Abstract
The increasing consumption of unregulated herbal and dietary supplements has presented clinicians with new challenges in assessing and managing acute liver injury. Patients may present in various ways ranging from asymptomatic transaminitis to acute liver failure. Several natural products have been found to mitigate drug-induced liver injury, which has led to the creation of numerous registries to outline all its aspects further. We describe the case of a 36-year-old female who developed a clinically significant acute liver injury with a cholestatic pattern due to an over-the-counter herbal liver detox tea. This is the first case reported of a hepatotoxic effect from any of these compounds or ingredients in the detox tea: burdock root, stinging nettle leaf, cleavers herb, dandelion root, lemon peel, and lemon myrtle leaf (Backhousia citriodora). Idiosyncratic drug-induced liver injury (DILI) remains poorly understood; however, recognizing potential toxins is imperative to understanding toxicogenomics and identifying those at risk.
Keywords: Drug-induced liver injury, Cholestatic pattern, Herbal and dietary supplements, Hepatotoxic
Introduction
Idiosyncratic drug-induced liver injury (DILI) poses an increasingly more significant challenge for physicians as the use of home herbal and dietary supplements (HDS) grows. DILI is the most common cause of acute liver failure in the USA [1]. Idiosyncratic DILI occurs unexpectedly in drugs and HDS in a nondose-dependent manner, whereas predicted DILI occurs in known hepatotoxic agents in a dose-dependent manner [2]. Compounds that lead to idiosyncratic DILI can lead to hepatocellular damage through either an immunologic or metabolic idiosyncrasy, which leads to toxic metabolites [3]. It can present as hepatocellular, cholestatic, or a mixed pattern of liver injury [2]. It is primarily a diagnosis of exclusion after other etiologies of hepatic injury such as infections, autoimmune disease, and other clinically relevant syndromes such as Wilson's disease have been excluded [4]. Here we present a case of DILI, which occurred 1 month after starting a herbal liver detoxification tea with active ingredients being burdock root, stinging nettle leaf, cleavers herb, dandelion root, lemon peel, and lemon myrtle leaf (Backhousia citriodora) not previously known to cause hepatotoxicity. We hope to increase awareness of potentially fatal adverse hepatotoxic effects of the aforementioned herbal ingredients to expedite treatment and improve safety outcomes.
Case Report
Here we present a 36-year-old female who presented for evaluation of elevated aminotransferases found on laboratory evaluation for abdominal pain. Her abdominal pain began 1 month before arrival and was described as cramping in nature, which was located diffusely across her abdomen without any radiation. The pain was intermittent and she could not recall any alleviating, aggravating, or possible precipitating factors. She otherwise denied any nausea, vomiting, fevers, chills, or change in bowel or urinary habits. She had not had any recent travel, sick contacts, or any changes to her diet. Of note, she reported using herbal tea for liver detoxification for 1 month, with active ingredients including burdock root, stinging nettle leaf, cleavers herb, dandelion root, lemon peel, and lemon myrtle leaf (B. citriodora). Her medical history and home medications were noncontributory. She had no previous surgeries and denied and significant family history. She also denied any tobacco, alcohol, or recreational drug use.
On presentation, her vitals were unremarkable and her physical exam was remarkable for diffuse tenderness to deep palpation. Murphy's sign was found to be negative. Her laboratory evaluation was remarkable for alkaline phosphatase found to be 147 U/L, AST 701 U/L, ALT 1329 U/L, and total bilirubin 6.4 mg/dL. Previous records found alkaline phosphatase, AST, ALT, and total bilirubin levels within normal limits on routine laboratory evaluation 2 months prior to arrival. Acetaminophen levels, amylase, and lipase were within normal limits.
Additionally, her serum serology of IgG, Ig4, antismooth muscle antibody ceruloplasmin, ANA titers, and alpha-1 antitrypsin levels were within normal limits. Right upper quadrant ultrasound was remarkable for cholelithiasis without evidence of cholecystitis. Magnetic resonance cholangiopancreatography revealed extrahepatic bile duct dilation without evidence of common bile duct dilation.
