging study of 22 patients, there was evidence of diffuse myocardial edema and reduction in average left ventricular longitudinal strain after 3 months of ICI treatment.111 Two patients developed new nonischemic late gadolinium enhancement lesions suggestive of myocardial scarring.111 Despite these findings, 21 of 22 patients remained asymptomatic,111 suggestive that subclinical ICI-related myocarditis may be more prevalent than expected. High-sensitivity troponin has been suggested as a potential surveillance biomarker112 that could lead to early detection of subclinical cases and incorporated into several ongoing phase 3 ICI trials, although data supporting its routine use are limited.
Tailored cardiovascular surveillance protocols during and after completion of ICI therapy, which could include routine myocardial imaging and screening of cardiovascular risk factors, may guide monitoring and even commencement of cardiovascular preventive therapies. Adoption of healthy lifestyle measures should also be encouraged in patients treated with ICIs.
Key Points
-
•
Potential chronic cardiovascular irAEs include atherosclerotic cardiovascular disease and heart failure.
-
•
Studies evaluating cardiovascular surveillance after ICI therapy are needed.
-
•
Cardiovascular risk reduction should be strongly considered in patients treated with ICIs.
Conclusions
ICIs are a key component of standard anticancer systemic therapy across multiple cancer types, across (neo)adjuvant and metastatic settings. Currently, ICIs targeting CTLA-4, PD-1/PD-L1, and LAG3 are approved for clinical use, with ongoing studies investigating novel immune-directed agents and combinations. Side effects, known as irAEs, can be acute or chronic. Acute cardiac irAEs during ICI treatment such as myocarditis can potentially be fatal but are rare, while potential chronic cardiac irAEs, such as atherosclerotic cardiovascular disease and left ventricular dysfunction, may increasingly be reported with improving cancer survivorship (a recent JACC: CardioOncology review provides a more detailed discussion on cardiovascular irAEs161). More comprehensive mechanistic understanding of irAEs coupled with longer term follow-up data will be central to improve prevention and management of irAEs, particularly chronic cardiac sequelae, as this is a major competing cause of death. Attention should be directed toward developing ICI-specific models of follow-up for cancer survivors, with a key focus on a multidisciplinary approach to patient care.
Funding Support and Author Disclosures
Dr Tan is supported by a postgraduate scholarship from the National Health and Medical Research Council of Australia, a PhD scholarship from the National Heart Foundation of Australia, and an Australian Government Research Training Program Scholarship. Dr Day is a recipient of the Royal Australasian College of Physicians Foundation 2022 Basser Research Entry Scholarship; and has received research support (clinical trials for the institution) from Beigene, Bristol-Myers Squibb, EpimAb, Harbour BioMed, Maxinovel, MSD, Olema Pharmaceuticals, Pfizer, PhamAbcine, and Roche. Dr Nicholls has received research support from AstraZeneca, Amgen, Anthera, CSL Behring, Cerenis, Eli Lilly, Esperion, Resverlogix, Novartis, InfraReDx, and Sanofi-Regeneron; and served as a consultant for Amgen, Akcea, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Esperion, Kowa, Merck, Takeda, Pfizer, Sanofi-Regeneron, and Novo Nordisk. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors wish to thank Professor Nitesh Nerlekar (Victorian Heart Institute) for his assistance with technical editing and proofreading of the manuscript.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Ledford H., Else H., Warren M. Cancer immunologists scoop medicine Nobel prize. Nature. 2018;562:20–21. doi: 10.1038/d41586-018-06751-0. [DOI] [PubMed] [Google Scholar]
- 2.Haslam A., Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnet M. Cancer - A biological approach. Br Med J. 1957;1:841–847. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Negrier S., Escudier B., Lasset C., et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d'Immunotherapie. N Engl J Med. 1998;338:1272–1278. doi: 10.1056/NEJM199804303381805. [DOI] [PubMed] [Google Scholar]
- 5.Appleton E., Hassan J., Chan Wah Hak C., et al. Kickstarting immunity in cold tumours: localised tumour therapy combinations with immune checkpoint blockade. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.754436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fares C.M., Van Allen E.M., Drake C.G., Allison J.P., Hu-Lieskovan S. Mechanisms of resistance to immune checkpoint blockade: why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ Book. 2019;39:147–164. doi: 10.1200/EDBK_240837. [DOI] [PubMed] [Google Scholar]
- 7.Krummel M.F., Allison J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walunas T.L., Lenschow D.J., Bakker C.Y., et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 9.Masteller E.L., Chuang E., Mullen A.C., Reiner S.L., Thompson C.B. Structural analysis of CTLA-4 function in vivo. J Immunol. 2000;164:5319–5327. doi: 10.4049/jimmunol.164.10.5319. [DOI] [PubMed] [Google Scholar]
