Abstract
Staphylococcus aureus is responsible for a large percentage of infections associated with implanted biomedical devices. The molecular basis of primary adhesion to artificial surfaces is not yet understood. Here, we demonstrate that teichoic acids, highly charged cell wall polymers, play a key role in the first step of biofilm formation. An S. aureus mutant bearing a stronger negative surface charge due to the lack of d-alanine esters in its teichoic acids can no longer colonize polystyrene or glass. The mutation abrogates primary adhesion to plastic while production of the glucosamine-based polymer involved in later steps of biofilm formation is not affected. Our data suggest that repulsive electrostatic forces can lead to reduced staphylococcal biofilm formation, which could have considerable impact on the design of novel implanted materials.
Staphylococcus aureus is one of the most frequently isolated bacterial pathogens, causing severe morbidity and often fatal infections. Like coagulase-negative Staphylococcus epidermidis, S. aureus has the capacity to adhere to catheters and other indwelling devices and form a biofilm, which is then difficult to combat with host defenses or antibiotics (for recent reviews, see references 7, 24, 26, and 28). The alarming rise in nosocomial staphylococcal bacteremia can be largely attributed to the increasing use of intravascular catheters (14). Biofilm formation is thought to be a two-step process that requires the primary adhesion of bacteria to a substrate surface followed by the formation of multiple cell layers (28). The glucosamine-based polysaccharide intercellular adhesin (PIA), or poly-N-succinylglucosamine (PNSG), is responsible for cell-cell adhesion; mutant S. epidermidis or S. aureus strains that no longer produce PIA (PNSG) are unable to form a biofilm, while the initial adhesion to plastic surfaces is unaffected (4, 10, 29). PIA (PNSG) is a linear β-1,6-linked glucosaminoglycan, a high percentage of which is N-acetylated and/or succinylated (20, 21). The icaADBC operon is responsible for its biosynthesis (6).
The mechanisms responsible for initial adhesion to a plastic surface, however, are not yet well understood. They are influenced by the physicochemical properties of both the plastic material and the bacterial cell surface. In S. epidermidis, the cell wall lytic enzyme AtlE, which affects the hydrophobicity of the cell surface, has been implicated in the initial adhesion to plastic and glass (8, 9). The role of teichoic acids, highly charged cell wall polymers, in biofilm formation, however, has remained elusive. S. aureus teichoic acids are composed of alternating phosphate and ribitol (wall teichoic acids) or glycerol (lipoteichoic acids) groups, which are replaced with d-alanine and N-acetylglucosamine (5). We have recently described an S. aureus mutant lacking d-alanine in the teichoic acids due to a disruption in the dltABCD operon, which is responsible for d-alanine incorporation. The lack of d-alanine esters caused a stronger negative net charge on the bacterial cell surface that affected the resistance to cationic antimicrobial peptides such as defensins from human phagocytes (22), the susceptibility to vancomycin, and the activity of autolysins (23).
The d-alanine substituents of S. aureus teichoic acids are necessary for biofilm formation.
The biofilm-forming capacities of the S. aureus Sa113 dltA mutant (ATCC 35556 dltA::spc) and its wild-type parental strain (ATCC 35556) were determined as described previously (4). Briefly, tryptic soy broth (Life Technologies, Karlsruhe, Germany) supplemented with 0.25% glucose was inoculated with 1/200 volume of overnight cultures, which had been adjusted to the same A578 of 0.1. Samples (200 μl) were added to the wells of 96-well polystyrene (Greiner Labortechnik, Frickenhausen, Germany) or glass (Dynatec, Denkendorf, Germany) microtiter plates. After cultivation for 24 h at 37°C, biofilm formation was detected by (i) staining bacteria that remained attached to the surface of flat-bottomed polystyrene or glass microtiter plates with 0.1% safranin after the plates were gently washed twice with phosphate-buffered saline and (ii) observing confluent growth in U-bottomed polystyrene microtiter plates. Wild-type and mutant cells showed the same capacity to bind safranin (data not shown). These experiments as well as the studies described below were carried out at least three times and yielded reproducible results.
