Highlights
-
•
Impaired phonological working memory is common in children with autism (ASD).
-
•
Children with and without ASD repeated nonwords with 2 to 5 syllables in fMRI.
-
•
The ASD group showed reduced activation in supplemental motor area (SMA).
-
•
Nonword length was classified inaccurately in speech production regions in ASD.
-
•
No functional alteration was found in speech motor regions for reading disability.
Keywords: Autism spectrum disorder, Phonological working memory, Nonword repetition, Motor cortex, Speech production
Abstract
Nonword repetition, a common clinical measure of phonological working memory, involves component processes of speech perception, working memory, and speech production. Autistic children often show behavioral challenges in nonword repetition, as do many individuals with communication disorders. It is unknown which subprocesses of phonological working memory are vulnerable in autistic individuals, and whether the same brain processes underlie the transdiagnostic difficulty with nonword repetition. We used functional magnetic resonance imaging (fMRI) to investigate the brain bases for nonword repetition challenges in autism. We compared activation during nonword repetition in functional brain networks subserving speech perception, working memory, and speech production between neurotypical and autistic children. Autistic children performed worse than neurotypical children on nonword repetition and had reduced activation in response to increasing phonological working memory load in the supplementary motor area. Multivoxel pattern analysis within the speech production network classified shorter vs longer nonword-repetition trials less accurately for autistic than neurotypical children. These speech production motor-specific differences were not observed in a group of children with reading disability who had similarly reduced nonword repetition behavior. These findings suggest that atypical function in speech production brain regions may contribute to nonword repetition difficulties in autism.
1. Introduction
During language development, children have a remarkable ability to rapidly learn to recognize and produce a multitude of new words (Bloom, 1973). Transforming language heard into language said depends on a sophisticated orchestration among the cognitive and neural systems that support speech perception, working memory, and speech production. The ability to briefly hold speech information in mind during this transformation is known as phonological working memory and is thought to play a key role in language development (Baddeley, Gathercole, & Papagno, 1998). In turn, this faculty is thought to be disrupted in many developmental communication disorders (e.g., Williams et al., 2013, Archibald, 2017, Gray et al., 2019).
Clinicians and researchers frequently use nonword repetition as a measure of phonological working memory, which has high sensitivity for developmental language disorders (Archibald and Joanisse, 2009, Estes et al., 2007, Weismer et al., 2000) and reading disorders (Ehrhorn et al., 2021, Melby-Lervag and Lervag, 2012). Nonword repetition is also atypical in many autistic1 children (Whitehouse and Bishop, 2008, Gabig, 2008, Williams et al., 2013, Nadig and Mulligan, 2017). It is currently unknown why children with autism spectrum disorder (ASD) have difficulty with nonword repetition, particularly given the heterogenous language profiles of autistic individuals (Kjelgaard & Tager-Flusberg, 2001). While nonword repetition performance is classically operationalized as an assessment of phonological working memory, this multifaceted behavior involves several subprocesses related to speech perception (e.g., encoding), short-term working memory (e.g., storage), and speech production (e.g., planning and articulation) (Hickok, 2009, Majerus, 2013). During the task of nonword repetition, it is likely that a distributed set of brain networks associated with each of these cognitive processes is engaged (Acheson et al., 2011, Fiez, 2016, Indefrey and Levelt, 2004, Scott and Perrachione, 2019). As such, each of these process-related networks may operate differently in autistic children, relating to different possible sources for the nonword repetition difficulties in this population. Here, we aimed to identify how functional response in each of these three brain networks differ in autism during nonword repetition, thereby shedding light on the neural bases of phonological working memory challenges in autistic individuals.
Phonological working memory is related to a wide range of language-related skills including vocabulary acquisition, sentence and discourse processing, and reading development (Adams and Gathercole, 1996, Baddeley et al., 1998, Gathercole et al., 1997, Gathercole, 2006). Several behavioral studies have found reduced phonological working memory in autistic individuals regardless of articulation skills (Gabig, 2008, Habib et al., 2019, Macizo et al., 2016, Williams et al., 2006). Further, reduced phonological working memory has been identified in autistic children who have lower scores on assessments of vocabulary, higher-order syntax, and semantics (Kjelgaard & Tager-Flusberg, 2001). Phonological working memory challenges are also seen in other populations with language difficulties, including dyslexia, dysfluency (stuttering), Down syndrome, and developmental language disorders (Estes et al., 2007, Peter et al., 2011, Melby-Lervag and Lervag, 2012, Ehrhorn et al., 2021). However, the underlying source of these difficulties may differ across diagnoses. For example, the types of errors made by autistic children during nonword repetition tend to differ from those made by children with specific language impairment (or developmental language disorders) (Nadig and Mulligan, 2017, Williams et al., 2013). These studies suggest that, while reduced phonological working memory in autistic children may have widespread consequences in language development, the source of their difficulties may differ from those of children with other developmental communication disorders.
Despite the importance of phonological working memory in language development, and the clinical value of its measurement via nonword repetition (Archibald and Gathercole, 2007, Dollaghan and Campbell, 1998), little is known about how phonological working memory is disrupted in autistic children, or why their difficulties with nonword repetition may manifest differently from those in other developmental communication disorders. Disentangling the sources of children’s nonword repetition difficulties via behavioral assays has proved challenging, as behavior alone cannot distinguish between difficulties perceiving, remembering, or reproducing the target speech. Neurally, however, the brain networks that support speech perception, working memory, and speech production are substantially distinct (Hickok and Poeppel, 2007), such that localized differences in brain activation may help inform which specific processes are disrupted. In this study, we investigated activation within each of these three brain networks (speech perception, working memory, speech production) to improve our understanding of their relationship to nonword repetition difficulties in autism.
Our primary aim in this research was to identify functional brain differences during nonword repetition in autistic versus neurotypical (NT) children. Previous research has documented sensory, cognitive, and motor differences in autism, such that each of the three primary sub-components of nonword repetition may be the source of impaired phonological working memory: Correspondingly, (1) activation differences in the speech perception network during nonword repetition would be consistent with evidence for perception-based differences in autism (e.g., Frith, 1989, Mottron and Burack, 2001, Pellicano and Burr, 2012); (2) activation differences in the working memory network during nonword repetition would be in-line with evidence for executive functioning differences in autism (e.g., Ozonoff et al., 1991, Joseph and Tager-Flusberg, 2004, Pellicano, 2012, Leung et al., 2016); and (3) activation differences in the speech production network during nonword repetition would be consistent with evidence for motor-based differences in autism (e.g., Whyatt and Craig, 2013, Bhat et al., 2011). Upon identifying brain regions of difference between the autistic and NT groups, we then tested the specificity of any difference in a group of non-autistic children with reading disability who also exhibited impaired nonword repetition.