- 10.Bagchi S., Yuan R., Engleman E.G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 11.Garbe C., Eigentler T.K., Keilholz U., Hauschild A., Kirkwood J.M. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011;16:5–24. doi: 10.1634/theoncologist.2010-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11:3801. doi: 10.1038/s41467-020-17670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larkin J., Chiarion-Sileni V., Gonzalez R., et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 14.Lebbe C., Meyer N., Mortier L., et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV CheckMate 511 Trial. J Clin Oncol. 2019;37:867–875. doi: 10.1200/JCO.18.01998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alsaab H.O., Sau S., Alzhrani R., et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert C., Schachter J., Long G.V., et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 17.Graydon C.G., Mohideen S., Fowke K.R. LAG3's enigmatic mechanism of action. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.615317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C.-T., Workman C.J., Flies D., et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Woo S.R., Turnis M.E., Goldberg M.V., et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tawbi H.A., Schadendorf D., Lipson E.J., et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24–34. doi: 10.1056/NEJMoa2109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Food and Drug Administration . U.S. Department of Health and Human Services; 2022. Drugs@FDA: FDA-Approved Drugs. Drug Databases. [Google Scholar]
- 22.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Seymour L., Bogaerts J., Perrone A., et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robert C., Ribas A., Hamid O., et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36:1668–1674. doi: 10.1200/JCO.2017.75.6270. [DOI] [PubMed] [Google Scholar]
- 25.Topalian S.L., Hodi F.S., Brahmer J.R., et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. 2019;5:1411–1420. doi: 10.1001/jamaoncol.2019.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marron T.U., Ryan A.E., Reddy S.M., et al. Considerations for treatment duration in responders to immune checkpoint inhibitors. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2020-001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Betof Warner A., Palmer J.S., Shoushtari A.N., et al. Long-term outcomes and responses to retreatment in patients with melanoma treated with PD-1 blockade. J Clin Oncol. 2020;38:1655–1663. doi: 10.1200/JCO.19.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rassy E., Pistilli B., Robert C. Association of adjuvant immunotherapy duration with chronic immune-related adverse events. JAMA Oncol. 2021;7:1573–1574. doi: 10.1001/jamaoncol.2021.2954. [DOI] [PubMed] [Google Scholar]
- 29.Bai X., Hu J., Betof Warner A., et al. Early use of high-dose glucocorticoid for the management of irAE is associated with poorer survival in patients with advanced melanoma treated with Anti-PD-1 monotherapy. Clin Cancer Res. 2021;27:5993–6000. doi: 10.1158/1078-0432.CCR-21-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galluzzi L., Humeau J., Buque A., Zitvogel L., Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17:725–741. doi: 10.1038/s41571-020-0413-z. [DOI] [PubMed] [Google Scholar]
- 31.Gandhi L., Garassino M.C. Pembrolizumab plus chemotherapy in lung cancer. N Engl J Med. 2018;379:e18. doi: 10.1056/NEJMc1808567. [DOI] [PubMed] [Google Scholar]
- 32.Paz-Ares L., Luft A., Vicente D., et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 33.Socinski M.A., Jotte R.M., Cappuzzo F., et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 34.Horn L., Mansfield A.S., Szczesna A., et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 35.Paz-Ares L., Dvorkin M., Chen Y., et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 36.Burtness B., Harrington K.J., Greil R., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 37.Cortes J., Cescon D.W., Rugo H.S., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828. doi: 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 38.Schmid P., Cortes J., Pusztai L., et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 39.Bang Y.J., Kang Y.K., Catenacci D.V., et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22:828–837. doi: 10.1007/s10120-018-00909-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janjigian Y.Y., Shitara K., Moehler M., et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun J.M., Shen L., Shah M.A., et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759–771. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 42.Doki Y., Ajani J.A., Kato K., et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386:449–462. doi: 10.1056/NEJMoa2111380. [DOI] [PubMed] [Google Scholar]
