Abstract
Background
In patients with resected gallbladder cancer (GBC), the role of adjuvant chemotherapy (aCT) remains ill-defined, especially in elderly patients. This study evaluates the value of aCT in elderly patients with GBC and assesses response according to tumor stage.
Methods
Patients of ≥65 years of age with resected GBC diagnosed from 2004–2015 were identified using a Surveillance, Epidemiology and End Results (SEER)/Medicare linked database. After propensity score matching, survival of patients treated with aCT was compared to survival of patients who did not receive aCT using Kaplan-Meier and Cox proportional hazards analysis.
Results
Of 2,179 patients with resected GBC, 876 (25%) received aCT. In the full cohort of 810 propensity-score matched patients, survival did not differ between patients treated with aCT (17.6 months ) and without aCT (19.5 months, P=0.7720). Subgroup analysis showed that survival was significantly better after aCT in T3/T4 disease (12.3 vs. 7.2 months, P=0.013). Interaction analysis showed that benefit of aCT was primarily seen in combined T3/T4, node-positive disease (HR 0.612 , P=0.006).
Conclusions
In this large cohort of elderly patients with resected GBC, aCT was not associated with increased survival. However, aCT may provide a survival benefit in T3/4, node-positive disease.
Keywords: Gallbladder cancer (GBC), chemotherapy, survival, elderly
Introduction
Gallbladder cancer (GBC) has an extremely poor prognosis (1-4). In resected patients, recurrence rates are as high as 65%, and 5-year overall survival (OS) is only 15–30% (5-7). Initial recurrence after resection is often locoregional, but distant relapse in the form of liver spread also occurs frequently (7,8). Adjuvant chemotherapy (aCT) or chemoradiotherapy (aCRT) could hypothetically grant a significant survival benefit by providing both locoregional and distant control.
Unfortunately, high-quality evidence regarding the benefit of aCT in GBC is sparse (9,10). Merely one prospective study, investigating gemcitabine and cisplatin as adjuvant treatment for GBC was conducted (11). However, only 100 patients were included and no sample size calculation was conducted. Hence, the only available prospective evidence consists of randomized controlled trials (RCTs) including patients with all forms of biliary tract cancer (BTC). Of these trials, only the BILCAP study (adjuvant capecitabine vs. observation alone) showed a statistically significant increase in survival in the per-protocol analysis alone (53 vs. 36 months, P=0.028) (8,12,13). Some have argued that the lack of apparent efficacy may be because aCT is only effective in patients with poor prognostic factors such as node-positive and R1 disease, a subgroup which was highly represented in the BILCAP trial (8). Moreover, one should excise caution when extrapolating results of trial including all patients with BTC to patients with GBC alone, as BTC is a heterogenous group of tumors with varying etiology, staging systems and molecular landscapes (8). Finally, studies show that there is a fundamental underrepresentation of especially geriatric patients in clinical trials, likely due to the utilization of stringent eligibility criteria (14,15). Consequently, outcomes of RCTs cannot necessarily be extrapolated to the increasingly frail and elderly general population (16,17). In order to improve external validity of clinical trials and provide clinicians with appropriate evidence for clinical decision making, experts have suggested supplementing RCT outcomes with data from observational cohorts.
Evidently, research specifically aimed at investigating the value of aCT in elderly patients with GBC is warranted. Population-based data provide an opportunity to analyze treatment and survival in a large number of patients and form a representative sample of the real-world population. Statistical methods such as propensity score matching can help to reduce the influence of treatment selection bias on results (18). We describe a propensity score-matched analysis of data from the Surveillance, Epidemiology and End Results (SEER) registry. The objective of this study was to determine the association of aCT in elderly GBC patients with survival and to identify clinically relevant subgroups of patients that may benefit from aCT. We present the follow article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-348/rc) (19).
Methods
Data sources and study design
The SEER program is a population-based database maintained by the National Cancer Institute (NCI), encompassing approximately 34% of the population of the United States (https://seer.cancer.gov). Data provided by SEER can be supplemented by Medicare claims data in order to capture information not recoded by SEER, such as specific information on chemotherapy treatment. Medicare is a federally-funded health insurance program for citizens aged ≥65 years, with disabilities or end-stage renal disease (20). The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the University of Michigan Institutional Review Board and a waiver for informed consent was provided, given that SEER data is de-identified.
Study population
The cohort was created using data from the 2018 SEER-Medicare release. The cohort was restricted to patients diagnosed from 2004 to 2015. Patients with resected, non-metastatic GBC were included using site and histology codes from the International Classification of Disease for Oncology, Third Edition (ICD-O-3). Only patients with non-metastatic disease who underwent resection of the primary tumor site were included. Patients with Tis/T0 or Nx disease, overlapping lesions or uncommon histologies were excluded (Table S1). The cohort was limited to patients aged 65 years or older with Medicare part A and B coverage and no Health Maintenance Organization (HMO) enrollment during 12 months prior and 6 months after diagnosis (or until death) in order to assure completeness of Medicare claims.