1.1. Neural systems supporting nonword repetition
1.1.1. Encoding: The speech perception network
Speech perception involves encoding acoustic speech signals and mapping them onto abstract linguistic representations (Samuel, 2011, Poeppel, 2015). Speech perception tasks engage a network of widely distributed brain regions, including primary and association auditory areas (e.g., bilateral superior temporal gyrus (STG) and superior temporal sulcus (STS)) involved in spectrotemporal and phonological processing, respectively. Sensorimotor transformations are believed to occur via a posterior/dorsal auditory stream, encompassing planum temporale and parietal operculum, with articulatory representations and actions encoded in left inferior frontal gyrus and ventral (pre)motor cortex, and in parietal operculum (Hickok and Poeppel, 2007).
Various sensory-based hypotheses of autism have posited that autistic individuals may make enhanced use of low-level sensory features at the expense of higher-level gestalt representations (Mottron and Burack, 2001, Happe and Frith, 2006). Indeed, some behavioral evidence has borne out this hypothesis in the domain of speech and language. Some autistic individuals pay greater attention to lower-level acoustic cues at the cost of higher-level linguistic information (Järvinen-Pasley et al., 2008, Soulières et al., 2007). Further, a functional magnetic resonance imaging (fMRI) meta-analysis study of the brain bases of speech perception revealed that activation in temporal-lobe language areas (e.g., STG), was common to both autistic and NT groups, but activation in frontal language areas (e.g., superior frontal gyrus, left medial frontal gyrus, and right inferior frontal gyrus) was found exclusively in the NT group (Tryfon et al., 2018).
1.1.2. Storage: The working memory network
Working memory refers to the temporary storage and manipulation of mental representations of information (e.g., speech sounds and sequences) on the timescale of seconds. In their influential phonological loop theory, Baddeley and Hitch (1974) proposed that short-term storage of verbal information occurs through interactions between a phonological buffer and subvocal rehearsal process (Baddeley, 1992, Baddeley and Hitch, 1974, Baddeley, 2003, Hitch and Baddeley, 1976). However, this psychological model does not posit specific brain regions associated with it. Classical fMRI and positron emission tomography (PET) studies using working memory tasks from cognitive psychology (e.g., digit span, n-back) identified a network of frontal and parietal regions as important for working memory (e.g., Buchsbaum and D’Esposito, 2019). However, studies that have used tasks focused on immediate repetition of nonwords tend to find a network comprising of mostly speech perception and speech production regions, such as STG, IFG, supplementary motor area (SMA), posterior middle frontal gyrus, and ventral motor cortex (Scott and Perrachione, 2019, Perrachione et al., 2017, Strand et al., 2008). To this end, it remains an open question whether and how nonword repetition (and therefore phonological working memory) requires the participation of domain-general (or multiple demand) working memory areas such as dorsolateral prefrontal cortex and parietal lobe (Jacquemot and Scott, 2006, Majerus, 2013, Hickok, 2009, Fiez, 2016.) Furthermore, it is unknown whether nonword repetition difficulties in developmental communication disorders like ASD place differential demands on speech vs domain-general (multiple demand) working memory networks for performing this task.
There is evidence to suggest that reduced executive functioning – including working memory – is associated with many challenges observed in autistic individuals (e.g., Pellicano, 2012, Leung et al., 2016). Meta-cognitive skills such as working memory and planning have been shown to predict social skills in autistic but not NT children (Leung et al., 2016). Behavioral research investigating spatial, verbal, and nonverbal working memory in autistic individuals has shown functional challenges, including reduced accuracy and slower performance (for a meta-analysis, see: Wang et al., 2017, Rabiee et al., 2018, Sachse et al., 2013, Cui et al., 2010). Conflicting research, however, has shown intact verbal working memory as measured by n-back letter tasks (Williams et al., 2005) and intact visual working memory as measured by a visual change detection task (Lynn et al., 2022). Multiple studies have reported reduced n-back performance and altered patterns of brain activation in autistic children and adults (Vogan et al., 2018, Yeung et al., 2019, Koshino et al., 2005, Barendse et al., 2013).
1.1.3. Planning and articulation: The speech production network
Speech production involves the planning and execution of articulatory movements, as well as the continuous monitoring of the auditory and somatosensory experience of one’s own speech. The computational and neuroanatomical Directions into Velocities of Articulators (DIVA) model provides a unified theory of speech production and implicates a network of cortical and subcortical regions, including motor regions (primary motor cortex, supplementary motor area (SMA), basal ganglia) and sensory regions (e.g., STG) (Tourville & Guenther, 2011). Neuroimaging studies have shown bilateral activation of motor cortex, premotor cortex, somatosensory cortex, and SMA to be most reliably associated with overt articulation, with a rough somatotopic organization of articulators in the motor cortex (Conant and Chang, 2013, Long et al., 2016, Guenther, 2016). The primary sensory regions play a key role in the auditory monitoring of one’s speech and articulation by mapping auditory targets, auditory states, and errors.
Despite the diagnostic emphasis on social and communication skills in autism, motor impairments have been increasingly identified as an underdiagnosed, yet clinically meaningful, feature of autism (Craig et al., 2021, Whyatt and Craig, 2013, Zampella et al., 2021). Motor delays and atypical movement development (e.g., stereotyped movement) have been identified as some of the earliest signs of autism, which often emerge even before the classical social communication differences (West, 2019, Posar and Visconti, 2022). Speech and language (expressive and receptive) abilities in autistic individuals have been shown to be correlated with oromotor, visuomotor, manual motor, fine motor, and gross motor skills (Gernsbacher et al., 2008, Bhat, 2021, Bal et al., 2019, Mody et al., 2017, Bedford et al., 2016). Behaviorally, studies of the motor speech profiles of autistic individuals have identified atypical speech production execution, including difficulty with complex syllable production tasks (Adams, 1998) and imprecise articulation (Wynn et al., 2022). Despite the mounting evidence suggesting motor differences in autism, including fine motor control related to speech production, the research that investigates the behavioral and brain bases of speech production in autism remains highly limited. A magnetoencephalography (MEG) study examining neural dynamics during phonemic production tasks revealed significant differences between ASD and NT in magnitude and latency of activation in several brain regions including primary motor cortex, motor planning areas (e.g., SMA), sensorimotor integration areas (e.g., orbitofrontal cortex), and the dorsolateral prefrontal cortex (Pang et al., 2016). A diffusor tensor imaging study in adults with autism revealed a weaker connection between the ventral premotor cortex and the SMA (Peeva et al., 2013). An fMRI study of activation during a speech production task (picture naming) showed no differences in brain activation between NT and autistic children, but increased intra-subject variability in brain activation in the autistic group (Heller Murray et al., 2022). Taken together, these outcomes suggest brain and behavioral differences between autistic and NT groups during basic and complex oro-motor and phonemic tasks.