- 43.Simonaggio A., Epaillard N., Pobel C., Moreira M., Oudard S., Vano Y.A. Tumor microenvironment features as predictive biomarkers of response to immune checkpoint inhibitors (ICI) in metastatic clear cell renal cell carcinoma (mccRCC) Cancers (Basel) 2021;13:231. doi: 10.3390/cancers13020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rini B.I., Plimack E.R., Stus V., et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 45.Motzer R., Alekseev B., Rha S.Y., et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 46.Choueiri T.K., Powles T., Burotto M., et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384:829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Motzer R.J., Penkov K., Haanen J., et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 49.Makker V., Colombo N., Casado Herraez A., et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386:437–448. doi: 10.1056/NEJMoa2108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shahabi V., Postow M.A., Tuck D., Wolchok J.D. Immune-priming of the tumor microenvironment by radiotherapy: rationale for combination with immunotherapy to improve anticancer efficacy. Am J Clin Oncol. 2015;38:90–97. doi: 10.1097/COC.0b013e3182868ec8. [DOI] [PubMed] [Google Scholar]
- 51.Johnson D.B., Nebhan C.A., Moslehi J.J., Balko J.M. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254–267. doi: 10.1038/s41571-022-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conroy M., Naidoo J. Immune-related adverse events and the balancing act of immunotherapy. Nat Commun. 2022;13:392. doi: 10.1038/s41467-022-27960-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Cancer Institute . National Institutes of Health; 2017. Common Terminology Criteria for Adverse Events Version 5.0. [Google Scholar]
- 54.Khoja L., Day D., Wei-Wu Chen T., Siu L.L., Hansen A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28:2377–2385. doi: 10.1093/annonc/mdx286. [DOI] [PubMed] [Google Scholar]
- 55.Da L., Teng Y., Wang N., et al. Organ-specific immune-related adverse events associated with immune checkpoint inhibitor monotherapy versus combination therapy in cancer: a meta-analysis of randomized controlled trials. Front Pharmacol. 2019;10:1671. doi: 10.3389/fphar.2019.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Day D., Hansen A.R. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs. 2016;30:571–584. doi: 10.1007/s40259-016-0204-3. [DOI] [PubMed] [Google Scholar]
- 57.Bertrand A., Kostine M., Barnetche T., Truchetet M.E., Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang P.F., Chen Y., Song S.Y., et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 2017;8:730. doi: 10.3389/fphar.2017.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brahmer J.R., Lacchetti C., Schneider B.J., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneider B.J., Naidoo J., Santomasso B.D., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO Guideline Update. J Clin Oncol. 2021;39:4073–4126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 61.Haanen J., Carbonnel F., Robert C., et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 62.Thompson J.A., Schneider B.J., Brahmer J., et al. Management of immunotherapy-related toxicities, version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:387–405. doi: 10.6004/jnccn.2022.0020. [DOI] [PubMed] [Google Scholar]
- 63.Maher V.E., Fernandes L.L., Weinstock C., et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol. 2019;37:2730–2738. doi: 10.1200/JCO.19.00318. [DOI] [PubMed] [Google Scholar]
- 64.Eggermont A.M.M., Kicinski M., Blank C.U., et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519–527. doi: 10.1001/jamaoncol.2019.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shankar B., Zhang J., Naqash A.R., et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6:1952–1956. doi: 10.1001/jamaoncol.2020.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Das S., Johnson D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dolladille C., Ederhy S., Sassier M., et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6:865–871. doi: 10.1001/jamaoncol.2020.0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Q., Zhang J., Xu L., et al. Safety and efficacy of the rechallenge of immune checkpoint inhibitors after immune-related adverse events in patients with cancer: a systemic review and meta-analysis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.730320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haanen J., Ernstoff M., Wang Y., et al. Rechallenge patients with immune checkpoint inhibitors following severe immune-related adverse events: review of the literature and suggested prophylactic strategy. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palaskas N., Lopez-Mattei J., Durand J.B., Iliescu C., Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonaca M.P., Olenchock B.A., Salem J.E., et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blank C., Gajewski T.F., Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conway J.R., Kofman E., Mo S.S., Elmarakeby H., Van Allen E. Genomics of response to immune checkpoint therapies for cancer: implications for precision medicine. Genome Med. 2018;10:93. doi: 10.1186/s13073-018-0605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doroshow D.B., Bhalla S., Beasley M.B., et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18:345–362. doi: 10.1038/s41571-021-00473-5. [DOI] [PubMed] [Google Scholar]