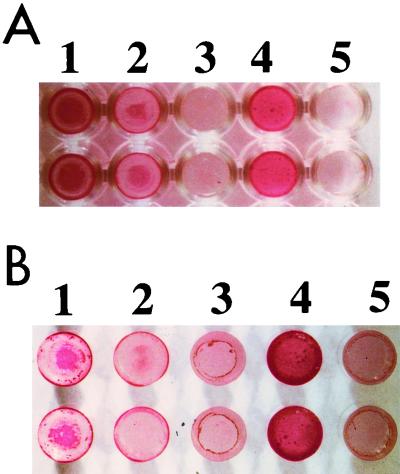
While the S. aureus wild-type strain was positive in both assays, the dltA mutant lacking d-alanine esters in its teichoic acids formed cell pellets in U-bottomed plates instead of confluent cell layers (data not shown) and staining with safranin demonstrated that the bacteria did not adhere to the plastic surface (Fig. 1A). Biofilm formation by the dltA mutant was even weaker than that of the intercellular adhesion mutant (ATCC 35556 icaADBC::tet) (4) (Fig. 1A, wells 2 and 3, respectively); adhesion of the dltA mutant was comparable to the phenotype of the nonadhering species Staphylococcus carnosus (Fig. 1A, well 5). Upon complementation of the dltA mutant with plasmid pRBdlt1 bearing a wild-type copy of the dltABCD operon, the capacity to form a biofilm was restored (Fig. 1A, well 4). Very similar results were obtained with glass wells, indicating that d-alanine-modified teichoic acids play a role in adherence to polar as well as apolar surfaces (Fig. 1B). Interestingly, dltA mutant cells bearing pRBdlt1 formed a stronger biofilm on plastic and glass surfaces than did the wild-type strain. This increase corresponds to the higher number of d-alanine esters in the teichoic acids of the complemented mutant (22), which is probably due to the relatively high copy number of plasmid pRBdlt1.
FIG. 1.
Biofilm formation by S. aureus strains in polystyrene (A) and glass wells (B). Bacterial biofilms formed during overnight cultivation in microtiter plates were stained with safranin. Wells: 1, S. aureus ATCC 35556 wild type; 2, icaADBC mutant; 3, dltA mutant; 4, dltA mutant complemented with plasmid pRBdlt1; 5, S. carnosus TM300.
Production of PIA/PNSG is not affected in the dltA mutant.
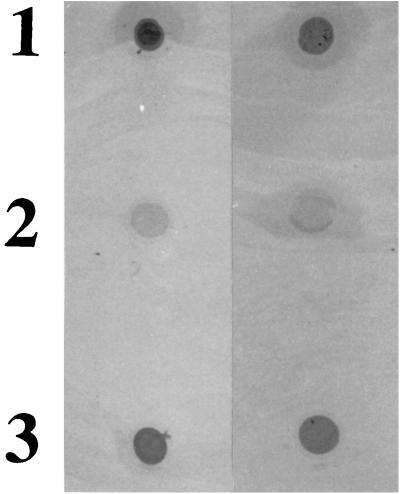
In order to elucidate which phase of biofilm formation is abrogated in the dltA mutant, we first analyzed production of the polysaccharide responsible for cell-cell adhesion, essentially as previously described (4). Briefly, polysaccharide was released from equal numbers of bacteria grown as described above by being boiled in 0.5 M EDTA (pH 8.0) for 5 min. Traces of protein A were degraded by incubation with 4 mg of proteinase K (Roche Biochemicals, Mannheim, Germany)/ml for 30 min at 37°C. The proteinase was subsequently removed by extraction with a phenol-chloroform-isoamylalcohol solution (25:24:1 [vol/vol/vol]) buffered with 20 mM Tris-HCl (pH 8.0). Five microliters from each sample was then spotted onto a nitrocellulose membrane and subjected to antibody detection analysis using a rabbit antiserum raised against S. epidermidis PIA (a gift from Dietrich Mack, University Hospital Eppendorf, Hamburg, Germany) (6). Both the wild-type and dltA mutant strains produced a product detectable with PIA-specific antiserum (Fig. 2, spots 1 and 3), while the icaADBC mutant remained negative (Fig. 2, spot 2), demonstrating that abrogation of plastic colonization in the dltA mutant is not due to the absence of intercellular adhesin.
FIG. 2.
Detection of PIA (PNSG). Cell surface extracts from overnight cultures of S. aureus ATCC 35556 were spotted onto a nitrocellulose filter and detected using an antiserum raised against S. epidermidis PIA. Spots: 1, wild type; 2, icaADBC mutant; 3, dltA mutant. Rows: results of two experiments with same samples.
The initial binding to plastic surfaces is impaired in the dltA mutant.