Demographic and clinicopathologic variables
The following demographic variables were analyzed; age, race (White vs. Black vs. Asian/Pacific vs. Alaskan/native American), year of diagnosis, zip-code level percentage of residents with a high-school education (in quartiles), zip-code level median household income (in quartiles) and percentage living in poverty by zip-code. The number of Elixhauser comorbidities was derived from the outpatient and inpatient claims data (21,22).
Clinicopathological characteristics included for analysis were tumor location, differentiation grade, nodal status (N0 vs. N+) and pT-stage. Tumor T, N and M stage were reported according to the 7th edition of the American Joint Committee on Cancer (AJCC)-staging manual (23).
Surgery, chemotherapy, and radiotherapy (RT) treatment identification
The date of surgery was identified using current procedural terminology (CPT) codes, healthcare common procedure coding system (HCPCS) codes, and international classification of diseases version 9 (ICD-9) procedure codes. Chemotherapy administration was identified using CPT codes, HCPCS codes and ICD-9 procedure codes. Oral equivalents of chemotherapeutic drugs were identified using National Drug Codes. RT was identified using CPT and revenue center codes for radiation therapy. All codes are available upon request to the authors.
Adjuvant treatment classification
Patients were classified into two groups according to the initial treatment strategy; aCT versus no aCT. aCT was defined as a claim for chemotherapy within six months of surgery, similar to other studies (24,25). A single claim for chemotherapy was used to reflect that a patient had received aCT. Claims for radiation therapy spanning ≥7 days were used to reflect that a patient had received RT.
Statistical analysis
Categorical variables were reported as counts with percentages and compared using the chi-squared test or Fisher’s exact test, where appropriate. Numeric variables were reported as means with ranges and compared using the student’s T-test or Mann-Whitney U test, where appropriate. The primary outcome of all analyses was OS. For all survival analyses, patients whom died within 30 days of surgery were excluded in order to correct for immortal-time bias. Sensitivity analysis in patients who survived >6 months was conducted to further reduce treatment selection and survivor bias since these patients may have had poor performance status or significant postoperative complications, resulting in a poor prognosis and potentially precluding them from receiving aCT. Survival was assessed using Kaplan-Meier curves and compared using the log-rank test. Missing data was classified as “unknown”.
Propensity-score matching (PSM) was used to adjust for treatment selection bias and compare OS of patients treated with aCT to that of patients with no aCT. The conditional probability of receiving chemotherapy (i.e., propensity score) was estimated using a multivariable logistic regression model including age, gender, education, median household income, Elixhauser score, tumor site, tumor size, tumor grade, pT/pN classification, tumor location and extent of lymph node resection. One-to-one nearest-neighbor PSM without replacement (caliper width 0.1) was then used to create a balanced cohort. Standardized mean differences were used to conduct balance diagnostics; all had a value of <0.1, indicating good balance according to Austin et al. (18).
In the matched cohort, additional sensitivity analyses were conducted to investigate the association between N-classification and T-classification with treatment effect of aCT and survival. Cox-regression analysis modelling the interaction between N-classification, T-classification, differentiation grade, receipt of aCT and survival was conducted. To this end, patients were grouped according to pT/pN classification and differentiation grade. A model was composed using the combined stage/grade groups as an interaction term. A P value of <0.05 was considered statistically significant. All analyses were performed using SAS/STAT software, version 9.4 of the SAS system for Windows (Copyright © 2013, SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
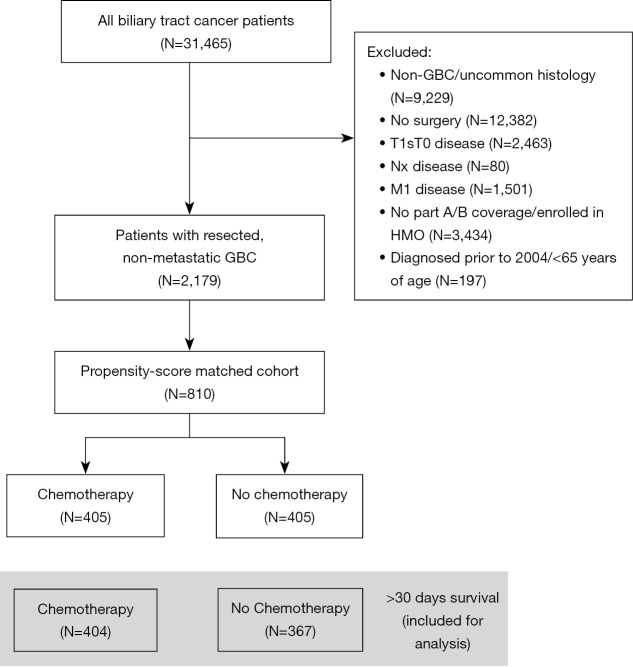
A total of 31,465 patients with BTC was identified, of whom 2,179 patients had GBC, underwent resection and met the inclusion criteria (Figure 1, Table 1). Of these patients, 411 (19%) were treated with aCT. Median follow-up of patients alive at last follow-up was 54 months. Median age at diagnosis was 77 years, and most patients presented with AJCC T1/T2 (N=1,438, 66%) and N0 (N=1,653, 76%) disease.
Figure 1.
Flow diagram. GBC, gallbladder cancer.