- 75.Yarchoan M., Hopkins A., Jaffee E.M. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Snyder A., Makarov V., Merghoub T., et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schumacher T.N., Scheper W., Kvistborg P. Cancer neoantigens. Annu Rev Immunol. 2019;37:173–200. doi: 10.1146/annurev-immunol-042617-053402. [DOI] [PubMed] [Google Scholar]
- 78.Van Allen E., Miao D., Schilling B., et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riaz N., Morris L., Havel J.J., Makarov V., Desrichard A., Chan T.A. The role of neoantigens in response to immune checkpoint blockade. Int Immunol. 2016;28:411–419. doi: 10.1093/intimm/dxw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andre T., Shiu K.K., Kim T.W., et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 81.Overman M.J., McDermott R., Leach J.L., et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marabelle A., Le D.T., Ascierto P.A., et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rini B.I., Powles T., Atkins M.B., et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393:2404–2415. doi: 10.1016/S0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 84.Hakimi A.A., Voss M.H., Kuo F., et al. Transcriptomic profiling of the tumor microenvironment reveals distinct subgroups of clear cell renal cell cancer: data from a randomized phase III trial. Cancer Discov. 2019;9:510–525. doi: 10.1158/2159-8290.CD-18-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gopalakrishnan V., Spencer C.N., Nezi L., et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Routy B., Le Chatelier E., Derosa L., et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 87.Rizzo A., Cusmai A., Giovannelli F., et al. Impact of proton pump inhibitors and histamine-2-receptor antagonists on non-small cell lung cancer immunotherapy: a systematic review and meta-analysis. Cancers (Basel) 2022;14:1404. doi: 10.3390/cancers14061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiong X., Chen S., Shen J., et al. Cannabis suppresses antitumor immunity by inhibiting JAK/STAT signaling in T cells through CNR2. Signal Transduct Target Ther. 2022;7:99. doi: 10.1038/s41392-022-00918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu J., Wang S., Zheng B., Qiu X., Wang H., Chen L. Modulation of gut microbiota to enhance effect of checkpoint inhibitor immunotherapy. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.669150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valero C., Lee M., Hoen D., et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun. 2021;12:729. doi: 10.1038/s41467-021-20935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sacdalan D.B., Lucero J.A., Sacdalan D.L. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther. 2018;11:955–965. doi: 10.2147/OTT.S153290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ameratunga M., Chenard-Poirier M., Moreno Candilejo I., et al. Neutrophil-lymphocyte ratio kinetics in patients with advanced solid tumours on phase I trials of PD-1/PD-L1 inhibitors. Eur J Cancer. 2018;89:56–63. doi: 10.1016/j.ejca.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 93.Li M., Spakowicz D., Burkart J., et al. Change in neutrophil to lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J Cancer Res Clin Oncol. 2019;145:2541–2546. doi: 10.1007/s00432-019-02982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhat T., Teli S., Rijal J., et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11:55–59. doi: 10.1586/erc.12.159. [DOI] [PubMed] [Google Scholar]
- 95.National Cancer Institute . U.S. Department of Health and Human Services; 2022. NCI Dictionaries. [Google Scholar]
- 96.Patrinely J.R., Jr., Johnson R., Lawless A.R., et al. Chronic immune-related adverse events following adjuvant anti-PD-1 therapy for high-risk resected melanoma. JAMA Oncol. 2021;7:744–748. doi: 10.1001/jamaoncol.2021.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghisoni E., Wicky A., Bouchaab H., et al. Late-onset and long-lasting immune-related adverse events from immune checkpoint-inhibitors: an overlooked aspect in immunotherapy. Eur J Cancer. 2021;149:153–164. doi: 10.1016/j.ejca.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 98.Xin Yu J., Hubbard-Lucey V.M., Tang J. Immuno-oncology drug development goes global. Nat Rev Drug Discov. 2019;18:899–900. doi: 10.1038/d41573-019-00167-9. [DOI] [PubMed] [Google Scholar]
