Abstract
Background
Mitogen-activated protein kinase kinase (MEK) is activated by mutated KRAS in >90% of pancreatic ductal adenocarcinoma (PDAC). MEK and focal adhesion kinase (FAK) are frequently co-activated in PDAC providing a rationale for combining trametinib, an oral allosteric MEK1/2 inhibitor, with GSK2256098, an oral FAK inhibitor.
Methods
Advanced PDAC patients whose disease progressed after first line palliative chemotherapy were treated with GSK2256098 250 mg twice daily and trametinib 0.5 mg once daily orally. The primary endpoint was clinical benefit (CB; complete response, partial response, or stable disease ≥24 weeks). Twenty-four patients were planned to enroll using a 2-stage minimax design (P0=0.15, P1=0.40; alpha =0.05, power 0.86). The combination would be considered inactive if 2/12 or fewer patients achieved CB at the end of stage 1, and would be considered active if >7/24 response-evaluable patients achieved CB by the end of stage 2. Serial blood samples were collected for circulating tumor DNA (ctDNA) mutation profiling.
Results
Sixteen patients were enrolled and 11 were response evaluable. Of those 11, 10 had progressive disease as best tumor response and one had stable disease for 4 months. No treatment related grade ≥3 adverse events (AEs) were observed. The median progression free survival (PFS) was 1.6 (95% CI: 1.5–1.8) months and the median overall survival (OS) was 3.6 (95% CI: 2.7–not reached) months. One response-inevaluable patient achieved clinical stability for 5 months with reduction in CA19-9 and ctDNA levels with a MAP2K1 treatment resistance mutation detected in ctDNA at clinical progression.
Conclusions
The combination of GSK2256098 and trametinib was well tolerated but was not active in unselected advanced PDAC.
Keywords: Pancreatic adenocarcinoma (PDAC), GSK2256098, trametinib, FAK inhibition, MEK inhibition
Introduction
Patients with advanced pancreatic ductal adenocarcinoma (PDAC) have a very poor prognosis with a median overall survival (OS) of <12 months as they have limited therapeutic options (1,2). Beyond chemotherapy, targeted treatment options are limited in PDAC. Olaparib maintenance therapy improves progression free survival (PFS) of PDAC patients harbouring a germline breast cancer gene (BRCA1/2) mutation that represent ~5% of the PDAC population (3). Erlotinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, combined with gemcitabine minimally improves survival (~2 weeks improvement in median OS) when compared to gemcitabine alone (4). Currently, second line treatment options are very limited for the majority of patients with advanced PDAC. For patients who encounter disease progression after gemcitabine based first line chemotherapy, nanoliposomal irinotecan plus fluorouracil and folinic acid is widely used as a second line chemotherapy based on results of NAPOLI-1 phase III trial although the survival benefit is very modest and its benefit in patients who were treated with upfront gemcitabine/nab-paclitaxel chemotherapy is not clear (5). For those who were treated with first line modified FOLFRINOX, gemcitabine based chemotherapy is widely used as second line treatment but again randomized data is lacking to support any one standard treatment in this setting. Only for a very small subset of patients with PDAC who have unique molecular aberrations, universally accepted second line treatments exit. For patients with microsatellite instability (MSI) high tumors that constitute <1% of PDAC population, pembrolizumab is an Food and Drug Administration (FDA) approved indication (6). Zenocutuzumab, a HER3 bi-specific antibody, was recently granted FDA fast track approval for NRG1-fusion PDAC (<1%) (7), and Larotrectinib and Entrectinib, TRK inhibitors are FDA approved for those with NTRK-fusion PDAC (<1%) (8,9).
Although KRAS is an obvious drug target in PDAC, direct inhibition of mutant KRAS has been challenging. Recently, antitumor activity of KRAS p.G12C inhibitor has been demonstrated (10) but <2% of patients with PDAC harbour a KRAS p.G12C mutation and for the majority, RAS targeted therapeutic option is still lacking. Thus, for the majority of patients with KRAS driven PDAC, inhibiting downstream pathways of KRAS is an important therapeutic strategy for investigation. Previous studies, however, have shown that inhibiting downstream MAPK enzymes MEK1/MEK2 alone is ineffective in treating PDAC, possibly due to cross-talk through alternative signaling pathways including PI3K/AKT (11,12) and focal adhesion kinase (FAK) (13). Multiple pan-PI3K and MEK inhibitor or AKT and MEK inhibitor combinations have been tested in phase I clinical trials, with frequently observed overlapping toxicities that have limited the maximum doses that can be achieved with these inhibitors in combination (14-17). Moreover, alternate dosing schedules, such as intermittent administration of PI3K or AKT inhibitors, have not shown improved tolerability or anti-tumor activity (14,18) limiting the scope for further development of this combination strategy.