1.2. Specificity of phonological working memory difficulties in autism
An important goal in studying the brain bases of nonword repetition is to better understand how the functional substrates of phonological working memory impairments are shared versus distinct across autism and other neurodevelopmental disorders (cf. Lu et al., 2016). For example, individuals with reading disabilities often demonstrate behavioral difficulties with nonword repetition (Baird et al., 2011), as well as its associated processes (e.g., speech perception: Manis et al., 1997, Joanisse et al., 2000, Boets et al., 2011; working memory: Smith-Spark et al., 2016, Jeffries and Everatt, 2004; speech production: Catts, 1989, Carroll et al., 2014, Lambrecht Smith et al., 2010). It is possible that any brain differences observed during nonword repetition in autism are not specific to autism but rather underlie nonword repetition and/or communication challenges more broadly. A previous neuroanatomical study found that shared atypicality in the white-matter microstructural connectivity was related to the degree of phonological working memory impairment for both children with reading disabilities (RD) and those with autism (Lu et al., 2016). Testing whether non-autistic populations with parallel challenges in nonword repetition show similar differences in brain functions as autistic children will shed light on the debate about the relationship between language or reading impairment and autism.
1.3. Current study
The primary aim of this study was to identify the brain bases of phonological working memory impairments in autism. Children with and without autism performed a nonword repetition task in the scanner while we measured their neural activation using fMRI. With complementary univariate and multivariate pattern analyses, we examined whether the atypical phonological working memory abilities seen in autism are associated with functional differences in each of the three key brain networks thought to be involved in nonword repetition: speech perception, working memory, and speech production. We also used targeted analyses to examine whether children with reading disability also demonstrate the same brain differences identified in the comparison between autistic and NT children.
2. Materials and methods
2.1. Participants
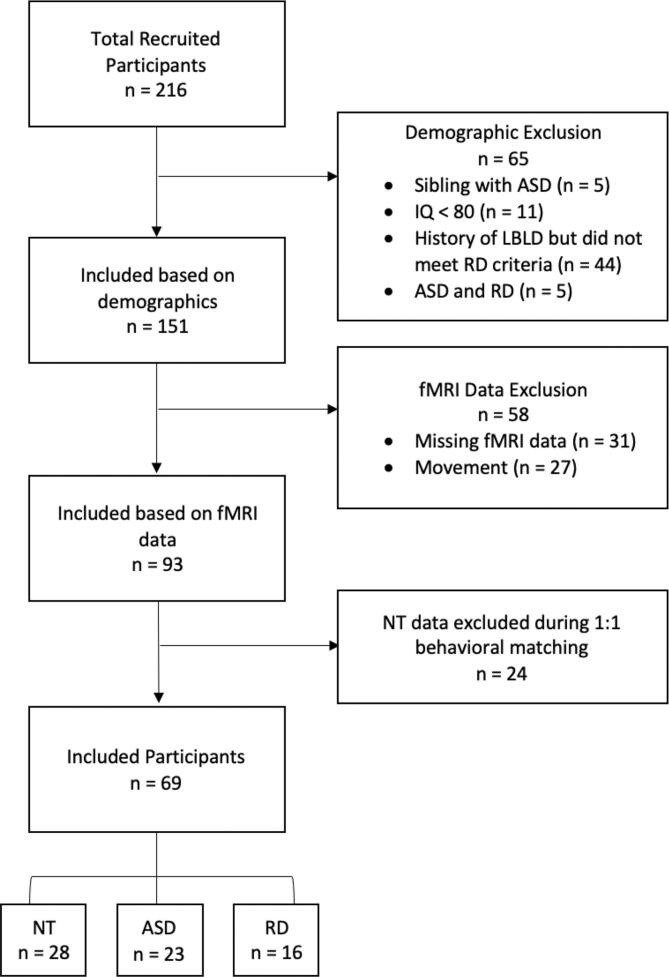
Children (age 5 to 18 years) with and without autism were recruited from the greater Boston area of the United States for this study. From an initial sample of n = 216 participants, a total of n = 69 children met criteria for inclusion in the neurotypical (NT, n = 28) or autism (n = 23) groups. We also included a control group of participants with a reading disability (RD, n = 16) for targeted secondary analyses. Inclusion criteria for all participants required them to be native speakers of American English, right-handed, and born after 32 weeks gestational age. All included participants had parent-reported normal hearing and had nonverbal cognitive ability within normal limits, as measured by nonverbal IQ scores of ≥ 80 on the Kaufman Brief Intelligence Test (KBIT; Kaufman & Kaufman, 2004). Included participants had no history of head injury, comorbid psychiatric or neurological conditions, or any genetic disorders associated with autism (e.g., Fragile X syndrome). Written informed consent was obtained from a guardian of all participants. Children provided informed written assent and received monetary compensation for their time. All procedures were approved and overseen by the Committee on the Use of Humans as Experimental Subjects (COUHES) at MIT and conducted in accordance with the Declaration of Helsinki.
2.1.1. Behavioral assessments
Participants completed a brief battery of standardized clinical measures of cognitive, language, and reading ability, including the Kaufman Brief Intelligence Test (KBIT) Expressive Vocabulary, Definitions, and Matrix subtests (Kaufman & Kaufman, 2004); the Clinical Evaluation of Language Fundamentals (CELF-4) core language subtests (Semel et al., 2004); the Comprehensive Test of Phonological Processing (CTOPP) Elision, Blending Words, and Nonword Repetition subtests (Bruno & Walker, 1999); the Children’s Test of Nonword Repetition (CNRep) (Gathercole et al., 1994); the Woodcock Reading Mastery Test – Revised Normative Update (WRMT) Word Identification (WI) and Word Attack (WA) subtests (Woodcock, 2011); and the Test of Word Reading Efficiency (TOWRE-2) Sight Word Efficiency and Phonemic Decoding subtests (Tarar et al., 2015, Torgesen et al., 2012). The CTOPP and CNRep are tests of phonological working memory and are typically used to identify children at-risk for developmental language disorders (Gathercole, 1995, Archibald and Joanisse, 2009).
2.1.2. Diagnostic confirmation
Participants were included in the autism group if they had a clinical diagnosis of autism, which was also confirmed by trained research staff using an Autism Diagnostic Observation Schedule (ADOS/ADOS-2) Module 3 or 4 (Lord et al., 2000, Lord et al., 2012). A score of 7 or higher was deemed consistent with an autism diagnosis. To quantify the degree of the autism symptomatology, we converted participants’ ADOS scores to autism calibrated severity scores (CSS) ranging from 1 to 10 (Gotham et al., 2006, Hus and Lord, 2014). Participants in the NT group scored within normal limits on the ADOS-2 and had no first-degree relatives with autism or reading disabilities. Participants in the RD group had standard scores below 90 (below the 25th percentile) on at least two of the four subtests of timed or untimed word or nonword reading from the WRMT and TOWRE. Participants in the RD group also scored within normal limits on the ADOS (Table 1). See Supplementary Methods S.1.1.1 and Supplementary Table 1 for more information on ADOS diagnostic confirmation and ADOS scores.
Table 1.