Table 1. Baseline characteristics of resected patients with GBC, 2004–2015.
| Characteristic | Unmatched cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| No adjuvant therapy (N=1,768, 81%) | Adjuvant chemotherapy (N=411, 19%) | P value | No adjuvant therapy (N=405, 50%) | Adjuvant chemotherapy (N=405, 50%) | P value | ||
| Age, years | <0.001 | 0.586 | |||||
| 65–70 | 210 (11.9%) | 83 (20.2%) | 100 (24.7%) | 81 (20.0%) | |||
| 70–75 | 306 (17.3%) | 126 (30.7%) | 110 (27.2%) | 122 (30.1%) | |||
| 75–80 | 385 (21.8%) | 106 (25.8%) | 100 (24.7%) | 106 (26.2%) | |||
| 80–84 | 427 (24.2%) | 63 (15.3%) | 61 (15.1%) | 63 (15.6%) | |||
| 85+ | 440 (24.9%) | 33 (8.0%) | 34 (8.4%) | 33 (8.1%) | |||
| Elixhauser comorbidity score | 0.099 | 0.831 | |||||
| 0–2 | 411 (23.2%) | 103 (25.1%) | 96 (23.7%) | 100 (24.7%) | |||
| 3–4 | 485 (27.4%) | 129 (31.4%) | 136 (33.6%) | 128 (31.6%) | |||
| ≥5 | 872 (49.3%) | 179 (43.6%) | 173 (42.7%) | 177 (43.7%) | |||
| Gender, male | 538 (30.4%) | 143 (34.8%) | 0.086 | 144 (35.6%) | 141 (34.8%) | 0.833 | |
| Race | 0.190 | 0.200 | |||||
| White | 1,374 (77.7%) | 336 (81.8%) | 307 (75.8%) | 331 (81.7%) | |||
| Black | 174 (9.8%) | 27 (6.6%) | 46 (11.4%) | 27 (6.7%) | |||
| Asian/pacific islander | 78 (4.4%) | 19 (4.6%) | 17 (4.2%) | 18 (4.4%) | |||
| Hispanic | 84 (4.8%) | 17 (4.1%) | 19 (4.7%) | 17 (4.2%) | |||
| Alaskan/native American | 11 (0.6%) | <11 (0%) | <11 (0%) | <11 (0%) | |||
| Other/unknown | 47 (2.7%) | 12 (2.9%) | 16 (3.9%) | 11 (2.7%) | |||
| Tumor size | 0.082 | 0.496 | |||||
| ≤5 cm | 926 (52.4%) | 232 (56.4%) | 211 (52.1%) | 227 (56.0%) | |||
| >5 cm | 198 (11.2%) | 53 (12.9%) | 60 (14.8%) | 52 (12.8%) | |||
| Unknown | 644 (36.4%) | 126 (30.7%) | 134 (33.1%) | 126 (31.1%) | |||
| pT stage | <0.001 | 0.899 | |||||
| T1 | 417 (23.6%) | 39 (9.5%) | 37 (9.1%) | 39 (9.6%) | |||
| T2 | 817 (46.2%) | 165 (40.1%) | 169 (41.7%) | 165 (40.7%) | |||
| T3 | 499 (28.2%) | 185 (45.0%) | 180 (44.4%) | 180 (44.4%) | |||
| T4 | 28 (1.6%) | 21 (5.1%) | 19 (4.7%) | 20 (4.9%) | |||
| Tx | <11 (0%) | <11 (0%) | <11 (0%) | <11 (0%) | |||
| Regional lymph node surgery | <0.001 | 0.846 | |||||
| No nodes removed | 1,080 (61.1%) | 185 (45.0%) | 183 (45.2%) | 184 (45.4%) | |||
| 1–3 nodes | 497 (28.1%) | 152 (37.0%) | 144 (35.6%) | 151 (37.3%) | |||
| 4+ nodes | 172 (9.7%) | <70 (<17.0%) | <75 (<18.5%) | <65 (<16.0%) | |||
| Unknown | 19 (1.1%) | <11 (0%) | <11 (0%) | <11 (0%) | |||
| pN stage | <0.001 | 0.166 | |||||
| N0 | 1,468 (83.0%) | 249 (60.6%) | 266 (65.7%) | 247 (61.0%) | |||
| N+ | 300 (17.0%) | 162 (39.4%) | 139 (34.3%) | 158 (39.0%) | |||
| Differentiation grade | <0.001 | 0.525 | |||||
| Well | 314 (17.8%) | 49 (11.9%) | 38 (9.4%) | 49 (12.1%) | |||
| Moderate | 763 (43.2%) | 161 (39.2%) | 171 (42.2%) | 159 (39.3%) | |||
| Poor/undifferentiated | 561 (31.7%) | 178 (43.3%) | 177 (43.7%) | 174 (43.0%) | |||
| Unknown | 130 (7.4%) | 23 (5.6%) | 19 (4.7%) | 23 (5.7%) | |||
| Type of chemotherapy | <0.001 | <0.001 | |||||
| Gemcitabine-based† | NA | 135 (35.2%) | NA | 134 (35.6%) | |||
| Gemcitabine-cisplatin | NA | 58 (14.1%) | NA | 55 (13.6%) | |||
| FU-based | NA | 167 (40.6%) | NA | 165 (40.7%) | |||
| Other | NA | 41 (10.0%) | NA | 41 (10.1%) | |||
| Timing of chemotherapy | <0.001 | <0.001 | |||||
| Adjuvant | NA | 384 (93.4%) | NA | 378 (93.3%) | |||
| Neo-adjuvant | NA | 27 (6.6%) | NA | 27 (6.7%) | |||
| Radiotherapy (yes) | 123 (7.0%) | 178 (43.3%) | 36 (8.9%) | 176 (43.5%) | |||
| Resection received‡ | <0.001 | <0.001 | |||||
| Cholecystectomy | 1,712 (96.8%) | 398 (96.5%) | 386 (95.3%) | 392 (96.8%) | |||
| Liver resection | 395 (22.3%) | 133 (32.4%) | 157 (38.8%) | 130 (32.1%) | |||
| Bile duct resection | 117 (6.6%) | 45 (10.9%) | 51 (12.6%) | 44 (10.9%) | |||