FAK, a non-receptor tyrosine kinase, is localized at sites of contact with the extracellular matrix and is involved in integrins and growth factor receptors signalling (19,20). The antitumor activity of FAK inhibition in PDAC was shown in several preclinical studies (21-24). The co-activation of MEK and FAK pathways are frequently found in PDAC (25,26) providing a rationale for combinatorial inhibition of these targets in this disease. KRAS oncogenic mutation activates MEK pathway in >90% of PDAC and FAK is expressed in 40–50% of PDAC. As such we estimate that ~40% of PDAC will have co-activation of MEK and FAK pathway (27). Moreover, in preclinical models, FAK was shown to be a critical synthetic lethal partner of KRAS mutant lung adenocarcinomas that are CDKN2A, or TP53 deficient (28). Loss of the tumor suppressor Merlin, encoded by the NF2 gene, is also a synthetic lethal partner for FAK inhibition (29). In PDAC, KRAS mutations frequently co-occur with alterations in TP53, CDKN2A, and NF2 (30-32). Collectively, these data provide a rationale for testing dual pathway blockade with FAK inhibitor (GSK2256098) and MEK inhibitor (trametinib) in advanced PDAC patients. Herein we conducted a phase II study to evaluate the antitumor activity of GSK2256098 and trametinib combination in advanced PDAC patients. We present the following article in accordance with the TREND reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-86/rc).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) with the Good Clinical Practice guidelines of the International Conference on Harmonisation. The study was approved by the Ontario Cancer Research Ethics Board (Project ID 1148) and all the patients gave informed consent for participation in the study. This study is registered with clinicaltrials.gov with registration number NCT02428270.
The subjects were recruited at four centres in Ontario, Canada; Princess Margaret Cancer Centre, Juravinski Cancer Centre, London Health Sciences Centre, and the Ottawa Hospital Regional Cancer Centre. Patients who were 18 years or older with histologically or cytologically-confirmed diagnosis of advanced PDAC that progressed either clinically, radiographically, or serologically after one prior line of therapy for metastatic or locally advanced disease based upon investigator assessment were eligible. Patients who experienced disease recurrence within 6 months after completion of adjuvant chemotherapy were also eligible. Patients were required to have measurable disease by RECIST 1.1 with tumor lesion(s) amenable to biopsy, Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1, adequate organ function(s), and able to swallow and retain oral medications. Key exclusion criteria included prior exposure to either a MEK inhibitor, RAF inhibitor or a FAK inhibitor, > grade 1 unresolved toxicity from previous anti-cancer therapy, presence of active gastrointestinal disease or other condition that could affect gastrointestinal absorption or predispose to gastrointestinal ulceration (subjects with prior Whipple procedure are eligible), evidence of mucosal or internal bleeding, prolonged QTcF interval ≥480 msecs (or ≥500 msec with right bundle branch block) and left bundle branch block, history of retinal vein occlusion, and history of interstitial lung disease or pneumonitis.
Study treatment and assessments
Eligible patients were treated on a continuous oral daily dosing schedule of trametinib 0.5 mg once daily and GSK2256098 250 mg twice daily in 28-day cycles. This dosing schedule was based on the maximum tolerated dose (MTD) of high trametinib/low GSK2256098 combination established in the phase Ib study FAK114746 (NCT01938443) that had been established at the time that this trial was initiated (33). The pharmacokinetic studies in this phase Ib study demonstrated that there was drug to drug interaction between GSK2256098 and trametinib resulting in an increase in plasma concentrations of trametinib 2–4 times higher than would be expected based on trametinib pharmacokinetic data following monotherapy dosing. For GSK2256098, a maximum reduction of one dose level from the starting dosage to 100 mg twice daily was allowed during the course of the study treatment. Further dose modification due to clinically significant adverse event (AE) mandated discontinuation of study treatment. No dose reduction was allowed for trametinib. If treatment-related AEs led to discontinuation of one investigational agent, patients were allowed to continue on the other investigational agent as monotherapy in the event of ongoing clinical benefit (CB). A maximum treatment interruption of 28 days due to intercurrent illness or toxicity was allowed. CTCAE (common toxicity criteria for AEs) v.4.03 was used to grade AEs. Radiological response was assessed every 2 cycles. For cardiac safety monitoring, ECG was performed at baseline and on day 1 of each subsequent treatment cycle and measurement of left ventricular ejection fraction by echocardiography or multigated acquisition (MUGA) scan was performed at baseline, at cycle 4 day 1 and at day 1 of every three subsequent cycles and at the final study visit. Ophthalmologic examination was also performed at baseline and as clinically indicated during the study because of the risk of trametinib-related serous retinopathy. Data were captured using Medidata Rave® platform. Patients continued on study treatment until disease progression or the development of unacceptable toxicity.
Correlative studies
Mandatory fresh tumor biopsies were performed at baseline before starting the study treatment and at cycle 1 day 22±7 days. Blood samples for circulating tumor DNA (ctDNA) analysis were collected at baseline, at cycle 1 day 22±7 days coinciding with the second mandatory biopsy, at C1D1 of even numbered cycles thereafter, and at the end of treatment. Blood samples were collected in EDTA tubes and processed within two hours of collection for plasma. ctDNA was extracted from 8–10 mL of plasma using a column purification method according to manufacturer instructions (QIAamp Circulating Nucleic Acid Kit; Germantown, MD, USA). Twenty nanogram (20 ng) of ctDNA extracted from each plasma sample was tested with a targeted amplicon library NGS assay (OncomineTM Lung ctDNA Assay, Illumina, San Diego, CA, USA) in the Advanced Molecular Diagnostics Laboratory, Princess Margaret Cancer Centre. The assay includes target regions of KRAS and additional genes (ALK, BRAF, EGFR, ERBB2, MAP2K1, MET, NRAS, PIK3CA, ROS1, TP53), with detection of single nucleotide variants and short indels (<20 base pairs) variants. Sequencing libraries were constructed using manufacturer protocols, with sequencing on the Ion S5TM XL system (Ion 530 or 540 Chip Kit; ThermoFisher, Waltham, MA, USA). Raw data analysis, alignment and variant calling were processed by Torrent Suite software v5.2, followed by variant filtering and annotation by Ion Reporter software v5.2 (ThermoFisher). Serum tumor marker CA19-9 was also measured at baseline and on day 1 of even numbered cycles.