Phenotypic cognitive, behavioral, language, and reading characteristics in the primary analysis group (NT and ASD), as well as for the RD groups. All p-values in this table are from one-tailed t-tests (ASD < NT, RD < NT, and RD < ASD for age and all assessments, except for ADOS where ASD > NT and ASD > RD).
| NT |
ASD |
ASD vs NT |
RD |
RD vs NT |
RD vs ASD |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 28 (8F) |
n = 23 (5F) |
n = 16 (7F) |
||||||||
| Mean | SD | Mean | SD | p-value | Mean | SD | p-value | p-value | ||
| Demographics | Age | 11.9 | 3.2 | 12.2 | 3.4 | 0.389 | 11.5 | 3.1 | 0.345 | 0.266 |
| IQ | KBIT | 112.8 | 14.2 | 110.6 | 16.2 | 0.302 | 104.3 | 12.3 | 0.022 | 0.088 |
| Language | CELF (CLS) | 116.2 | 9.8 | 103.2 | 19.2 | 0.004 | 97.3 | 17.9 | 0.001 | 0.166 |
| NWR | In-scanner NWR | 87.7 | 8.8 | 75.0 | 22.7 | <0.001 | 73.8 | 21.2 | 0.000 | 0.262 |
| CTOPP (NWR) | 10.0 | 2.7 | 7.7 | 2.1 | 0.001 | 8.4 | 2.7 | 0.035 | 0.199 | |
| CNRep (Raw) | 34.7 | 3.2 | 30.1 | 6.4 | 0.002 | 30 | 6.2 | 0.005 | 0.475 | |
| Reading | TOWRE (SWE) | 108.3 | 9.9 | 97.5 | 10.5 | 0.001 | 83.1 | 7.2 | 0.000 | <0.001 |
| TOWRE (PDE) | 110.3 | 13.3 | 101.7 | 12.4 | 0.012 | 83.7 | 5.0 | 0.000 | <0.001 | |
| WRMT (WI) | 113.0 | 9.8 | 102.7 | 15.0 | 0.004 | 88.1 | 9.7 | 0.000 | 0.001 | |
| WRMT (WA) | 108.7 | 12.0 | 101.9 | 14.1 | 0.038 | 91.1 | 6.9 | 0.000 | 0.002 | |
| WJ | 114.3 | 11.6 | 104.6 | 17.2 | 0.015 | 83.7 | 8.7 | 0.000 | <0.001 | |
| Autism | ADOS CSS | 1.19 | 1.28 | 10.18 | 4.48 | <0.001 | 2.08 | 2.64 | 0.05 | <0.001 |
Note. NT = neurotypical; ASD = autism spectrum disorder; F = female; RD = reading disability; CELF (CLS) = Core Language Score (Clinical Evaluation of Language Fundamentals, Fourth edition); NWR = nonword repetition; CNRep = Children's Test of Nonword Repetition; CTOPP = Comprehensive Test of Phonological Processing 2; KBIT = Kaufman Brief Intelligence Test 2 (Matrix subtest); WRMT = Woodcock Reading Mastery Tests; WI = Word Identification; WA = Word Attack; TOWRE = Test of Word Reading Efficiency; SWE = Sight Word Efficiency; PDE = Phonemic Decoding Efficiency. WJ = Woodcock-Johnson III Tests of Achievement (Sentence Reading Fluency subtest); ADOS = Autism Diagnostic Observation Schedule; CSS = Calibrated Severity Score (averaged across ADOS Modules 3 and 4).
2.1.3. Included matched sample
After screening for demographic inclusion criteria (see 2.1), data quality (see MRI data pre-processing, below), and matching for demographic characteristics, 69 children were included in this study. See Fig. 1 for a breakdown of reasons for exclusion. All matching was done using the optmatch package in R (Hansen and Klopfer, 2006), and a summary of the demographic, cognitive, language, and reading characteristics of all participants is shown in Table 1. Supplementary Table 2 provides a breakdown of the racial and ethnic makeup of the participants. In matching all participants, we used the pair matching function of the optmatch tool in R. The pair matching function works by minimizing the mean paired Euclidean distance between designated variables (i.e., scores). To verify our matching, we used the MatchIt package (Ho et al., 2011) in R to calculate propensity scores for our included groups. All group comparisons had variance ratios within an acceptable range (0.5–2), as established by the literature (Kover and Atwood, 2013). The NT vs ASD groups had a variance ratio of 0.83 for the variables of age, sex, and IQ. The NT vs RD groups had a variance ratio of 1.71 for the variables of age, sex, and IQ. We also conducted supplementary analyses with an additional control group of ASD and NT children matched on the Core Language Score of the CELF. The language-matched sample had a variance ratio of 1.56 for the variables of age, sex, IQ, and CELF core language score. See Supplementary Methods 1.1.2 and Supplementary Table 3 for additional information on the language-matched groups. Hypotheses related to our primary aim (neurofunctional differences during nonword repetition) were tested using groups of autistic (n = 23) and NT (n = 28) children who were matched by age, sex, and non-verbal IQ. Hypotheses related to our secondary aim (altered neural responses’ specificity to autism) were tested by comparing the NT and ASD groups to a third group of children with reading disability (RD; n = 16) and by comparing language-matched autistic vs NT subgroups.
Fig. 1.
Flowchart depicting exclusionary criteria and numbers of included participants in each group.
Note. ASD = autism spectrum disorder; IQ = intelligence quotient; LBLD = language based learning disorder; RD = reading disability; fMRI = functional magnetic resonance imaging; NT = neurotypical.
2.2. Stimuli
We used the nonword (i.e., pseudoword) stimuli that were previously generated and described (Perrachione et al., 2017). There were 96 nonwords total, balanced across syllable lengths (2, 3, 4, and 5 syllables), with 24 nonwords per syllable-length condition. The positional phoneme and biphone phonotactic probabilities of the stimuli did not vary significantly across syllable length (both F(1, 133) < 0.23, p > 0.61). The nonwords were generated to closely parallel the structural and statistical properties of real English words. A full list of the nonword stimuli can be found in Appendix A.
Recordings were made from the speech of a female native speaker of standard American English. Before making the audio recordings of the nonword stimuli, the speaker was extensively familiarized with the nonwords to ensure natural, correct pronunciation. The speaker read the stimuli in citation format (individually and in isolation) and the speech was digitally recorded using a SM58 microphone (Shure Inc., Niles, IL) and Edirol UA-25EX sound card (Roland Corp., Los Angeles, CA), sampling at 44.1 kHz (as described by Perrachione et al., 2017). Each token was normalized for root-mean-square amplitude to 70 dB using Praat (Boersma, 2001).