| Pancreatic resection | <11 (0%) | <11 (0%) | <11 (0%) | <11 (0%) | |||
| Lymph node resection | 345 (19.5%) | 115 (28.0%) | 139 (34.3%) | 112 (27.7%) | |||
Patients were matched on age, gender, Elixhauser comorbidity score, date of diagnosis, tumor size, pT- and pN stage, differentiation grade, education, income and poverty status. †, 20 of these patients received 5-FU and gemcitabine; ‡, patients may have received multiple procedures which have been coded separately: i.e., a cholecystectomy and a liver resection as a second, separate procedure. GBC, gallbladder cancer.
Patients who received aCT were younger than patients that did not receive aCT. Patients that did receive aCT were diagnosed with more advanced tumors and more node-positive disease as opposed to patients that did not receive aCT. Adjuvant RT was more frequently administered in patients that received aCT compared to patients that did not receive aCT. After propensity score matching, 810 cases were matched: 405 patients with aCT and 405 patients who did not receive aCT. Baseline characteristics of the matched cohort did not differ significantly between patients with and without aCT (Table 1, Table S2). Mean standardized difference in propensity score was 0.91 before matching and <0.1 across all variables after matching, indicating good balance (Figure S1).
Survival in patients with and without aCT before and after propensity score matching
In the unmatched cohort median OS was 18.0 months and 5-year survival was 27%. Median OS of patients that received aCT did not differ from survival in patients that did not receive aCT (aCT 17.7 months vs. no aCT 18.1 months, P=0.284).
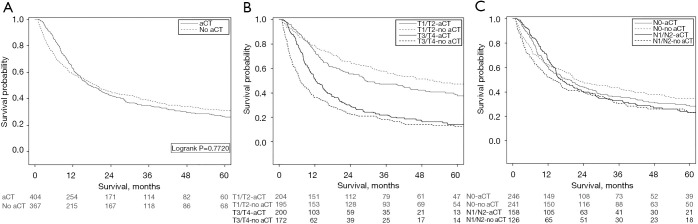
In the matched cohort included for survival analysis (N=772), median OS was 18.3 months and 5-year survival was 28%. Survival did not differ between patients treated with aCT (17.6 months) compared to patients with no aCT (19.5 months, P=0.7720, Figure 2A). In adjusted Cox regression analysis, administration of chemotherapy was not significantly associated with survival (HR 0.89, 95% CI: 0.74–1.08, P=0.232).
Figure 2.
Survival curves of patients treated with and without aCT. (A) Survival of matched cohort excluding mortality <30 days (N=771, P=0.7720). (B) Survival of matched cohort excluding mortality <30 days, stratified by T-stage (N=399). Log rank P=0.0984 in T1/T2, aCT vs. no aCT. Log rank P=0.0129 in T3/T4 (N=372), aCT vs. no aCT. (C) Survival of matched cohort excluding mortality <30 days, stratified according to N stage. Log rank P=0.3150 in N0 (N=487), aCT vs. no aCT. Log rank P=0.2527 in N1/N2 (N=284), aCT vs. no aCT. aCT, adjuvant chemotherapy.
Subgroup analysis of survival in patients with and without chemotherapy according T-classification and N-classification
In the subgroup of patients with T1/T2 disease, no statistically significant difference between patients treated with aCT (29.2 months) and without aCT (51.8 months, P=0.0984, Figure 2B) was found. In T3/T4 disease, median OS in patients with aCT was 12.3 months, versus 7.2 months in patients without (P=0.013) (Figure 2B). When classifying patients according to N-stage (N0 and N1/N2), no difference in survival could be demonstrated between patients who did and did not receive aCT (Figure 2C).
Effect of different chemotherapy regimens
In uncorrected analysis, there was no significant (P=0.542) difference in median OS between different aCT regimens; FU-based 20.5 months, gemcitabine-based 15.7 months, gemcitabine-cisplatin 16.7 months and other chemotherapy combinations 12.7 months. Adjusted multivariable analysis also did not show significant differences in survival between chemotherapy regimens (Table 2).