For tumor RNA sequencing, biospecimens underwent laser captured microdissection for tumor enrichment as previously described (34). RNASeq analysis was performed at the Ontario Institute of Cancer Research per published standard protocols (34). Briefly, reads were aligned to the human reference genome (hg38) and transcriptome (Ensembl v84) using STAR v.2.5.2a (35). Picard v. 1.121 (https://github.com/broadinstitute/picard) was used for marking duplicated reads. Gene expression was calculated in fragments per kilobase of exon per million reads mapped (FPKM) using commands in cufflinks package v. 2.2.1 (36). Variants were called using GATK 3.4.0 (37) and annotated by Annovar. The gene set enrichment analysis was done using GSEA v4.1.0 (38) with differentially expressed data between before and after therapy using DESeq2 (39) package in R v3.6.2 (Vienna, Austria; https://www.R-project.org/).
Statistical analyses
The intent-to-treat (ITT) population comprised all registered subjects regardless of whether treatment was administered. Primary efficacy results reported here were, however, based on the response evaluable patients, defined as those who completed the first reassessment CT scan after the first two cycles of study treatment. Interim data were evaluated by the study review committee to monitor efficacy and safety to allow for early stopping due to futility. An optimal two-stage, single arm phase II Simon design was employed with a plan to enrol up to a total of 24 patients, 12 patients in each stage (Minimax design, P0=0.15, P1=0.40; alpha =0.05, power 0.86). The primary endpoint was CB, defined as complete response, partial response, or stable disease for >24 weeks measured by RECIST v1.1. The estimated 6-month CB rate of 15% under the null hypothesis was based upon the published data with 5-fluorouracil and folinic acid or leucovorin from the CONKO-003 trial (40) and PANCREOX (41) randomized trials of second-line therapy in advanced pancreatic cancer following progression on gemcitabine. The CB rate of 40% under the alternative hypothesis was selected for this single-arm, non-randomized trial to provide a sufficiently high anti-tumor activity signal to justify further development. Enrolment was planned to be terminated early if two or fewer patients achieved CB after 12 patients were evaluable for response (stage I). If three or more of 12 patients in stage I achieved CB, then 12 additional response evaluable patients would be enrolled into stage II. The GSK2256098 and trametinib combination would be deemed worthy of further evaluation if seven or more of 24 response-evaluable patients achieved CB at the end of stage II. Descriptive statistics were used to summarize efficacy and safety results. Time-to-event outcomes including PFS and OS were analysed using the Kaplan-Meier method. Two-sided 95% confidence intervals were constructed for outcomes of interest. Data analysis was performed using SPSS (Statistical Package for the Social Sciences) software version 25.
Results
Patients
Between June 2016 and June 2017, a total of 16 patients were enrolled into the study. Nine were female and seven were male. The median age was 64 (range, 50–74) years. All patients had metastatic PDAC. Of 16 enrolled, 14 (88%) had modified FOLFIRINOX as the first line chemotherapy and the remaining two (12%) had gemcitabine/nab-paclitaxel. Eight patients were ECOG PS of 0 at study entry and the remaining eight were PS 1. Patient characteristics are summarized in Table 1.
Table 1. Patient characteristics.
| Characteristics | Values |
|---|---|
| Total number of patients, n [%] | 16 [100] |
| Male | 7 [44] |
| Female | 9 [56] |
| Median age, years (range) | 63 [50–74] |
| ECOG PS, n [%] | |
| 0 | 8 [50] |
| 1 | 8 [50] |
| Histology, n [%] | |
| Adenocarcinoma | 16 [100] |
| Stage, n [%] | |
| IV | 16 [100] |
| Baseline CA19-9, median (IQR) | 3,491 [283–10,000] |
| Previous first line palliative chemotherapy, n [%] | |
| Modified FOLFIRINOX | 14 [88] |
| Gemcitabine/nab-Paclitaxel | 2 [12] |
ECOG, Eastern Cooperative Oncology Group; PS, performance status; IQR, interquartile range.