2.3. Experimental session
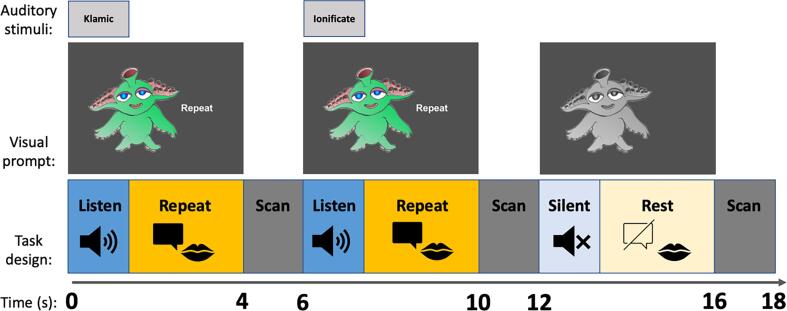
This experiment was part of a larger project that included other fMRI and MRI acquisition protocols. Participants first engaged in a practice scan to become familiarized with the equipment and behavioral tasks (see Supplementary Methods S1.2 for more information). Children completed the nonword repetition task during a sparse-sampling fMRI paradigm, which allowed the auditory stimuli to be presented, and verbal responses to be uttered, during silent periods in between functional volume acquisitions. Children were instructed to listen to the “alien language” and repeat the words. An image of a colorful alien was used as a visual prompt for the child to repeat the word during the repeating trials, and a gray alien image signaled the child to remain silent during the resting trials (Fig. 2). The participants did not hear or say anything during the rest trials.
Fig. 2.
Task design and accompanying visual prompt.
Auditory stimuli were presented binaurally using Sensimetrics S-14 earphones (Sensimetrics Corporation, Woburn, MA) at a comfortable listening level. Stimulus presentation was controlled by the software PsychoPy (Peirce et al., 2019). The task was conducted in three runs. Each run included 32 trials, including 8 trials of each syllable load (two, three, four, five syllables) and 8 trials of rest. Within each run, the trials were presented in pairs of each condition (e.g., the participant completed two trials of four syllables, then two trials of two syllables, then two trials of rest). These condition-pairs were pseudo-randomized in each run, such that all participants were presented with the stimuli and rest conditions in the same order, by run. Each trial lasted 6 s and consisted of 4 s of silence, during which the auditory stimulus was presented, and overt verbal repetition recorded, followed by a 2 s fMRI volume acquisition (Fig. 2). Rest trials also lasted 6 s, during which the participant saw the grayed alien image and remained silent.
2.4. Neuroimaging data collection and analysis
2.4.1. Scanning parameters
Data were acquired on a Siemens Trio 3T scanner (Siemens AG, Berlin and Munich, Germany) with a 32-channel phased array head coil. A whole-head, high-resolution T1-weighted, multi-echo magnetization-prepared rapid gradient-echo (ME-MPRAGE) anatomical volume was obtained (acquisition parameters: repetition time [TR] = 2530 ms, echo time [TE] = {1.64, 3.44, 5.24, 7.04 ms}, flip angle = 7.0°, inversion time [TI] = 1400 ms, voxel resolution = 1.0 mm3, field of view [FOV] = 220, 220 mm, 176 sagittal slices.
Three functional runs containing 108 volumes each were collected using sparse-sampled T2*-weighted gradient-echo planar imaging (EPI) scans (acquisition parameters: TR = 6000 ms, acquisition time [TA] = 2000 ms, TE = 30 ms, flip angle = 90°, voxel resolution = 3.0 mm3, FOV = 192×192 mm, and 32 transverse slices acquired parallel to the anterior commissure–posterior commissure [AC-PC] plane, providing whole-brain coverage). Each functional run was preceded by two additional TRs during which no data were recorded to allow for stabilization of longitudinal magnetization. Each run was 4 min, 12 s long. Sparse-sampling (Hall et al., 1999) was chosen to present auditory stimuli in silence, as this is the case when nonword repetition is administered clinically. The sparse scanning protocol allows the stimuli to be presented, and overt verbal responses to occur, during silent intervals between EPI volume acquisitions, precluding motion artifacts from children’s head motion, as well as from structural changes in oral and nasal cavity, during speech production. The reduction in acoustic stimulation during scanning improves not only dynamic range in auditory brain responses (Gaab et al., 2007), but has been shown to reduce spurious activation in executive regions related to the cognitive demands of noise exclusion (Peelle, 2014, Gaab et al., 2008). A sparse-sampling rate of TR = 6 s, with four seconds of sparse delay, was selected to allow sufficient time for children to listen to and repeat the nonwords in silence while still sampling BOLD signal at the peak of the hemodynamic response at each trial (Perrachione & Ghosh, 2013). The delay in volume acquisition leads to substantially improved contrast-to-noise ratio (CNR) for each sparse volume relative to analogous continuously-sample EPI volume acquisition. Furthermore, we used an optimized procedure for modeling sparse data using convolved hemodynamic response function (HRF), which allows detection of neural activation to events across discontinuous volume acquisitions (see Perrachione & Ghosh, 2013, for evidence that even slow sparse designs can capture event-related functional activation through HRF convolution).
2.4.2. MRI data pre-processing
The fMRI DICOM data were converted to NIFTI files following Brain Imaging Data Structure (BIDS) formatting using HeuDiConv (https://github.com/nipy/heudiconv; Gorgolewski et al., 2017, Yarkoni et al., 2019). Anatomical and functional images were preprocessed using fMRIPrep (v.1.4.1), including T1 correction, brain extraction, normalization to the fsaverage5 template, tissue segmentation, and motion correction procedures [(Esteban et al., 2019); fMRIPrep Available from: https://doi.org/10.5281/zenodo.852659, RRID:SCR_016216], a Nipype-based tool (Gorgolewski et al., 2017); Nipype available from: https://doi.org/10.5281/zenodo.581704, RRID:SCR_002502). Slice timing correction was not applied due to the sparse data collection method that was used. See Supplementary Methods (S1.3.1) for additional details on the use of fMRIPrep.
Framewise displacement (FD; Power et al., 2014), together with other commonly used confounds, such as motion parameters, was calculated for each functional run using the implementation in Nipype. FD and spatial s.d. of the data after temporal differencing (known as ‘DVARS’) are calculated for each functional run, both with their implementations in Nipype (Esteban et al., 2019). Volumes with an FD ≥ 2 mm were regressed out from the analysis (Siegel et al., 2014) and participants with ≥ 33 % of motion outlier volumes were removed from the analyses (Supplementary Table 4 for the group-average number of removed volumes and Fig. 1 for the total number of removed participants due to motion). The average number of removed volumes and average FD by group are reported in Supplementary Table 4. The two groups were not statistically different on either the number of outliers or motion parameters (Supplementary Table 4).
2.4.3. fMRI analyses
Analyses and hypotheses for this project were pre-registered using Open Science Framework (https://osf.io/xerpc).