Table 2. Cox regression of prognostic factors for patients that received chemotherapy in the matched cohort.
| Factor | HR | 95% CI | P value |
|---|---|---|---|
| Age (per year) | 1.02 | 1.00–1.04 | 0.044 |
| Elixhauser comorbidity score | |||
| 0–2 | 1 | 1 | |
| 3–4 | 0.83 | 0.60–1.16 | 0.264 |
| ≥5 | 1.13 | 0.82–1.54 | 0.447 |
| Differentiation grade | |||
| Well | 1 | 1 | |
| Moderate | 1.16 | 0.78–1.72 | 0.478 |
| Poor | 1.40 | 1.01–1.81 | 0.094 |
| Undifferentiated | 1.30 | 0.64–2.27 | 0.386 |
| Poverty indicator | |||
| 0–5% | 1 | 1 | |
| 5–10% | 1.192 | 0.84–1.70 | 0.329 |
| 10–20% | 1.341 | 0.95–1.90 | 0.099 |
| >20% | 1.096 | 0.74–1.62 | 0.647 |
| Race | |||
| White | 1 | 1 | |
| Black | 1.07 | 0.65–1.79 | 0.782 |
| Asian/pacific islander | 0.85 | 0.43–1.70 | 0.643 |
| Hispanic | 0.69 | 0.35–1.35 | 0.275 |
| Alaskan/native American | 0 | 0–1000.00 | 0.956 |
| Year of diagnosis | |||
| 2004–2005 | 1 | 1 | |
| 2006–2007 | 0.97 | 0.64–1.48 | 0.888 |
| 2008–2009 | 1.20 | 0.81–1.79 | 0.362 |
| 2010–2011 | 1.00 | 0.66–1.52 | 0.992 |
| 2012–2014 | 0.81 | 0.53–1.23 | 0.324 |
| pN stage (yes vs. no) | |||
| N0 | 1 | 1 | |
| N1/N2 | 1.58 | 1.13–2.20 | 0.008 |
| pT stage | |||
| T1 | 1 | 1 | |
| T2 | 1.37 | 0.87–2.16 | 0.171 |
| T3 | 2.28 | 1.46–3.56 | <0.001 |
| T4 | 4.66 | 2.40–9.04 | <0.001 |
| Tumor size | |||
| ≤5 cm | 1 | 1 | |
| >5 cm | 1.04 | 0.78–1.39 | 0.776 |
| Radiotherapy (yes) | 0.73 | 0.59–0.91 | 0.004 |
| Type of chemotherapy | |||
| Gemcitabine-based | 1 | ||
| 5FU-based | 1.07 | 0.88–1.41 | 0.464 |
| Gemcitabine-cisplatin | 0.83 | 0.61–1.12 | 0.180 |
| Other | 1.37 | 0.98–1.90 | 0.110 |
HR, hazard ratio; CI, confidence interval.
Interaction analysis of combined T-/N- classification and differentiation grade with aCT in the matched cohort
An association with superior OS after treatment with aCT was seen in patients with T4 disease (HR 0.475, 95% CI: 0.242–0.932), but not in patients with node-positive disease (HR 0.795, 95% CI: 0.598–1.057) (Table S3). Patients were grouped according to different combinations of T-classification (T1/T2 vs. T3/T4) and N-classification (N0 vs. N+) to analyze their interaction with chemotherapy and survival (Table 3). Multivariable analysis showed that aCT was only associated with longer OS in patients with a combination of T3/T4 and N+ disease (HR 0.61, 95% CI: 0.43–0.87, Table 3).
Table 3. Interaction analysis between T-/N- classification and receipt of aCT and survival in patients who survived >30 and >180 days postoperatively.
| Group | aCT (N) | No aCT (N) | HR | 95% CI | P value |
|---|---|---|---|---|---|
| T1/T2, N0 | |||||
| >30 days | 138 | 140 | 1.18 | 0.85–1.65 | 0.328 |
| >180 days | 124 | 122 | 1.32 | 0.90–1.94 | 0.155 |
| T1/T2, N1/N2 | |||||
| >30 days | 66 | 55 | 1.27 | 0.79–2.06 | 0.324 |
| >180 days | 60 | 51 | 1.24 | 0.73–2.09 | 0.428 |
| T3/T4, N0 | |||||
| >30 days | 108 | 101 | 0.92 | 0.67–1.26 | 0.583 |
| >180 days | 83 | 63 | 1.30 | 0.86–1.96 | 0.217 |
| T3/T4, N1/N2 | |||||
| >30 days | 92 | 71 | 0.61 | 0.43–0.87 | 0.006 |
| >180 days | 78 | 35 | 1.03 | 0.65–1.63 | 0.911 |
aCT, adjuvant chemotherapy; HR, hazard ratio; CI, confidence interval.
When including differentiation grade in the model, an association between administration of aCT and longer median OS was seen in patients with T3/T4, N+, poorly/undifferentiated disease (HR 0.43, Table S4).