Efficacy
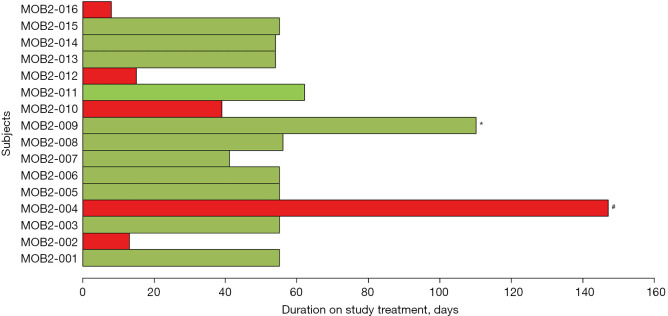
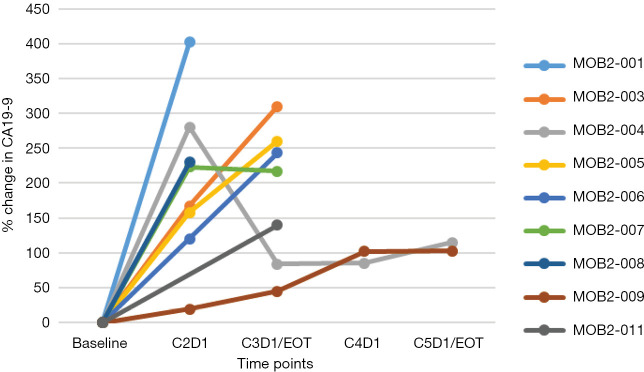
All patients (n=16) who had at least one dose of study treatments were safety evaluable. Five patients were not evaluable for response (four did not have the first reassessment CT scan scheduled at 8 weeks due to rapid clinical deterioration and one patient could not have on treatment contrast-enhanced imaging due to severe contrast allergy). Of 11 response evaluable patients, 10 had progressive disease as best tumor response and one had stable disease that lasted for 4 months. The median cycles of treatment patients received was 2 (range, 1–5). The median duration of treatment for all 16 enrolled patients was 55 (range, 8–147) days (Figure 1). At the data cut off at August 2017, at a median follow-up of 9 months, all but one patient had died. The median PFS was 1.6 (95% CI: 1.5–1.8) months and the median OS was 3.6 (95% CI: 2.7–not reached) months. Of interest, one response inevaluable patient due to the contrast allergy who had rapidly progressed on 1st line FOLFIRINOX chemotherapy achieved clinical stability for 5 months with a >50% decline in serum CA19-9 after 3 months of treatment and symptomatic improvement although there was initial increase in CA19-9. Percent changes in CA-19-9 during study treatment in response evaluable patients were shown in the Figure 2.
Figure 1.
Duration on study treatment for all patients who are treated with at least one dose of study treatment. The cases highlighted in green are response evaluable patients by RECIST v1.1 and those in red were non-evaluable patients. MOB2-009 highlighted with * was the only evaluable patient who achieved stable disease as the best tumor response and all other evaluable patients had progressive disease. MOB2-004 highlighted with # was on study treatment for 147 days with a CA-19-9 response and clinical stability, however, the patient was inevaluable for radiological response evaluation due to severe contrast allergy.
Figure 2.
Percent change in CA19-9 during study treatment in nine response evaluable patients. Of 11 response evaluable patients, one was CA19-9 non-secretor and another did not have complete CA19-9 results. EOT, end of treatment.
Safety
All patients discontinued due to disease progression. No treatment related grade ≥3 AEs were observed. The most common treatment related grade 2 AEs were acneiform rash (19%), diarrhea (13%), nausea (6%), fatigue (6%), proteinuria (6%), paronychia (6%), and retinal detachment (6%). The most common all grade treatment related AEs that occurred with >10% frequency included acneiform rash (75%), diarrhea (38%), nausea (31%), anorexia (13%), dry mouth (13%), limb edema (13%), maculopapular rash (13%), fatigue (13%), and proteinuria (13%). Treatment related AEs were summarized in Table 2.
Table 2. Most common all grade treatment related AEs (≥10% frequency).
| AEs | Grade 1 | Grade 2 | Total |
|---|---|---|---|
| Acneiform rash, n [%] | 9 [56] | 3 [19] | 12 [75] |
| Diarrhea, n [%] | 4 [25] | 2 [13] | 6 [38] |
| Nausea, n [%] | 4 [25] | 1 [6] | 5 [31] |
| Anorexia, n [%] | 2 [13] | 0 | 2 [13] |
| Dry mouth, n [%] | 2 [13] | 0 | 2 [13] |
| Edema limbs, n [%] | 2 [13] | 0 | 2 [13] |
| Rash maculopapular, n [%] | 2 [13] | 0 | 2 [13] |
| Fatigue, n [%] | 1 [6] | 1 [6] | 2 [13] |
| Proteinuria, n [%] | 1 [6] | 1 [6] | 2 [13] |
AEs, adverse events.
Results from correlative studies
Tumor biopsies from were available from 11 patients. Of these 11 paired, 4 only underwent pre-treatment biopsy and did not have an on-treatment biopsy due to clinical deterioration or patient/investigator refusal. Of the 7 paired pre- and post-treatment biopsies, 2 did not contain tumor and 2 additional paired biopsies had very low tumor content that did not yield sufficient RNA for sequencing. Paired pre-treatment and post-treatment RNA sequencing results were available only in three patients. No significant differentially expressed genes were identified between pre-treatment and post-treatment samples including MAPK pathway genes or pathways.
Baseline plasma samples were available for ctDNA analysis in all 11 evaluable patients and serial plasma samples in 10/11 patients. All patients had detectable KRAS mutations in plasma. Five patients had KRAS p.G12D mutation, four had KRAS p.G12V, two had KRAS p.G12R, and one had KRAS p.Q61R (one patient had KRAS p.G12V mutation as well as KRAS p.G12R mutation in plasma). Apart from KRAS and TP53 mutations (7/11 evaluable patients had a TP53 mutation in plasma), we did not observe mutations in other genes at baseline ctDNA analysis. The rise in allelic fraction of KRAS mutations compared to baseline was observed in end of treatment samples in most patients (Figure 3). However, we did not observe consistent correlation between mutant KRAS allelic fraction changes measured at earlier time points (baseline vs. C2D1) and radiology response after 2 cycles of treatment. Four patients whose disease progressed after two cycles of study treatment had a drop in mutant KRAS fraction at the end of first cycle (C2D1 time point) (Figure 3). Interestingly, in the patient described above who was on study treatment for five months because of clinical stability, end of treatment ctDNA analysis revealed a MAP2K1 (MEK1) p.P124L (c.370_371delCCinsTT) mutation that was not present in the pre-treatment ctDNA. This MAP2K1 variant has previously been identified in clones of cancer cells that are resistant to MEK inhibition in melanoma (42).