2.4.3.1. Networks of interest
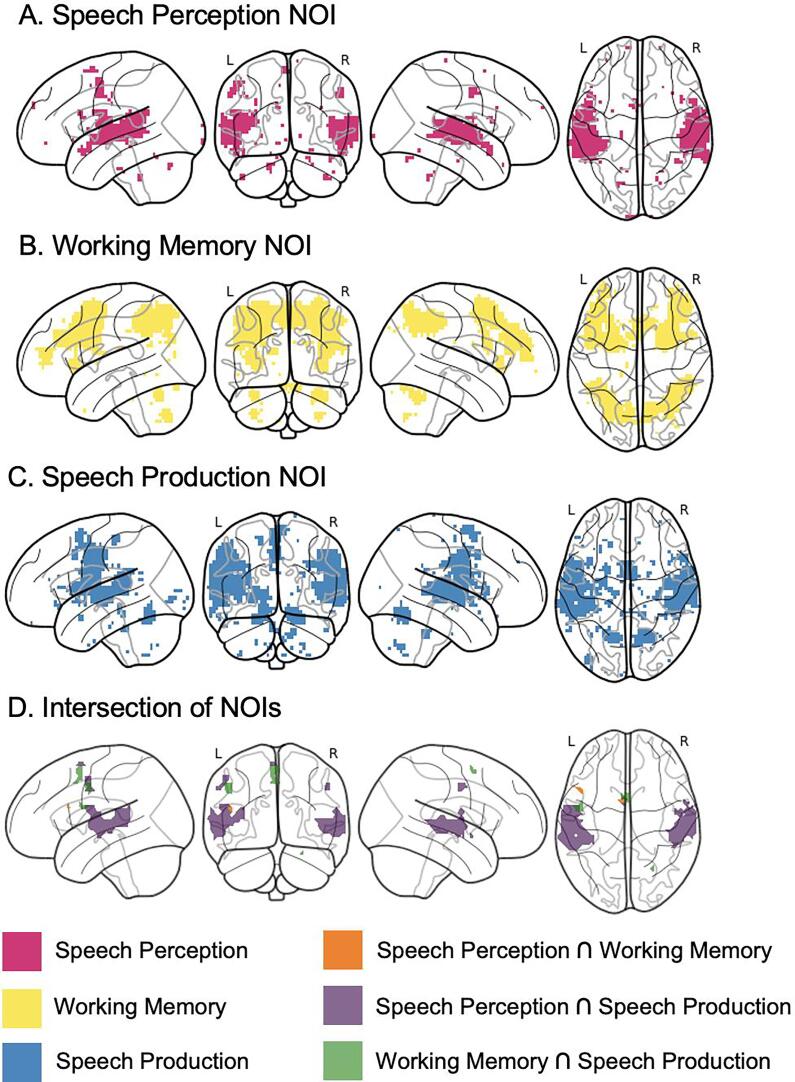
We conducted the group-level analyses within hypothesis-driven networks of interest (NOIs) representing the three underlying cognitive processes involved in nonword repetition, as described in the introduction (Fig. 3). The NOIs were regions associated with the following functional terms on Neurosynth.org (https://neurosynth.org): “speech perception” (derived from a meta-analysis of 97 studies), “working memory” (derived from a meta-analysis of 1091 studies), and “speech production” (derived from a meta-analysis of 107 studies). The use of a priori NOIs derived from meta-analyses is useful in reducing the effects of multiple comparisons. Univariate analyses (see 2.5.3.2 below) were performed within a union (i.e., combination) mask of the three NOIs to examine within- and between-group differences. Multivariate pattern analysis (MVPA; see 2.5.3.3 below) were separately conducted in each NOI, as well as the union and conjunction (i.e., intersection of at least 2 NOIs) regions. To confirm that we did not miss possible effects of condition or group occurring in regions outside of the a priori NOIs, and to examine possible compensatory activity in the autistic group, additional univariate and multivariate analyses in the complement regions to the NOIs (i.e., those outside the networks) were subsequently performed after the main analyses. The location and extent of the Neurosynth-derived NOIs were compared to significant activation in the NT and ASD groups for nonword repetition of all syllable lengths > rest (p < 0.001) in the current study (Supplementary Methods S1.3.2), and were found to be generally inclusive of the task-based activation patterns.
Fig. 3.
Networks of interest (NOI) derived from Neurosynth.org used for the univariate and multivariate analyses. Regarding overlap between NOIs, the speech perception network has a 58.7% overlap with the speech production network and a 1% overlap with the working memory network. The working memory network has a 0.4% overlap with the speech perception network and a 2.4% overlap with the speech production network. The speech production network has a 30.5% overlap with the perception network and a 3% overlap with the working memory network. For coordinates and automated anatomical labeling (based on the Automated Anatomical Labeling Atlas) of the regions included within each of the networks of interest, see Supplementary Table 5.
2.4.3.2. Univariate analyses
We used SnPM (version 13.1.08; https://nisox.org/Software/SnPM13/) to perform the non-parametric univariate analyses within the combination of the (1) speech perception, (2) working memory, and (3) speech production regions (Nichols & Holmes, 2002). We used within- and between-group cluster-wise nonparametric t-tests (5000 permutations, cluster-forming threshold p < 0.01, cluster-level FWE-corrected p < 0.05) to compare activation between task and rest (i.e., all nonword repetition syllable lengths > rest) and to compare activation as a function of phonological working memory-load (i.e., five > two-syllables). These analyses were followed by a more stringent threshold (cluster-forming threshold p < 0.001, cluster-level FWE-corrected p < 0.05) to compare activation as a function of phonological working memory-load (i.e., five syllable > two syllable). Given the wide age-range of our participants, we also conducted the above univariate analysis with age and motion as covariates, to ensure that neither factor was driving the observed effects. Univariate analyses were also conducted in the union and complement of the phonological working memory NOIs. See Supplementary Methods (S1.3.3) for methods used to identify significant anatomical brain regions.
To investigate whether the univariate differences between NT and ASD were also seen in the RD group, we extracted each participant’s beta values from the voxels in which we detected a primary group difference (NT (n = 28) > ASD (n = 23)). We conducted independent samples one-tailed t-tests to compare of the beta values for (1) RD vs ASD and (2) RD vs NT.
2.4.3.3. Multivariate pattern analysis (MVPA)
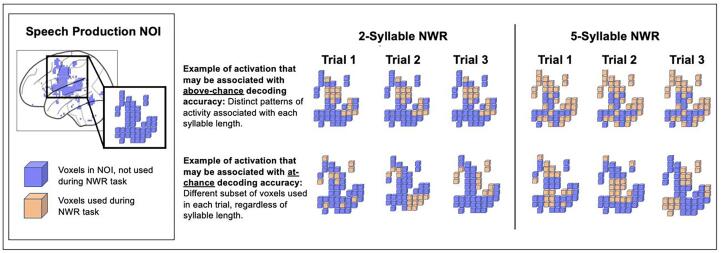
We used MVPA as an information-based decoding approach to ask how the decoding accuracy of information content (i.e., voxel-wise brain activity patterns associated with two vs five syllable nonword repetition) varies between autistic and NT children (Bae et al., 2020). MVPA is used to explore brain activation distinctions in processing between stimulus features and was chosen due to its usefulness in analyzing distributed patterns of activity that differentiate between multiple experimental conditions within typical and clinical populations (Kamitani and Tong, 2005, Bray, 2009). The accuracy of a decoder can be used as a measure of neural specificity or reliability for a particular task (in this study, nonword repetition of varying syllable lengths; Park et al., 2010, Haynes and Rees, 2006, Rissman and Wagner, 2012). Given the likely increased variability within children, a within-subject MVPA may yield additional insight regarding neural function and compensatory processing strategies at an individual level, beyond that of a traditional univariate approach (Hanke, 2009). By comparing the average decoding accuracy of each group, we can better understand the underlying neural specificity during nonword repetition for each group. Fig. 4 provides a schematic representation of how patterns of activity may be decoded between two conditions.