Discussion
Tumor recurrence after resection of GBC occurs in up to 65% of patients and ultimately determines survival (7). Although RCTs show that aCT reduces recurrence rates in other cancers, robust evidence to support its use in GBCs has not existed. Given the conflicting results from single-institute series and absence of RCTs including GBC alone, we sought to inform this debate by performing an analysis of SEER registry data on elderly patients with resected GBC. In this propensity-score matched cohort survival was not significantly associated with receipt of aCT. However, subgroup analysis revealed that in patients with T3/T4, node-positive, poorly differentiated disease the administration of aCT was significantly associated with increased survival.
No high-quality RCTs investigating the value of aCT for GBC have been published. Therefore, treatment guidelines are based on the conflicting results of a few randomized trials investigating the efficacy of aCT in all forms of BTC. Of three high-quality trials completed recently, only the BILCAP trial (adjuvant capecitabine compared to observation alone) showed positive results in the per-protocol analysis alone (53 vs. 36 months, P=0.028) and not in the primary, intention to treat analysis (8,26). It is important to note that in the BILCAP trial, a relatively high number of patients with R1 resections (38%) or node-positive disease (46%) was included; both factors are known poor prognostic factors and are associated with increased response to chemotherapy (24,26,27). We aimed to delineate clinically relevant subgroups of GBC patients that may benefit from chemotherapy and found that chemotherapy may only be beneficial in patients with T3/T4, node-positive and poorly differentiated disease. In this study, aCT in patients with low-risk (i.e., T1/T2, node-negative, well-differentiated) disease even appeared to be negatively associated with median OS. This finding can potentially be attributed to the fact that patients with irradical resection are more frequently treated with chemotherapy. Since other studies show that irradical resection is a poor prognostic factor, we suggest that aCT for low-risk patients should potentially only be considered in case of positive resection margins (28).
After the aforementioned RCTs, the next highest level of evidence is a recent meta-analysis of 21 studies including 6,712 patients, of which 1,797 were treated with chemotherapy, RT or a combination of both (29). The meta-analysis showed a positive effect of adjuvant therapy in all patients with BTC (OR 0.74), contradicting our finding that aCT is only associated with improved median OS in patients with high-risk (i.e., T3/T4, N+) features. However, only one RCT was included and all other studies were retrospective, single-center experiences, which may be subject to selection and immortal-time bias. Additionally, a large grade of heterogeneity was seen, and the authors were unable to report hazard ratios (adjusted for survival time) since many studies did not report actual survival times. Finally, a significant portion of the included studies had a high (>50%) rate of R1 resection or lymph-node positivity, explaining the high effect of aCT in their study. We used propensity score matching and exclusion of patients who deceased <30 days to account for both forms of bias, which may explain the lack of efficacy of aCT across the full cohort in our study.
Since the publication of the ABC-02 trial in 2010, gemcitabine and cisplatin (Gem-Cis) has been the regimen of choice in the treatment of locally advanced or metastatic GBC. (30-32). However, its efficacy has not been investigated in GBC alone, nor proven in the adjuvant setting (33,34). The only chemotherapeutic agent which has demonstrated to increase survival in resected patients compared to observation alone in a randomized controlled study is capecitabine (13). Most studies investigating other agents or combination regimens were single-arm studies or compared to observation alone and no studies have directly compared capecitabine to other commonly used agents. Therefore, it is difficult to establish whether other treatment regimens may be more effective than capecitabine. In the present study, after covariate adjustment all chemotherapeutic regimens (gemcitabine monotherapy, 5-FU, Gem-Cis or other combinations) were comparable in terms of association with median OS. This does not necessarily mean that no significant difference in efficacy between these regimens exists. New evidence shows that the expression of specific molecular profiles may be associated with extremely good response to certain forms of (targeted) therapy (35,36). Future research should focus on identifying specific molecular profiles and their association with response to chemotherapy.
There is no randomized evidence available regarding the use of adjuvant RT, alone or in combination with chemotherapy. In the aforementioned meta-analysis, chemotherapy alone appeared to be more effective in reducing the risk of death (HR 0.39 compared to surgery alone) than chemoradiotherapy (HR 0.61) (29). However, in patients with R1 disease adjuvant RT was associated with significantly better survival (HR 0.33). Our data shows that in patients treated with chemotherapy, additional radiation appeared to provide a survival benefit. However, only 212 patients received RT in our cohort and the majority of irradiated patients (83%) received concurrent chemotherapy, potentially providing significant bias. Therefore, we feel that our data are of insufficient quality to provide definitive recommendations on the use of adjuvant RT. Similarly, no randomized evidence on the efficacy of neoadjuvant chemotherapy is available. A very small number of patients in our cohort received neoadjuvant chemotherapy (N=27). Due to statistical limitations, no separate analyses investing patients treated with neoadjuvant chemotherapy were conducted.
The primary limitation of this study is the non-random allocation of treatment. PSM and exclusion of subjects whom were deceased within 30 days of surgery were used to limit the impact of selection bias and immortal time bias. Secondly, SEER does not register margin status after resection, which may be both a marker of disease biology and a risk factor for recurrence. The inability to correct for margin status is a significant confounding factor. Since chemotherapy is more likely to be administered to patients with positive resection margins, it is likely that the efficacy of aCT may be underestimated in this study as a positive resection margin is a significant predictor for poor survival (37,38). Finally, it is possible that the use of oral chemotherapeutic agents, especially capecitabine, is not fully captured because not all patients had Part D coverage. However, if anything this likely leads to an underestimation of treatment efficacy.