Figure 3.

Changes in KRAS mutation allelic fraction in plasma during study treatment from 10 evaluable patients who had serial plasma samples available for ctDNA analysis. The red lines represent nine patients who had progressive disease after 2 cycles of study treatment and a marked rise in mutant KRAS allelic fraction was observed in all but one. The green line represents the only patient who had stable disease as the best tumor response and it is noteworthy that mutant KRAS allelic fraction is very low in this patient with no significant change during study treatment. EOT, end of treatment.
Discussion
We tested the efficacy of combined inhibition of MEK and FAK pathways by trametinib and GSK2256098 respectively using a two-stage phase II trial design in unselected advanced PDAC patients in the second line setting. Although KRAS mutation may sensitize MEK inhibitor response (20), as KRAS is mutated in >90% of PDAC, we decided to adopt an unselected approach. All evaluable patients in our study had a KRAS mutation detected in ctDNA. Disappointingly, none of the 11 response-evaluable patients derived CB demonstrating the lack of anti-tumor activity of this combination in advanced PDAC.
The median PFS of evaluable patients in our study was 1.6 months and median OS 3.6 months. Both were inferior to those achieved with chemotherapy in the second line setting for patients with advanced PDAC. For example, PANCREOX randomized phase 3 study that investigated the efficacy of FOLOX6 vs. infusional 5-fluorouracil after failure of gemcitabine based first line chemotherapy demonstrated a median PFS of 3.1 months and a median OS of 6.1 months in patients who received infusional 5-fluorouracil (41). In the CONKO-003 trial, similar median PFS and median OS were reported with oxaliplatin/5-fluorouracil/folinic acid second line treatment in patients who experienced disease progression after gemcitabine monotherapy (40). In contrast to PANCREAOX and CONKO-003 studies, the majority of patients in our study had modified FOLFIRINOX chemotherapy in the first line setting. However, previous retrospective and prospective studies have also demonstrated a median PFS of ~3 months and a median OS of ~6–7 months achieved in patients treated with gemcitabine/nab-paclitaxel chemotherapy in the second line setting after the failure of first line modified FOLFIRINOX (43-46). The median OS of 3.6 months in our study is more akin to the survival achieved with the second line single agent gemcitabine chemotherapy post modified FOLFIRINOX failure (47). Of 16 patients recruited to the study, seven patients had rapid deterioration in clinical status and they all died soon after study completion. Of those seven patients, three came off study after 1 cycle due to rapid clinic progression and another four came off study after 2 cycles of treatment and they all died soon after study completion. Despite they all had good PS at the study entry, their rapid clinical progression reflects a higher disease burden and we believe this could be the main reason for poor median OS observed in our study. We unfortunately did not have comprehensive subsequent treatment information data for the remaining patients. The results from our study again demonstrate the poor survival outcomes and the lack of benefit to molecular targeted therapies in patients with advanced PDAC who progressed after first line chemotherapy and highlights the urgent unmet clinical need of more effective and less toxic therapies in this group of patients.
In this study, a continuous oral daily dosing schedule of trametinib 0.5 mg once daily and GSK2256098 250 mg twice daily in 28-day cycles was used. Although, the doses of trametinib and GSK2256098 used in this study were lower than the monotherapy doses, these doses were based on the results of phase Ib study FAK114746 (33) that showed drug to drug interaction between GSK2256098 and trametinib resulting in trametinib plasma concentrations 2–4 times higher than would be expected. Although intra-tumoral drug concentrations were not evaluated, we do not believe that the lack of therapeutic efficacy observed in our study is attributed to the dosing schedule of GSK2256098 and trametinib. We identified dynamic changes in MAPK/ERK pathway gene sets, however there were no significant differences in gene set expression before and after treatment after multiple testing correction, which may be due to the limited number of evaluable pre- and post-treatment biopsy pairs (n=3). Our results suggest that KRAS mutations may not be oncogenic driver alterations in chemotherapy resistant PDAC. This is supported by the lower rate of anti-tumor activity of KRAS G12C covalent inhibitors in KRAS G12C mutant PDAC compared with KRAS G12C mutant non-small cell lung cancer (48,49). There may also be other intracellular signalling pathways beyond FAK that interact with MEK and drive tumor growth in PDAC.
One of the main limitations of our study is the inability to evaluate potential biomarkers of response as no patient derived CB. One response unevaluable patient by RECIST1.1 due to the contrast allergy who achieved clinical stability for five months with a >50% decline in serum CA19-9 after 3 months of treatment and symptomatic improvement had genomic profiling through the COMPASS trial (NCT02750657) prior to the study entry and was found to have a basal-like tumor by RNA-sequencing and mutant KRAS amplification by DNA whole genome sequencing. Approximately 25% of patients with metastatic PDAC have basal-like tumors and they respond poorly to modified FOLFIRINOX chemotherapy (50). Emerging evidence suggests that these basal-like tumors have high replication stress (51) that is most likely KRAS oncogene driven and perhaps they may be more susceptible to MEK inhibition. However, currently, this assertion is purely speculative and requires further evaluation. Only three patients had RNA sequencing results from paired pre-treatment and post-treatment tumor biopsies and no significant differential expression of genes was observed. This may be due to the small sample size and no conclusion can be made from this analysis.