Fig. 4.
A simplified schematic representation of how patterns of activity may be decoded between two conditions (two syllable nonword repetition and five syllable nonword repetition) within the speech production NOI. The first example depicts distinct patterns of activation associated with each syllable length and would likely be associated with above chance decoding accuracy. The second example depicts an inconsistent pattern of voxel activation across syllable lengths, and would likely be associated with at-chance decoding accuracy.
We used the BIDS app version of PyMVPA for running the MVPA analysis (Sajjadtorabian, 2018; https://github.com/BIDS-Apps/PyMVPA). Using leave-one-out support vector machine classification, the classifier decoded between five-syllable and two-syllable word activation (i.e., between high and low phonological working memory load) for each participant. The contrast of five syllables vs two syllables was selected to examine how the autistic and NT groups use neural information to represent increased phonological working memory load in each NOI. The classifier was iterated through each NOI to reveal how well the pattern of activation in each NOI differentiates between high and low phonological working memory loads and whether there are group differences (similar methods used in: Koster-Hale et al., 2013).
All included participants had at least 67% of two and five syllable trials included to ensure sufficient trials for the analysis (Supplementary Table 4). There were no significant differences between groups or conditions in the number of included trials for decoding. Given our relatively low number of runs (n = 3) and trials, we generated estimates per individual trials rather than per run, which allowed for more, albeit noisier, estimates. We used feature-wise, chunk-wise z-scoring of the data which normalizes the dataset by scaling features into approximately the same range and removes the mean.
Average decoding accuracy was calculated for the ASD and NT groups within each of the three phonological working memory NOIs (Fig. 2), as well as the combination and conjunction areas. In both the autistic and NT groups, decoding accuracy for each network was compared against chance. An analysis of variance (ANOVA) was used to compare decoding accuracy between NT and ASD across the three primary NOIs: speech perception, working memory, and speech production. Post-hoc independent one-tailed t-tests were used to compare between decoding accuracy for ASD and NT within each of the three NOIs. The p-values for these t-tests were FDR-corrected for each between-group test conducted.
We then completed a targeted follow-up analysis by comparing MVPA decoding accuracy within the network in which we ultimately detected group differences in our primary group analysis (i.e., speech production network). Independent one-tailed t-tests were used to compare decoding accuracy between (1) RD vs ASD, and (2) RD vs NT.
2.5. Data availability statement
Pre-processed z-maps have been uploaded to GitHub as an anonymized data set (https://github.com/Amandaobrien8/ynicl_103299/). Additionally, one anonymized participant's data has been uploaded to GitHub with an accompanying tutorial to reproduce our MVPA results at a single-subject level.
3. Results
3.1. In-scanner behavioral results
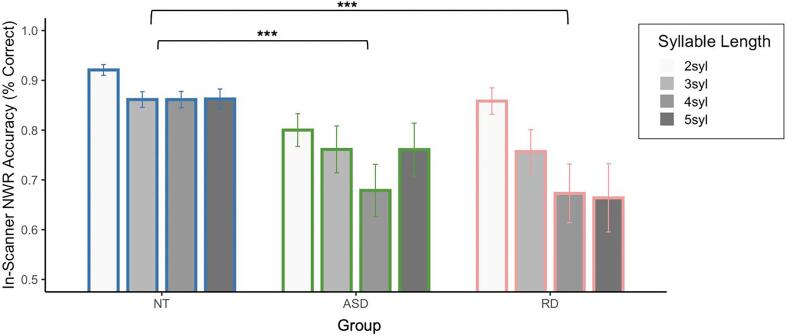
We used an ANOVA to test the effects of diagnosis and syllable length on nonword repetition accuracy among the RD, NT, and ASD groups (Fig. 5). The nonword repetition audio recordings from one participant in the RD group were missing due to technical issues. The ANOVA revealed significant main effects of groups (F(1, 256) = 30.32, p < 0.0001) and syllable length (F(3, 256) = 4.71, p = 0.003), but no interaction between diagnosis and syllable length (F(3, 256) = 1.28, p = 0.283).
Fig. 5.
In-scanner performance on nonword repetition task. Mean accuracy of nonword repetition within each condition for the NT, ASD, and RD groups. The NT had significantly higher overall performance than the ASD and the RD groups. There was no significant difference between the RD and ASD group. Error bars represent standard errors. Statistical significance marked by * is based on one-tailed T-tests on group differences. *** p < 0.001.
A comparison between the NT and ASD groups showed a main effect of diagnosis (F(1, 196) = 29.89, p < 0.0001) with the ASD group performing worse, a marginal effect of syllable length (F(3, 196) = 2.43, p = 0.066), with better performance at shorter lengths, and no interaction between diagnosis and syllable length (F(3, 196) = 0.699, p = 0.55). A comparison between the NT and RD groups showed a main effect of diagnosis with the RD group performing worse (F(1, 164) = 40.29, p < 0.0001), a main effect of syllable length (F(3, 164) = 5.629, p = 0.0011), with better performance at shorter lengths, and a marginal interaction between diagnosis and syllable length (F(3, 164) = 2.284, p = 0.081), suggesting a steeper decrease of accuracy with increasing syllable length in RD than NT. A comparison between the ASD and RD groups showed no effect of diagnosis (F(1, 144) = 0.115, p = 0.735), a main effect of syllable length (F(3, 144) = 3.099, p = 0.0288), and no interaction between diagnosis and syllable length (F(3, 144) = 0.787, p = 0.503). Within each group, the effect of syllable-length was significant in the NT (F(3, 88) = 3.378, p = 0.021) and RD (F(3, 56) = 3.029, p = 0.037) groups, but not in the ASD group (F(3, 88) = 1.17, p = 0.325).
3.2. Univariate comparison of group differences in task-based brain activation
We investigated group differences in activation during nonword repetition > rest between the NT and ASD groups by conducting nonparametric one-sample (within) and two-sample (between) t-tests (cluster-forming threshold p < 0.01, cluster-level FWE-corrected threshold p < 0.05). Both groups showed significant clusters of activation in locations known to be involved in nonword repetition, such as bilateral STG and SMA (task vs rest contrast; Supplementary Table 6). No between-group differences were detected in the complement non-phonological working memory NOI regions or within the union of the three NOIs (Fig. 3), suggesting that similar brain regions are involved in nonword repetition in both groups of children.