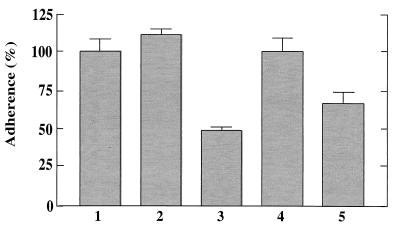
To analyze the initial binding of the bacteria to polystyrene, equal amounts of wild-type and mutant cells were prepared as described above for biofilm formation assays but were incubated for only 30 min in flat-bottomed polystyrene microtiter plates. After the washing step, the wells were air-dried and stained with safranin, and A490 was determined in a microtiter plate reader (SpectraMax 340; Molecular Devices, Sunnyvale, Calif.). The absorbance of wells incubated with 200 μl of sterile medium was subtracted. While similar amounts of wild-type and icaADBC mutant bacteria adhered to the wells, the dltA mutant exhibited more than 50% reduced level of initial binding, indicating that the initial step of biofilm formation was affected by the loss of the d-alanine esters (Fig. 3).
FIG. 3.
Initial adhesion to a polystyrene surface. Equal numbers of bacteria were incubated in polystyrene microtiter plates for 30 min. Adhering bacteria were visualized with safranin and quantified using a microtiter plate reader. The mean values and standard deviations of at least six wells from a representative experiment are shown. The mean value obtained for the wild-type strain was defined as 100%. Bars: 1, S. aureus ATCC 35556 wild type; 2, icaADBC mutant; 3, dltA mutant; 4, dltA mutant complemented with plasmid pRBdlt1; 5, S. carnosus TM300.
The lack of d-alanine esters affects the pattern of surface-bound proteins.
The composition and amount of cell wall-associated proteins can considerably influence the hydrophobicity and net charge of the bacterial cell envelope and the interaction with substrate surfaces (17). In order to compare the patterns of surface proteins, wild-type and dltA mutant bacteria were grown to stationary phase in tryptic soy broth and equal numbers of cells were boiled in 1% sodium dodecyl sulfate (SDS) for 5 min as described previously (23). Intact cells were removed by centrifugation, and surface proteins were separated on Tricine-SDS-polyacrylamide gels according to standard methods (1).
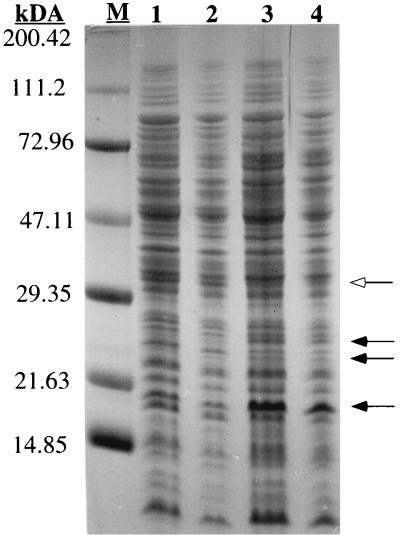
The patterns of surface-associated proteins in the two strains revealed only slight differences. Three bands at about 19, 24, and 26 kDa were more pronounced in the mutant while one band at about 33 kDa was stronger in the wild type (Fig. 4). These deviations may be the result of different capacities to retain the proteins in the cell wall or differences in proteolytic activities in the two strains. We have recently demonstrated that smaller amounts of autolytic enzymes, which bind to teichoic acids (3) and which may affect the primary adhesion to plastic or glass (9), are released by SDS treatment from the dltA mutant than from wild-type bacteria (23). The four prominent protein bands indicated in Fig. 4, however, exhibited no autolytic activity in zymographic analyses (data not shown), which were performed with SDS-polyacrylamide gels containing Micrococcus luteus or S. carnosus cells as described previously (23).
FIG. 4.
Surface-associated proteins. Surface proteins from S. aureus ATCC 35556 strains were released with 1% SDS, separated on an SDS–15% polyacrylamide gel, and stained with Coomassie blue. Lanes: 1 and 2, extracts from the wild type; 3 and 4, extracts from dltA mutant. Protein extracts (50 or 25 μl) were applied to lanes 1 and 3 or 2 and 4, respectively. The masses of protein markers (M) are indicated on the left. Protein bands with different intensities in the two strains are indicated by closed (more pronounced in the wild type) and open (more pronounced in the dltA mutant) arrows.
Conclusions.