KRAS mutation was found in plasma of all evaluable patients and TP53 mutation in 7/11 patients by using targeted DNA next generation sequencing. This again highlights the potential clinical utility of liquid biopsy in disease monitoring of patients with advanced PDAC as demonstrated in previous studies (52-54). However, we did not observe consistent correlation between KRAS allelic fraction changes at early time points measured prior to the first radiological disease assessment and radiological response in our patients. This is most likely because we are using a relative measure of KRAS mutant quantity, i.e., allelic fraction, rather than the absolute quantity. The most interesting finding pertinent to ctDNA analyses in our study was finding a resistant MAP2K1 mutation at disease progression in a patient who had clinical stability of 5 months on the study treatment. However, we could not be certain whether this is a mutation acquired during the treatment or already existing mutation that became more abundant in ctDNA at disease progression. Nonetheless, this clearly exemplifies the important and perhaps the most relevant utility of liquid biopsy in elucidating drug resistance mechanisms in individual patients.
Despite the fact that both MAPK and FAK pathways play critical biological roles in PDAC initiation and progression, combined inhibition of these two pathways using MEK inhibitor trametinib and FAK inhibitor GSK2256098 did not show meaningful anti-tumor activity in patients with advanced PDAC in our study. Other novel strategies are needed to effectively target KRAS driven MAPK pathway activation in PDAC and possibly interaction between tumor microenvironment and cancer cells to improve clinical outcomes. It is also of critical importance to identify biomarkers for patient selection for these novel approaches to make progress in this challenging disease.
Supplementary
The article’s supplementary files as
Acknowledgments
LLS and PLB are co-contact principal investigators for NCI UM1 Grant CA186644.
Funding: This study was conducted with the support of the Ontario Institute for Cancer Research through funding provided by the Government of Ontario. This research was supported by the Canadian Cancer Society (grant No. 702807) to PLB. This work was also funded by a Conquer Cancer Foundation of ASCO Career Development Award to PLB. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the American Society of Clinical Oncology® or the Conquer Cancer Foundation. The Advanced Molecular Diagnostics Laboratory receives support from the Princess Margaret Cancer Foundation. Drug supply was provided by GlaxoSmithKline and Novartis.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) with the Good Clinical Practice guidelines of the International Conference on Harmonisation. The study was approved by the Ontario Cancer Research Ethics Board (Project ID 1148) and informed consent was taken from all the patients.
Footnotes
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-86/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-86/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-86/coif). EM received honoraria from Novartis for advisory board and educational activities in melanoma. SW received consulting fees from Amgen and Seattle Genetics and honoraria from GSK, Merk, Eisai, and Pfizer for speaking engagements and participation in advisory boards (these engagements did not relate to pancreatic cancer, nor did they involve the agents studied in this clinical trial). LLS has consulting/advisory arrangements with Merck, Pfizer, Celgene, AstraZeneca, Morphosys, Roche, Oncorus, Symphogen, Seattle Genetics, GlaxoSmithKline, Voronoi, Arvinas, Tessa, Navire, Relay, Rubius, Janpix, Daiichi Sanyko; stock ownership of Agios (spouse); leadership position in Treadwell Therapeutics (spouse); and institution receives clinical trials support from Novartis, Bristol-Myers Squibb, Pfizer, Boerhinger-Ingelheim, GlaxoSmithKline, Roche/Genentech, Karyopharm, AstraZeneca, Merck, Celgene, Astellas, Bayer, Abbvie, Amgen, Symphogen, Intensity Therapeutics, Mirati Therapeutics, Shattucks, Avid; data safety monitor board participation with Mirati Therpeutics. PLB has uncompensated consulting/advisory arrangements with Merck, Lilly, BMS, SeaGen, Gilead, Amgen, Genentech/Roche; institution receives clinical trials support from Novartis, Bristol-Myers Squibb, Pfizer, GlaxoSmithKline, Roche/Genentech, AstraZeneca, Merck, Amgen, Sanofi, Nektar Therapeutics, Bicara Therapeutics, Sanofi, Lilly; data safety monitor board participation with Lilly. The other authors have no conflicts of interest to declare.