This study has multiple strengths. Primarily, this study provides insight into the outcomes of a uniquely large cohort of elderly GBC patients treated with aCT. Due to the use of population-based data and the inclusion of elderly patients with comorbidities, this paper provides an excellent reflection of contemporary clinical practice and outcomes of patients with GBC. Specifically, median age of GBC patients in Western countries is 72 and only 29% of patients is under 65 years of age (39,40). Median age of patients included in the BILCAP trial was 62 and virtually all patients had a WHO performance status of 0 or 1. In our cohort the median age was 77 and patients had multiple comorbidities, which may more accurately reflect the average GBC patient. In contrast to most retrospective and registry studies that do not include information on patient comorbidity, we used Medicare claims data to calculate the Elixhauser comorbidity score. This method is viable to assess and correct for performance status in statistical models (41,42). Finally, we were able to compare different chemotherapeutic regimens.
Conclusions
These data show that aCT may provide a survival benefit in high-risk elderly patients with advanced GBC, including T3/T4 tumors and node-positive disease. Future research efforts should focus on improving the selection of GBC patients who might have a higher likelihood of benefit from adjuvant treatment.
Supplementary
The article’s supplementary files as
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The paper has been previously published as a book, titled: “Diagnosis and treatment of gallbladder cancer: an overview of contemporary practise”.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the University of Michigan Institutional Review Board and a waiver for informed consent was provided, given that SEER data is de-identified.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-348/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-348/coif). The authors have no conflicts of interest to declare.
References
- 1.SEER. Cancer statistics 2019. Available online: https://seer.cancer.gov/statfacts/html/livibd.html
- 2.Yao KJ, Jabbour S, Parekh N, et al. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol 2016;16:117. 10.1186/s12876-016-0527-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagorney DM, Donohue JH, Farnell MB, et al. Outcomes after curative resections of cholangiocarcinoma. Arch Surg 1993;128:871-7; discussion 877-9. 10.1001/archsurg.1993.01420200045008 [DOI] [PubMed] [Google Scholar]
- 4.Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014;6:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaga MM, Brasoveanu V, Stroescu C, et al. Pattern of the First Recurrence Has No Impact on Long-Term Survival after Curative Intent Surgery for Perihilar Cholangiocarcinomas. Gastroenterol Res Pract 2018;2018:2546257. 10.1155/2018/2546257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saiura A, Yamamoto J, Kokudo N, et al. Intrahepatic cholangiocarcinoma: analysis of 44 consecutive resected cases including 5 cases with repeat resections. Am J Surg 2011;201:203-8. 10.1016/j.amjsurg.2008.12.035 [DOI] [PubMed] [Google Scholar]
- 7.Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer 2003;98:1689-700. 10.1002/cncr.11699 [DOI] [PubMed] [Google Scholar]
- 8.Lamarca A, Edeline J, McNamara MG, et al. Current standards and future perspectives in adjuvant treatment for biliary tract cancers. Cancer Treat Rev 2020;84:101936. 10.1016/j.ctrv.2019.101936 [DOI] [PubMed] [Google Scholar]
- 9.Lamarca A, Valle JW. Should Patients with Resected Bile Duct Cancer Receive an Adjuvant Treatment? Journal of OncoPathology 2014;2:57-68. 10.13032/tjop.2052-5931.100109 [DOI] [Google Scholar]
- 10.McNamara MG, Walter T, Horgan AM, et al. Outcome of adjuvant therapy in biliary tract cancers. Am J Clin Oncol 2015;38:382-7. 10.1097/COC.0b013e31829e19fb [DOI] [PubMed] [Google Scholar]
- 11.Saluja SS, Nekarakanti PK, Mishra PK, et al. Prospective Randomized Controlled Trial Comparing Adjuvant Chemotherapy vs. No Chemotherapy for Patients with Carcinoma of Gallbladder Undergoing Curative Resection. J Gastrointest Surg 2022;26:398-407. 10.1007/s11605-021-05143-6 [DOI] [PubMed] [Google Scholar]
- 12.Rangarajan K, Simmons G, Manas D, et al. Systemic adjuvant chemotherapy for cholangiocarcinoma surgery: A systematic review and meta-analysis. Eur J Surg Oncol 2020;46:684-93. 10.1016/j.ejso.2019.11.499 [DOI] [PubMed] [Google Scholar]
- 13.Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:663-73. 10.1016/S1470-2045(18)30915-X [DOI] [PubMed] [Google Scholar]