- 99.Vuong J.T., Stein-Merlob A.F., Nayeri A., Sallam T., Neilan T.G., Yang E.H. Immune checkpoint therapies and atherosclerosis: mechanisms and clinical implications: JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;79:577–593. doi: 10.1016/j.jacc.2021.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Joller N., Hafler J.P., Brynedal B., et al. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mariotto A.B., Enewold L., Zhao J., Zeruto C.A., Yabroff K.R. Medical care costs associated with cancer survivorship in the United States. Cancer Epidemiol Biomarkers Prev. 2020;29:1304–1312. doi: 10.1158/1055-9965.EPI-19-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Verma V., Sprave T., Haque W., et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer. 2018;6:128. doi: 10.1186/s40425-018-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ding H., Xin W., Tong Y., et al. Cost effectiveness of immune checkpoint inhibitors for treatment of non-small cell lung cancer: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zaorsky N.G., Churilla T.M., Egleston B.L., et al. Causes of death among cancer patients. Ann Oncol. 2017;28:400–407. doi: 10.1093/annonc/mdw604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koczwara B., Meng R., Miller M.D., et al. Late mortality in people with cancer: a population-based Australian study. Med J Aust. 2021;214:318–323. doi: 10.5694/mja2.50879. [DOI] [PubMed] [Google Scholar]
- 106.Dolladille C., Ederhy S., Allouche S., et al. Late cardiac adverse events in patients with cancer treated with immune checkpoint inhibitors. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Drobni Z.D., Alvi R.M., Taron J., et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142:2299–2311. doi: 10.1161/CIRCULATIONAHA.120.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dolladille C., Akroun J., Morice P.M., et al. Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: a safety meta-analysis. Eur Heart J. 2021;42:4964–4977. doi: 10.1093/eurheartj/ehab618. [DOI] [PubMed] [Google Scholar]
- 109.Bonsu J.M., Guha A., Charles L., et al. Reporting of Cardiovascular events in clinical trials supporting FDA approval of contemporary cancer therapies. J Am Coll Cardiol. 2020;75:620–628. doi: 10.1016/j.jacc.2019.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tan S., Day D., Nicholls S.J., Segelov E. Atherosclerotic cardiovascular risk with combination avelumab and axitinib. J Clin Oncol. 2022;40:3467–3469. doi: 10.1200/JCO.22.00712. [DOI] [PubMed] [Google Scholar]
- 111.Faron A., Isaak A., Mesropyan N., et al. Cardiac MRI depicts immune checkpoint inhibitor-induced myocarditis: a prospective study. Radiology. 2021;301:602–609. doi: 10.1148/radiol.2021210814. [DOI] [PubMed] [Google Scholar]
- 112.Waliany S., Neal J.W., Reddy S., et al. Myocarditis surveillance with high-sensitivity troponin I during cancer treatment with immune checkpoint inhibitors. J Am Coll Cardiol CardioOnc. 2021;3:137–139. doi: 10.1016/j.jaccao.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Joyce J.A., Fearon D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 114.Linsley P.S., Brady W., Grosmaire L., Aruffo A., Damle N.K., Ledbetter J.A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin-2 mRNA accumulation. J Exp Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Azuma M., Ito D., Yagita H., et al. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366:76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 116.Hodi F.S., O'Day S.J., McDermott D.F., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weber J.S., D'Angelo S.P., Minor D., et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 118.Gutzmer R., Stroyakovskiy D., Gogas H., et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835–1844. doi: 10.1016/S0140-6736(20)30934-X. [DOI] [PubMed] [Google Scholar]
- 119.Hughes B.G.M., Munoz-Couselo E., Mortier L., et al. Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629 study): an open-label, nonrandomized, multicenter, phase II trial. Ann Oncol. 2021;32:1276–1285. doi: 10.1016/j.annonc.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 120.Migden M.R., Rischin D., Schmults C.D., et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 121.Stratigos A.J., Sekulic A., Peris K., et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22:848–857. doi: 10.1016/S1470-2045(21)00126-1. [DOI] [PubMed] [Google Scholar]
- 122.Nghiem P., Bhatia S., Lipson E.J., et al. Durable tumor regression and overall survival in patients with advanced merkel cell carcinoma receiving pembrolizumab as first-line therapy. J Clin Oncol. 2019;37:693–702. doi: 10.1200/JCO.18.01896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.D'Angelo S.P., Russell J., Lebbe C., et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2018.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mok T.S.K., Wu Y.L., Kudaba I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 125.Borghaei H., Paz-Ares L., Horn L., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brahmer J., Reckamp K.L., Baas P., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sezer A., Kilickap S., Gumus M., et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397:592–604. doi: 10.1016/S0140-6736(21)00228-2. [DOI] [PubMed] [Google Scholar]