References
- 1.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 2.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golan T, Hammel P, Reni M, et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med 2019;381:317-27. 10.1056/NEJMoa1903387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. 10.1200/JCO.2006.07.9525 [DOI] [PubMed] [Google Scholar]
- 5.Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, arvard ed, open-label, phase 3 trial. Lancet 2016;387:545-57. 10.1016/S0140-6736(15)00986-1 [DOI] [PubMed] [Google Scholar]
- 6.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schram AM, O’Reilly EM, O’Kane GM, et al. Efficacy and safety of zenocutuzumab in advanced pancreas cancer and other solid tumors harboring NRG1 fusions. J Clin Oncol 2021;39:3003. 10.1200/JCO.2021.39.15_suppl.3003 [DOI] [Google Scholar]
- 8.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018;378:731-9. 10.1056/NEJMoa1714448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:271-82. 10.1016/S1470-2045(19)30691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canon J, Rex K, Saiki AY, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019;575:217-23. 10.1038/s41586-019-1694-1 [DOI] [PubMed] [Google Scholar]
- 11.Hofmann I, Weiss A, Elain G, et al. K-RAS mutant pancreatic tumors show higher sensitivity to MEK than to PI3K inhibition in vivo. PloS One 2012;7:e44146. 10.1371/journal.pone.0044146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alagesan B, Contino G, Guimaraes AR, et al. Combined MEK and PI3K inhibition in a mouse model of pancreatic cancer. Clin Cancer Res 2015;21:396-404. 10.1158/1078-0432.CCR-14-1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eggers JP, Grandgenett PM, Collisson EC, et al. Cyclin-dependent kinase 5 is amplified and overexpressed in pancreatic cancer and activated by mutant K-Ras. Clin Cancer Res 2011;17:6140-50. 10.1158/1078-0432.CCR-10-2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurzrock R, Patnaik A, Rosenstein L, et al. Phase I dose-escalation of the oral MEK1/2 inhibitor GSK1120212 (GSK212) dosed in combination with the oral AKT inhibitor GSK2141795 (GSK795). J Clin Oncol 2011;29:3085. 10.1200/jco.2011.29.15_suppl.308521383288 [DOI] [Google Scholar]
- 15.Bedard PL, Tabernero J, Janku F, et al. A phase Ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor trametinib (GSK1120212) in patients with selected advanced solid tumors. Clin Cancer Res 2015;21:730-8. 10.1158/1078-0432.CCR-14-1814 [DOI] [PubMed] [Google Scholar]
- 16.Tolcher AW, Baird RD, Patnaik A, et al. A phase I dose-escalation study of oral MK-2206 (allosteric AKT inhibitor) with oral selumetinib (AZD6244; MEK inhibitor) in patients with advanced or metastatic solid tumors. J Clin Oncol 2011;29:3004. 10.1200/jco.2011.29.15_suppl.3004 [DOI] [Google Scholar]
- 17.Shapiro G, LoRusso P, Kwak EL, et al. Clinical combination of the MEK inhibitor GDC-0973 and the PI3K inhibitor GDC-0941: A first-in-human phase Ib study testing daily and intermittent dosing schedules in patients with advanced solid tumors. J Clin Oncol 2011;29:3005. 10.1200/jco.2011.29.15_suppl.3005 [DOI] [Google Scholar]
- 18.Grilley-Olson JE, Bedard PL, Fasolo A, et al. A phase Ib dose-escalation study of the MEK inhibitor trametinib in combination with the PI3K/Mtor inhibitor GSK2126458 in patients with advanced solid tumors. Invest New Drugs 2016;34:740-9. 10.1007/s10637-016-0377-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLean GW, Carragher NO, Avizienyte E, et al. The role of focal-adhesion kinase in cancer – a new therapeutic opportunity. Nat Rev Cancer 2005;5:505-15. 10.1038/nrc1647 [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Adjei AA. The clinical development of MEK inhibitors. Nat Rev Clin Oncol 2014;11:385-400. 10.1038/nrclinonc.2014.83 [DOI] [PubMed] [Google Scholar]
- 21.Kurenova E, Liao J, He DH, et al. The FAK scaffold inhibitor C4 disrupts FAK-VEGFR-3 signaling and inhibits pancreatic cancer growth. Oncotarget 2013;4:1632-46. 10.18632/oncotarget.1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, He DH, Zajac-Kaye M, et al. A small molecule FAK kinase inhibitor, GSK2256098, inhibits growth and survival of pancreatic ductal adenocarcinoma cells. Cell Cycle 2014;13:3143-9. 10.4161/15384101.2014.949550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan Y, Zheng M, Zhong L, et al. A preclinical evaluation of SKLB261, a multikinase inhibitor of EGFR/Src/VEGFR2, as a therapeutic agent against pancreatic cancer. Mol Cancer Ther 2015;14:407-18. 10.1158/1535-7163.MCT-14-0485 [DOI] [PubMed] [Google Scholar]
- 24.Kanteti R, Mirzapoiazova T, Riehm JJ, et al. Focal adhesion kinase a potential therapeutic target for pancreatic cancer and malignant pleural mesothelioma. Cancer Biol Ther 2018;19:316-27. 10.1080/15384047.2017.1416937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol 1999;71:435-78. 10.1016/S0079-6107(98)00052-2 [DOI] [PubMed] [Google Scholar]
- 26.Sawai H, Okada Y, Funahashi H, et al. Activation of focal adhesion kinase enhances the adhesion and invasion of pancreatic cancer cells via extracellular signal-regulated kinase-1/2 signaling pathway activation. Mol Cancer 2005;4:37. 10.1186/1476-4598-4-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuyama K, Doi R, Mori T, et al. Clinical significance of focal adhesion kinase in resectable pancreatic cancer. World J Surg 2006;30:219-26. 10.1007/s00268-005-0165-z [DOI] [PubMed] [Google Scholar]