Her herbal tea was stopped and a loading dose of n-acetylcysteine was administered. Further doses of n-acetylcysteine were held due to nausea and vomiting. Subsequently, her ALT, AST, alkaline phosphatase, and bilirubin levels trended down during her 12-day hospital course. The trend in these values is shown in Table 1. Additionally, her abdominal pain progressively resolved as well. A liver biopsy revealed a cholestatic pattern of liver injury, as shown in Figure 1. Her elevated AST, ALT, alkaline phosphatase, bilirubin levels, and abdominal pain were attributed to DILI from herbal tea ingestion.
Table 1.
Serum levels of AST, ALT, alkaline phosphatase, and bilirubin
| On presentation | Hospital day 5 | Hospital day 10 | Hospital day 12 (discharge) | |
|---|---|---|---|---|
| AST, U/L | 701 | 139 | 82 | 23 |
| ALT, U/L | 1,329 | 569 | 366 | 194 |
| Alkaline phosphatase, U/L | 147 | 123 | 87 | 92 |
| Total bilirubin, mg/dL | 6.4 | 3.6 | 2.5 | 1.2 |
Fig. 1.
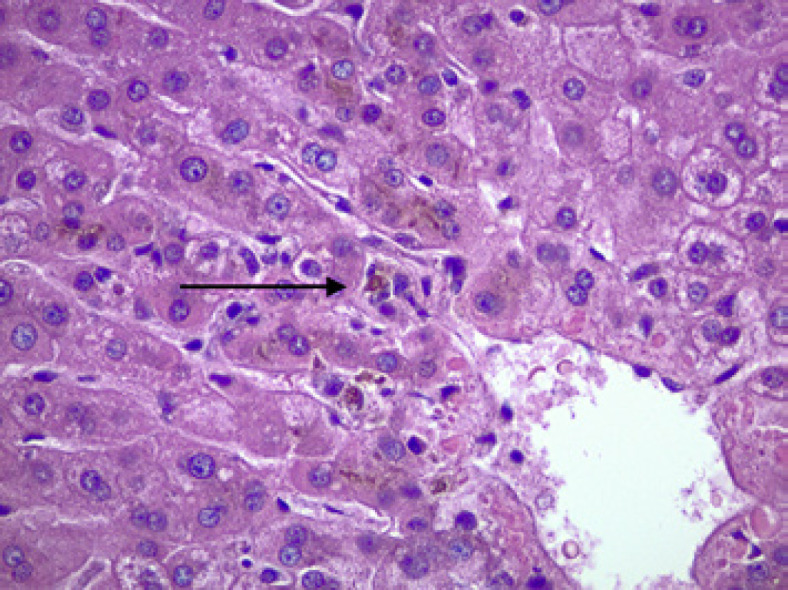
A cholestatic pattern of liver injury is shown here. The arrow indicates areas of cholestasis. Photo Credit: Kunchang Song, MD.
Conclusion
DILI is described as a toxic injury to the liver commonly implicated by prescription drugs, recreational drugs, and HDS. Patients can present asymptomatically or with abdominal pain and jaundice and can be found to have mild to severe elevations in serum aminotransferases [4]. DILI can be classified as predictable or unpredictable (idiosyncratic). Predictable DILI usually occurs in a dose-dependent manner from drugs usually known to be hepatotoxic. In contrast, idiosyncratic DILI is a diagnosis of exclusion occurring unexpectedly from medication and HDS [5]. Some studies have shown that idiosyncratic DILI, in particular, accounts for 12% of all cases of acute liver failure in the USA [1]. Offending agents have been found to cause DILI within several days to 6 months of initiation [6]. After 1 month of starting a new herbal tea, our patient presented with elevated levels of aminotransferases, alkaline phosphatase, and bilirubin found to be consistent with idiosyncratic DILI. This herbal tea was advertised for hepatic detoxification with reported ingredients of burdock root, stinging nettle leaf, cleavers herb, dandelion root, lemon peel, and lemon myrtle. This will be the first case reporting a hepatotoxic effect from these compounds or ingredients. We hope to bring awareness of the possible adverse effects of the aforementioned herbal ingredients in order to diagnose potentially fatal DILI expediently and add to the growing work of possible hepatotoxic herbal and dietary supplements.