We show here for the first time that the charge of teichoic acids plays a pivotal role in the initial step of biofilm formation. The cell surface of S. aureus, as in most bacteria, has a moderately negative net charge at neutral pH (27), which is probably due to the fact that the teichoic acids contain fewer positively charged d-alanine residues than negatively charged phosphate groups (22). Nevertheless, S. aureus can adhere to hydrophobic or slightly negatively charged surfaces such as polystyrene or glass, respectively. The direct interaction of bacteria and surfaces is dependent on van der Waals forces, which are generally attractive, and interionic forces, which can be either attractive or repulsive (25). Even if bacteria and surfaces are charged alike, van der Waals forces can overcome repulsion and lead to adhesion (18, 19). The much stronger net negative charge of the dltA mutant, however, probably leads to a pronounced increase in the repulsive forces, thereby disabling any adherence of the bacteria to polystyrene or glass. On the other hand, altered teichoic acid net charge may affect the adhesive properties of bacterial cells in an indirect way. For instance, the absence of d-alanine esters in teichoic acids has been shown to alter the folding of exoproteins in Bacillus subtilis (13). Although the pattern of cell wall-associated proteins was only slightly different in the S. aureus mutant, altered protein conformations might lead to altered physicochemical properties of the cell surface and thereby compromise the interaction with artificial surfaces.
Our data suggest that increasing the repulsive forces between the plastic surface and the bacteria by modifying the properties of implanted materials may lead to reduced capacity to form a biofilm. Accordingly, several studies have demonstrated that the use of negatively charged materials, such as ionized plastics, Teflon, or heparinized surfaces, is of particular benefit in reducing colonization (2, 11, 12, 15). Moreover, since the teichoic acid content and the degree of d-alanylation vary among S. aureus strains (16), increased amounts of d-alanine esters may contribute to the capacity of staphylococci to colonize indwelling devices.
Acknowledgments
We thank Matthias Herrmann and Michael Otto for helpful discussions, Dietrich Mack for providing the PIA-specific antiserum, and Ulrike Pfitzner for photography.
This work was supported by the Interdisciplinary Clinical Research Center Tübingen and by grants from the Deutsche Forschungsgemeinschaft (GO 371/3-1) to A.P. and F.G.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1990. [Google Scholar]
- 2.Biedlingmaier J F, Samaranayake R, Whelan P. Resistance to biofilm formation on otologic implant materials. Otolaryngol Head Neck Surg. 1998;118:444–451. doi: 10.1177/019459989811800403. [DOI] [PubMed] [Google Scholar]
- 3.Bierbaum G, Sahl H-G. Induction of autolysis of Staphylococcus simulans 22 by Pep5 and nisin and influence of the cationic peptides on the activity of the autolytic enzymes. In: Jung G, Sahl H-G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM; 1991. p. 386-396. [Google Scholar]
- 4.Cramton S E, Gerke C, Schnell N F, Nichols W W, Götz F. The intercellular adhesin (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun. 1999;67:5427–5433. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer W. Lipoteichoic acid and teichoic acid biosynthesis. Targets of new antibiotics? In: Hakenbeck R, editor. New targets for new antimicrobial agents. Heidelberg, Germany: Spektrum Akademischer Verlag; 1997. p. 47-50. [Google Scholar]
- 6.Gerke C, Kraft A, Süβmuth R, Schweizer O, Götz F. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J Biol Chem. 1998;273:18586–18593. doi: 10.1074/jbc.273.29.18586. [DOI] [PubMed] [Google Scholar]
- 7.Götz F, Peters G. Colonization of medical devices by coagulase-negative staphylococci. In: Waldvogel F A, Bisno A L, editors. Infections associated with indwelling medical devices. Washington, D.C.: ASM Press; 2000. pp. 55–88. [Google Scholar]