- 128.Herbst R.S., Giaccone G., de Marinis F., et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 129.Hellmann M.D., Paz-Ares L., Bernabe Caro R., et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 130.Baas P., Scherpereel A., Nowak A.K., et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397:375–386. doi: 10.1016/S0140-6736(20)32714-8. [DOI] [PubMed] [Google Scholar]
- 131.Motzer R.J., Tannir N.M., McDermott D.F., et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bellmunt J., de Wit R., Vaughn D.J., et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Balar A.V., Kamat A.M., Kulkarni G.S., et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021;22:919–930. doi: 10.1016/S1470-2045(21)00147-9. [DOI] [PubMed] [Google Scholar]
- 134.Sharma P., Retz M., Siefker-Radtke A., et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 135.Balar A.V., Galsky M.D., Rosenberg J.E., et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Galsky M.D., Arija J.A.A., Bamias A., et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1547–1557. doi: 10.1016/S0140-6736(20)30230-0. [DOI] [PubMed] [Google Scholar]
- 137.Powles T., Duran I., van der Heijden M.S., et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391:748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 138.Powles T., Park S.H., Voog E., et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 139.Ferris R.L., Blumenschein G., Jr., Fayette J., et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhu A.X., Finn R.S., Edeline J., et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 141.Yau T., Kang Y.K., Kim T.Y., et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6 doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Overman M.J., Lonardi S., Wong K.Y.M., et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 143.Chung H.C., Ros W., Delord J.P., et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37:1470–1478. doi: 10.1200/JCO.18.01265. [DOI] [PubMed] [Google Scholar]
- 144.O'Malley D.M., Bariani G.M., Cassier P.A., et al. Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J Clin Oncol. 2022;40:752–761. doi: 10.1200/JCO.21.01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen R., Zinzani P.L., Lee H.J., et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134:1144–1153. doi: 10.1182/blood.2019000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Armand P., Engert A., Younes A., et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36:1428–1439. doi: 10.1200/JCO.2017.76.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Armand P., Rodig S., Melnichenko V., et al. Pembrolizumab in relapsed or refractory primary mediastinal large B-cell lymphoma. J Clin Oncol. 2019;37:3291–3299. doi: 10.1200/JCO.19.01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marabelle A., Fakih M., Lopez J., et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 149.Eggermont A.M., Chiarion-Sileni V., Grob J.J., et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–530. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 150.Eggermont A.M.M., Blank C.U., Mandala M., et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 151.Weber J., Mandala M., Del Vecchio M., et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 152.Spicer J., Wang C., Tanaka F., et al. Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC) J Clin Oncol. 2021;39:8503. [Google Scholar]
- 153.Felip E., Altorki N., Zhou C., et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398:1344–1357. doi: 10.1016/S0140-6736(21)02098-5. [DOI] [PubMed] [Google Scholar]
- 154.Antonia S.J., Villegas A., Daniel D., et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 155.Choueiri T.K., Tomczak P., Park S.H., et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. 2021;385:683–694. doi: 10.1056/NEJMoa2106391. [DOI] [PubMed] [Google Scholar]
- 156.Bajorin D.F., Witjes J.A., Gschwend J.E., et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384:2102–2114. doi: 10.1056/NEJMoa2034442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kelly R.J., Ajani J.A., Kuzdzal J., et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384:1191–1203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 158.Muntyanu A., Netchiporouk E., Gerstein W., Gniadecki R., Litvinov I.V. Cutaneous immune-related adverse events (irAEs) to immune checkpoint inhibitors: a Dermatology perspective on management. J Cutan Med Surg. 2021;25:59–76. doi: 10.1177/1203475420943260. [DOI] [PubMed] [Google Scholar]
- 159.Barroso-Sousa R., Barry W.T., Garrido-Castro A.C., et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4:173–182. doi: 10.1001/jamaoncol.2017.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cuzzubbo S., Javeri F., Tissier M., et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer. 2017;73:1–8. doi: 10.1016/j.ejca.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 161.Zhang L., Reynolds K.L., Lyon A.R., Palaskas N., Neilan T.G. The evolving immunotherapy landscape and the epidemiology, diagnosis, and management of cardiotoxicity: JACC: CardioOncology Primer. J Am Coll Cardiol CardioOnc. 2021;3(1):35–47. doi: 10.1016/j.jaccao.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]