- 14.Kennedy-Martin T, Curtis S, Faries D, et al. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials 2015;16:495. 10.1186/s13063-015-1023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Deudekom FJ, Postmus I, van der Ham DJ, et al. External validity of randomized controlled trials in older adults, a systematic review. PLoS One 2017;12:e0174053. 10.1371/journal.pone.0174053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Averitt AJ, Weng C, Ryan P, et al. Translating evidence into practice: eligibility criteria fail to eliminate clinically significant differences between real-world and study populations. NPJ Digit Med 2020;3:67. 10.1038/s41746-020-0277-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu S, Rosen KJ, Cupertino A, et al. Generalizability of Randomized Controlled Trials in Rectal Cancer. J Gastrointest Surg 2022;26:453-65. 10.1007/s11605-021-05192-x [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014;33:1242-58. 10.1002/sim.5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 20.NCI. SEER-Medicare: Brief Description of the SEER-Medicare Database. 2020. Available online: https://healthcaredelivery.cancer.gov/seermedicare/overview/
- 21.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998;36:8-27. 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 22.Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care 2004;42:355-60. 10.1097/01.mlr.0000118861.56848.ee [DOI] [PubMed] [Google Scholar]
- 23.Edge SB, Byrd DR, Compton CC, et al. editors. AJCC cancer staging manual (7th ed). 7 ed. New York: Springer, 2010. [Google Scholar]
- 24.Wang SJ, Lemieux A, Kalpathy-Cramer J, et al. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol 2011;29:4627-32. 10.1200/JCO.2010.33.8020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong JC, Murphy JD, Wang SJ, et al. Chemoradiotherapy before and after surgery for locally advanced esophageal cancer: a SEER-Medicare analysis. Ann Surg Oncol 2013;20:3999-4007. 10.1245/s10434-013-3072-9 [DOI] [PubMed] [Google Scholar]
- 26.Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J Clin Oncol 2019;37:1015-27. 10.1200/JCO.18.02178 [DOI] [PubMed] [Google Scholar]
- 27.Weber SM, DeMatteo RP, Fong Y, et al. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg 2002;235:392-9. 10.1097/00000658-200203000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farhat MH, Shamseddine AI, Tawil AN, et al. Prognostic factors in patients with advanced cholangiocarcinoma: role of surgery, chemotherapy and body mass index. World J Gastroenterol 2008;14:3224-30. 10.3748/wjg.14.3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40. 10.1200/JCO.2011.40.5381 [DOI] [PubMed] [Google Scholar]
- 30.Dierks J, Gaspersz MP, Belkouz A, et al. Translating the ABC-02 trial into daily practice: outcome of palliative treatment in patients with unresectable biliary tract cancer treated with gemcitabine and cisplatin. Acta Oncol 2018;57:807-12. 10.1080/0284186X.2017.1418532 [DOI] [PubMed] [Google Scholar]
- 31.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 32.Crinò L, Scagliotti G, Marangolo M, et al. Cisplatin-gemcitabine combination in advanced non-small-cell lung cancer: a phase II study. J Clin Oncol 1997;15:297-303. 10.1200/JCO.1997.15.1.297 [DOI] [PubMed] [Google Scholar]
- 33.Ebata T, Hirano S, Konishi M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg 2018;105:192-202. 10.1002/bjs.10776 [DOI] [PubMed] [Google Scholar]
- 34.Belkouz A, Wilmink JW, Haj Mohammad N, et al. Advances in adjuvant therapy of biliary tract cancer: an overview of current clinical evidence based on phase II and III trials. Crit Rev Oncol Hematol 2020;151:102975. 10.1016/j.critrevonc.2020.102975 [DOI] [PubMed] [Google Scholar]
- 35.Tariq NU, McNamara MG, Valle JW. Biliary tract cancers: current knowledge, clinical candidates and future challenges. Cancer Manag Res 2019;11:2623-42. 10.2147/CMAR.S157092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pellino A, Loupakis F, Cadamuro M, et al. Precision medicine in cholangiocarcinoma. Transl Gastroenterol Hepatol 2018;3:40. 10.21037/tgh.2018.07.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kemp Bohan PM, Kirby DT, Chick RC, et al. Adjuvant Chemotherapy in Resectable Gallbladder Cancer is Underutilized Despite Benefits in Node-Positive Patients. Ann Surg Oncol 2021;28:1466-80. 10.1245/s10434-020-08973-x [DOI] [PubMed] [Google Scholar]
- 38.Bergquist JR, Shah HN, Habermann EB, et al. Adjuvant systemic therapy after resection of node positive gallbladder cancer: Time for a well-designed trial? (Results of a US-national retrospective cohort study). Int J Surg 2018;52:171-9. 10.1016/j.ijsu.2018.02.052 [DOI] [PubMed] [Google Scholar]
- 39.Pinter M, Hucke F, Zielonke N, et al. Incidence and mortality trends for biliary tract cancers in Austria. Liver Int 2014;34:1102-8. 10.1111/liv.12325 [DOI] [PubMed] [Google Scholar]
- 40.Charbel H, Al-Kawas FH. Cholangiocarcinoma: epidemiology, risk factors, pathogenesis, and diagnosis. Curr Gastroenterol Rep 2011;13:182-7. 10.1007/s11894-011-0178-8 [DOI] [PubMed] [Google Scholar]
- 41.Austin SR, Wong YN, Uzzo RG, et al. Why Summary Comorbidity Measures Such As the Charlson Comorbidity Index and Elixhauser Score Work. Med Care 2015;53:e65-72. 10.1097/MLR.0b013e318297429c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menendez ME, Neuhaus V, van Dijk CN, et al. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res 2014;472:2878-86. 10.1007/s11999-014-3686-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as