- 8.Heilmann C, Gerke C, Perdreau-Remington F, Götz F. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect Immun. 1996;64:277–282. doi: 10.1128/iai.64.1.277-282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heilmann C, Hussein M, Peters G, Götz F. Evidence for autolysin-mediated primary attachement of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 10.Heilmann C, Schweizer O, Gerke C, Vanittanakom N, Mack D, Götz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996;20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 11.Hogt A H, Dankert J, Feijen J. Adhesion of coagulase-negative staphylococci to methacrylate polymers and copolymers. J Biomed Mater Res. 1986;20:533–545. doi: 10.1002/jbm.820200409. [DOI] [PubMed] [Google Scholar]
- 12.Homma H, Nagaoka S, Mezawa S, Matsuyama T, Masuko E, Ban N, Watanabe N, Niitsu Y. Bacterial adhesion on hydrophilic heparinized catheters, compared with adhesion on silicone catheters, in patients with malignant obstructive jaudice. J Gastroenterol. 1996;31:836–843. doi: 10.1007/BF02358611. [DOI] [PubMed] [Google Scholar]
- 13.Hyyrylainen H L, Vitikainen M, Thwaite J, Wu H, Sarvas M, Harwood C R, Kontinen V P, Stephenson K. d-Alanine substitution of teichoic acids as a modulator of protein folding and stability at the cytoplasmic membrane/cell wall interface of Bacillus subtilis. J Biol Chem. 2000;275:26696–26703. doi: 10.1074/jbc.M003804200. [DOI] [PubMed] [Google Scholar]
- 14.Ing B M, Baddour L M, Bayer A S. Bacteremia and infective endocarditis: pathogenesis, diagnosis, and complications. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 331–354. [Google Scholar]
- 15.Jansen B, Kohnen W. Prevention of biofilm formation by polymer modification. J Ind Microbiol. 1995;15:391–396. doi: 10.1007/BF01569996. [DOI] [PubMed] [Google Scholar]
- 16.Jenni R, Berger-Bächi B. Teichoic acid content in different lineages of Staphylococcus aureus NCTC8325. Arch Microbiol. 1998;170:171–178. doi: 10.1007/s002030050630. [DOI] [PubMed] [Google Scholar]
- 17.Jucker B A, Harms H, Zehnder A J B. Adhesion of the positively charged bacterium Stenotrophomonas (Xanthomonas) maltophilia 70401 to glass and Teflon. J Bacteriol. 1996;178:5472–5479. doi: 10.1128/jb.178.18.5472-5479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loosdrecht C M M, Lyklema J, Norde W, Schraa G, Zehnder A J B. Electrophoretic mobility and hydrophobicity as a measure to predict the initial steps of bacterial adhesion. Appl Environ Microbiol. 1987;53:1898–1901. doi: 10.1128/aem.53.8.1898-1901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loosdrecht C M M, Lyklema J, Norde W, Schraa G, Zehnder A J B. The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987;53:1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKenney D, Pouliot K L, Wang Y, Murthy V, Ulrich M, Döring G, Lee J C, Goldmann D A, Pier G B. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science. 1999;284:1523–1527. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- 22.Peschel A, Otto M, Jack R W, Kalbacher H, Jung G, Götz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins and other antimicrobial peptides. J Biol Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 23.Peschel A, Vuong C, Otto M, Götz F. The d-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolysins. Antimicrob Agents Chemother. 2000;44:2845–2847. doi: 10.1128/aac.44.10.2845-2847.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proctor R A. Toward an understanding of biomaterial infections: a complex interplay between the host and bacteria. J Lab Clin Med. 2000;135:14–15. doi: 10.1016/s0022-2143(00)70015-1. [DOI] [PubMed] [Google Scholar]
- 25.Rijnaarts H H M, Norde W, Bouwer E J, Lyklema J, Zehnder A J B. Reversibility and mechanism of bacterial adhesion. Colloids Surf B: Biointerface. 1995;4:5–22. [Google Scholar]
- 26.Rupp M E. Infections of intravascular catheters and vascular devices. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 379–399. [Google Scholar]
- 27.Sonohara R, Muramatsu N, Ohshima H, Kondo T. Difference in surface properties between Escherichia coli and Staphylococcus aureus as revealed by electrophoretic mobility measurements. Biophys Chem. 1995;55:273–277. doi: 10.1016/0301-4622(95)00004-h. [DOI] [PubMed] [Google Scholar]
- 28.von Eiff C, Heilmann C, Peters G. New aspects in the molecular basis of polymer-associated infections due to staphylococci. Eur J Clin Microbiol Infect Dis. 1999;18:843–846. doi: 10.1007/s100960050417. [DOI] [PubMed] [Google Scholar]
- 29.Ziebuhr W, Krimmer V, Rachid S, Lossner I, Götz F, Hacker J. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS256. Mol Microbiol. 1999;32:345–356. doi: 10.1046/j.1365-2958.1999.01353.x. [DOI] [PubMed] [Google Scholar]