Dietary and herbal supplements are increasingly being recognized as causes of hepatotoxicity. HDS accounts for at least 20% of cases of hepatotoxicity in the USA [7]. This may be attributed to how HDS are regulated compared to traditional medications. In the USA, herbal and dietary supplements are designated as a special food category. Therefore, they do not undergo the same efficacy and safety testing that medications undergo prior to entering the market [8]. Additionally, the purity of HDS is not regulated the way medications are, raising concern for adverse effects from impure compounds [9]. Subsequently, adverse outcomes from HDS are commonly found clinically among patients using these supplements. Therefore, clinicians need to be aware of the possible hepatotoxic effects of herbal and dietary supplements.
Clinical presentation of DILI is highly variable and requires a careful examination of history and laboratory evaluation. Patients can present asymptomatically with elevated transferases and nonspecific abdominal pain or even severe abdominal pain with jaundice. Diagnosis of DILI is established by laboratory evaluation of aminotransferases, alkaline phosphatase, and bilirubin, along with excluding other causes of hepatotoxicity and establishing a clear temporal relationship between the offending agent and hepatotoxicity [4]. DILI is suspected with elevations of ALT greater than five times the upper limit of normal (ULN), elevation of alkaline phosphatase greater than two times the ULN in the absence of bone pathology, or with elevations of ALT greater than three times the ULN in conjunction with elevations of total bilirubin greater than two times the ULN [10]. As DILI requires a diagnosis of exclusion, other causes of hepatotoxicity such as autoimmune hepatitis, Wilsons Disease, and alpha-1-antitrypsin deficiency need to be excluded. Finally, the culprit of suspected hepatotoxicity should reveal a temporal relationship with the onset of injury and resolution of injury upon discontinuation of the culprit toxic agent. Our patient's presentation was consistent with DILI, including a nonspecific clinical presentation of abdominal pain, significantly elevated aminotransferases, an exclusion of other causes of hepatotoxicity, as well as a temporal relationship of hepatotoxicity.
Liver biopsy is not routinely required in diagnosing DILI; however, it can aid in the pathogenesis. Some reports have shown that liver biopsy is performed in less than 50% of idiosyncratic DILI cases [11]. The most common patterns of hepatic injury are hepatic, cholestatic, and mixed hepatic-cholestatic [12]. Hepatic patterns reveal variable tubular and portal inflammation, cholestatic patterns reveal hepatocellular or canalicular bile deposition, and hepatic-cholestatic patterns reveal a mixture of hepatic and cholestatic patterns [12]. The different patterns of liver injury can suggest a possible offending agent as certain drugs and compounds have shown specific patterns of hepatic injury, and our patient, in particular, was found to have a cholestatic pattern of hepatic damage on liver biopsy.
Idiosyncratic DILI does not usually occur in a dose-dependent manner and is thought to be immune-mediated. It has been proposed that single nucleotide polymorphisms in HLA genes may contribute to the susceptibility of idiosyncratic DILI [13]. Treatment for DILI targets discontinuing the suspected offending agent and monitoring for improvements in aminotransferases. Additionally, n-acetylcysteine has been used to treat acetaminophen and nonacetaminophen-related hepatotoxicity, though studies have revealed inconclusive efficacy in nonacetaminophen-related hepatotoxicity [14]. Discontinuing the offending agent usually reverses liver injury when it is still irreversible. A loading dose of n-acetylcysteine was not tolerated well by our patient, and subsequent doses were held. However, after withholding her supplement tea, her ALT, alkaline phosphatase, and bilirubin levels progressively trended down during the entirety of her hospital course.