- 28.Konstantinidou G, Ramadori G, Torti F, et al. RHOA-FAK is a required signaling axis for the maintenance of KRAS-driven lung adenocarcinomas. Cancer Discov 2013;3:444-57. 10.1158/2159-8290.CD-12-0388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapiro IM, Kolev VN, Vidal CM, et al. Merlin deficiency predicts FAK inhibitor sensitivity: a synthetic lethal relationship. Sci Transl Med 2014;6:237ra68. 10.1126/scitranslmed.3008639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012;491:399-405. 10.1038/nature11547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495-501. 10.1038/nature14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cancer Genome Atlas Research Network . Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017;32:185-203.e13. 10.1016/j.ccell.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mak G, Soria JC, Blagden SP, et al. A phase Ib dose-finding, pharmacokinetic study of the focal adhesion kinase inhibitor GSK2256098 and trametinib in patients with advanced solid tumours. Br J Cancer 2019;120:975-81. 10.1038/s41416-019-0452-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connor AA, Denroche RE, Jang GH, et al. Association of Distinct Mutational Signatures With Correlates of Increased Immune Activity in Pancreatic Ductal Adenocarcinoma. JAMA Oncol 2017;3:774-83. 10.1001/jamaoncol.2016.3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29:15-21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trapnell C, Williams BA, Pertea G, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 2010;28:511-5. 10.1038/nbt.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poplin R, Ruano-Rubio V, DePristo MA, et al. Scaling accurate genetic variant discovery to tens of thousands of samples. bioRxiv 2018. doi: . 10.1101/201178 [DOI]
- 38.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545-50. 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15:550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 2014;32:2423-9. 10.1200/JCO.2013.53.6995 [DOI] [PubMed] [Google Scholar]
- 41.Gill S, Ko YJ, Cripps C, et al. PANCREOX: A Randomized Phase III Study of Fluorouracil/Leucovorin With or Without Oxaliplatin for Second-Line Advanced Pancreatic Cancer in Patients Who Have Received Gemcitabine-Based Chemotherapy. J Clin Oncol 2016;34:3914-20. 10.1200/JCO.2016.68.5776 [DOI] [PubMed] [Google Scholar]
- 42.Emery CM, Vijayendran KG, Zipser MC, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A 2009;106:20411-6. 10.1073/pnas.0905833106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Hochster H, Stein S, et al. Gemcitabine plus nab-paclitaxel for advanced pancreatic cancer after first-line FOLFIRINOX: single institution retrospective review of efficacy and toxicity. Exp Hematol Oncol 2015;4:29. 10.1186/s40164-015-0025-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertocchi P, Abeni C, Meriggi F, et al. Gemcitabine Plus Nab-Paclitaxel as Second-Line and Beyond Treatment for Metastatic Pancreatic Cancer: a Single Institution Retrospective Analysis. Rev Recent Clin Trials 2015;10:142-5. 10.2174/1574887110666150417115303 [DOI] [PubMed] [Google Scholar]
- 45.Nguyen KT, Kalyan A, Beasley HS, et al. Gemcitabine/nab-paclitaxel as second-line therapy following FOLFIRINOX in metastatic/advanced pancreatic cancer-retrospective analysis of response. J Gastrointest Oncol 2017;8:556-65. 10.21037/jgo.2017.01.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dadi N, Stanley M, Shahda S, et al. Impact of Nab-Paclitaxel-based Second-line Chemotherapy in Metastatic Pancreatic Cancer. Anticancer Res 2017;37:5533-9. [DOI] [PubMed] [Google Scholar]
- 47.Tsang ES, Wong HL, Wang Y, et al. Outcomes and Characteristics of Patients Receiving Second-line Therapy for Advanced Pancreatic Cancer. Am J Clin Oncol 2019;42:196-201. 10.1097/COC.0000000000000500 [DOI] [PubMed] [Google Scholar]
- 48.Strickler JH, Fakih M, Price TJ, et al. AMG 510, a novel small molecule inhibitor of KRAS(G12C), for patients (pts) with advanced gastrointestinal (GI) cancers: Results from the CodeBreaK100 phase I trial. Ann Oncol 2020;31:S1273-86. 10.1016/j.annonc.2020.10.103 [DOI] [Google Scholar]
- 49.Hong DS, Fakih MG, Strickler JH, et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med 2020;383:1207-17. 10.1056/NEJMoa1917239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aung KL, Fischer SE, Denroche RE, et al. Genomics-Driven Precision Medicine for Advanced Pancreatic Cancer: Early Results from the COMPASS Trial. Clin Cancer Res 2018;24:1344-54. 10.1158/1078-0432.CCR-17-2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dreyer SB, Upstill-Goddard R, Paulus-Hock V, et al. Targeting DNA Damage Response and Replication Stress in Pancreatic Cancer. Gastroenterology 2021;160:362-77.e13. 10.1053/j.gastro.2020.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takai E, Totoki Y, Nakamura H, et al. Clinical utility of circulating tumor DNA for molecular assessment in pancreatic cancer. Sci Rep 2015;5:18425. 10.1038/srep18425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riva F, Dronov OI, Khomenko DI, et al. Clinical applications of circulating tumor DNA and circulating tumor cells in pancreatic cancer. Mol Oncol 2016;10:481-93. 10.1016/j.molonc.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perets R, Greenberg O, Shentzer T, et al. Mutant KRAS Circulating Tumor DNA Is an Accurate Tool for Pancreatic Cancer Monitoring. Oncologist 2018;23:566-72. 10.1634/theoncologist.2017-0467 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as