DILI is a severe and potentially fatal adverse reaction that can sometimes progress to fulminant hepatic failure, possibly requiring a liver transplant [4]. With the rise of herbal and dietary supplement use, increasing cases of DILI have been uncovered. Because the regulation of herbal and dietary supplements does not require rigorous efficacy and safety trials, it is essential to recognize these potentially fatal adverse outcomes early and avoid delays in subsequent diagnosis and treatment. Here, we present a DILI case after consuming an herbal tea advertised for liver detoxification. Our review of the literature did not find any previously reported cases of DILI associated with any of the compounds listed in the ingredients for this tea, including burdock root, stinging nettle leaf, cleavers herb, dandelion root, lemon peel, and lemon myrtle leaf (B. citriodora). In reporting our findings, we hope to make clinicians aware of the possible fatal adverse outcomes from the ingredients mentioned above in this herbal tea and hope to aid in expedient diagnosis and treatment and add to the growing body of literature on HDS found to cause DILI.
Statement of Ethics
Ethical approval is not required in accordance with local or national guidelines. Written and informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to report.
Funding Sources
No funding was received for this article.
Author Contributions
Bilal Niazi wrote the case presentation and discussion/conclusion as well as performed a review of the remainder of the manuscript. Kareem Ahmed and Mohamed Ahmed wrote the abstract and introduction. Saad Ali and Sameh Elias assisted in review of the manuscript. Kunchang Song provided histology and review of the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Funding Statement
No funding was received for this article.
References
- 1.Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23((3)):217–226. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- 2.Hoofnagle JH, Björnsson ES. Drug-induced liver injury types and phenotypes. N Engl J Med. 2019;381((3)):264–273. doi: 10.1056/NEJMra1816149. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman HJ. The spectrum of hepatotoxicity. Perspect Biol Med. 1968;12((1)):135–161. doi: 10.1353/pbm.1968.0004. [DOI] [PubMed] [Google Scholar]
- 4.Chalasani NP, Maddur H, Russo MW, Wong RJ, Reddy KR, Practice Parameters. Committee of the American College of Gastroenterology. ACG clinical guideline diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2021;116((5)):878–898. doi: 10.14309/ajg.0000000000001259. [DOI] [PubMed] [Google Scholar]
- 5.Andrade RJ, Chalasani N, Björnsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, et al. Drug-induced liver injury. Nat Rev Dis Primers. 2019;5((1)):58. doi: 10.1038/s41572-019-0105-0. [DOI] [PubMed] [Google Scholar]
- 6.Voican CS, Corruble E, Naveau S, Perlemuter G. Antidepressant-induced liver injury a review for clinicians. Am J Psychiatry. 2014;171((4)):404–415. doi: 10.1176/appi.ajp.2013.13050709. [DOI] [PubMed] [Google Scholar]
- 7.Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology. 2017;65((1)):363–373. doi: 10.1002/hep.28813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avigan MI, Mozersky RP, Seeff LB. Scientific and regulatory perspectives in herbal and dietary supplement associated hepatotoxicity in the United States. Int J Mol Sci. 2016;17((3)):331. doi: 10.3390/ijms17030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipman TO. Herbal supplements. Curr Gastroenterol Rep. 2005;7((4)):302–307. doi: 10.1007/s11894-005-0023-z. [DOI] [PubMed] [Google Scholar]
- 10.Andrade RJ, Aithal GP, Björnsson ES, Kaplowitz N, Kullak-Ublick GA, Larrey D, et al. EASL clinical practice guidelines drug-induced liver injury. J Hepatol. 2019;70((6)):1222–1261. doi: 10.1016/j.jhep.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, et al. Drug-induced liver injury an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129((2)):512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB, et al. Hepatic histological findings in suspected drug-induced liver injury systematic evaluation and clinical associations. Hepatology. 2014;59((2)):661–670. doi: 10.1002/hep.26709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontana RJ. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology. 2014;146((4)):914–928. doi: 10.1053/j.gastro.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chughlay MF, Kramer N, Spearman CW, Werfalli M, Cohen K. N-acetylcysteine for non-paracetamol drug-induced liver injury a systematic review. Br J Clin Pharmacol. 2016;81((6)):1021–1029. doi: 10.1111/bcp.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.