3.3. Univariate comparison of group differences in brain activation as a function of nonword length
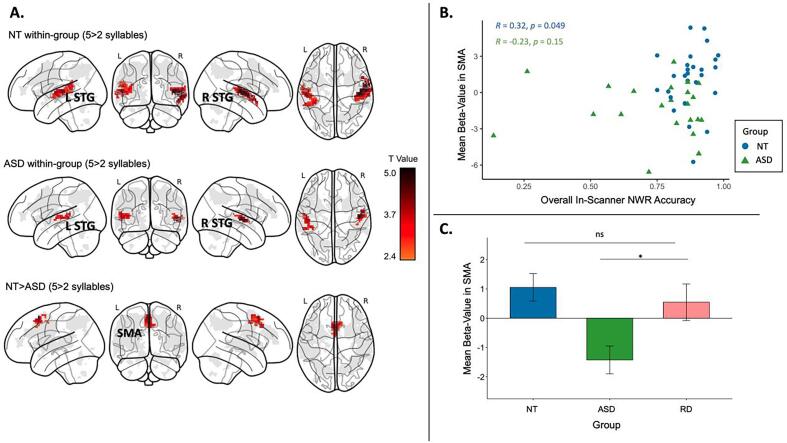
We examined the effect of syllable length (five syllables > two syllables) in each group using nonparametric one-sample t-tests (cluster-forming non-parametric threshold p < 0.01, cluster-level FWE-corrected threshold p < 0.05) within the union of the three NOIs. The NT group showed significantly greater activation in bilateral STG and left STS for longer vs shorter nonwords (Table 2, Fig. 6A). The ASD group also showed significantly greater activation in bilateral STG for the five > two syllables contrast.
Table 2.
Peak coordinates and anatomical locations of significant clusters for within and between group analyses for five-syllable > two-syllable contrast with a cluster defining threshold of p < 0.01 and FWE-corrected p < 0.05.
| Comparison | Cluster-level |
k | T | Voxel-level p(uncorr) | x | y | z | Anatomical location | |
|---|---|---|---|---|---|---|---|---|---|
| p(FWE-corr) | p(uncorr) | ||||||||
| NT (within group) | 0.004 | 0.0008 | 201 | 5.47 | 0.0002 | 45 | −21 | 5 | R STG |
| 0.047 | 0.0126 | 46 | 4.29 | 0.0002 | 54 | −42 | 1 | R STS | |
| 0.006 | 0.0011 | 178 | 4.19 | 0.0002 | −54 | −24 | 4 | L STG | |
| ASD (within group) | 0.011 | 0.0019 | 58 | 5.09 | 0.0002 | 54 | −9 | 1 | R STG |
| 0.007 | 0.0012 | 66 | 3.52 | 0.0010 | −51 | −27 | 5 | L STG | |
| NT > ASD | 0.014 | 0.0019 | 135 | 4.43 | 0.0002 | −3 | 15 | 55 | SMA |
Fig. 6.
Effects of syllable length in fMRI. A. Two vs five-syllable effect within the neurotypical (NT) and autism spectrum disorder (ASD) groups and the difference between the two groups (cluster-forming nonparametric p < 0.01, cluster-level FWE-corrected p < 0.05). B. Activation responses at SMA were positively correlated with in-scanner nonword repetition performance in the NT group, but not in the ASD group. C. The reading disability (RD) group did not vary significantly in SMA activation magnitude from the NT group but was significantly higher than the ASD group. Error bars represent standard errors. Spearman’s Rank correlation p-values are reported as one-tailed values. * p < 0.05; ** p < 0.01.
Between group analyses revealed that thehe NT group had significantly greater difference between the two- and five-syllable conditions in pre-SMA and SMA-proper (peak coordinates: −3, 15, 55; Table 2, Fig. 6A) compared to the ASD group. The reverse contrast of ASD > NT did not yield any significant clusters. The group difference remained consistent with use of a more stringent cluster-forming threshold (p < 0.001) and cluster-level FWE-corrected threshold of 0.05), and also remained significant when age and motion were added as covariates. Notably, the SMA in the NT group had greater activation to longer than shorter nonwords (t(27) = 2.24, p = 0.017), as measured by the beta weight from two and five syllables extracted from the SMA ROI. In contrast, the SMA in the ASD group had greater activation during shorter than longer nonwords (t(22) = 2.99, p = 0.003). No within-group syllable-load effect nor between-group differences were found in the complement regions outside of the phonological working memory NOIs.
To better understand the relationship between SMA activation and phonological working memory abilities in the ASD and NT groups, we correlated each participant’s load-dependent activation difference (beta weight from the contrast of five > two syllables) extracted from the SMA ROI with their overall in-scanner nonword repetition accuracy. Using a nonparametric Spearman’s rank correlation in each group, we found a significant positive correlation for the NT group between SMA activation and in-scanner performance (rho = 0.32, one-tailed p < 0.05) (Fig. 6B). In contrast, the ASD group did not have a significant correlation between SMA activation and in-scanner performance (rho = -0.23, one-tailed p = 0.15), which was a significantly weaker association compared to that in the NT group (z = 1.89, one-tailed p = 0.03).
We performed targeted follow-up analyses of activation within the SMA for two control comparisons (1) a smaller subset of the full ASD and NT groups who were additionally matched on CELF Core Language scores (language-matched groups; See Supplementary Methods S1.1.2 for details) and (2) the RD group, who did not differ from the ASD group on nonword repetition performance either in or out of the scanner. The language-matched NT group continued to show significantly greater SMA activation with increased nonword syllable load than the language-matched ASD group (t(33.3) = 3.89, one-tailed p < 0.001). The RD group showed significantly greater SMA activation during increased phonological working memory load (five > two syllables) than the ASD group (t(29) = 2.34, one-tailed p = 0.02), but did not differ from the NT group (t(31) = 0.52) (Fig. 6C).
3.4. Multivariate group comparison of neural decoding of nonword length
We used the accuracy of multivoxel trial-wise decoding between the two- and five-syllable conditions as a measure of how consistently each of the NOIs represented phonological working memory load within each individual. A between-group comparison of decoding accuracy within each NOI for the ASD and NT groups, using a group by NOI ANOVA, showed a main effect of diagnosis, with significantly lower decoding accuracy in the ASD group compared to the NT group (F(1, 147) = 7.65, p < 0.01), which remained significant after controlling for in-scanner performance (F(1, 146) = 7.34, p < 0.01).
There was above-chance decoding of phonological working memory load in all NOIs in the NT group, as revealed by a one-sample t-test against chance. In contrast, there was not above-chance decoding in any of the tested networks in the ASD group, including the combination, union, or complement networks (Table 3). After we controlled for multiple comparisons, a significant group difference was only observed in the speech-production NOI, shown as higher decoding accuracy in the NT group than the ASD group (t(47) = 2.5, FDR-corrected p < 0.05). Further, even for the two- vs four-syllable contrast where more robust behavioral differences were observed in the ASD group (See Fig. 5), neural decoding of these two conditions in the ASD group did not differ significantly from the classification of two- vs five-syllable load (Supplementary Table 8).
Table 3.
Decoding accuracy for NT and ASD groups within the NOIs, intersections of interest, complement regions, and combination of all networks. Between-group comparisons were conducted for the three NOIs. ∩: Intersection of two NOIs.
| Networks of Interest | NT |
ASD |
NT vs ASD |
||||
|---|---|---|---|---|---|---|---|
| Against Chance |
Against Chance |
FDR corrected p-value | |||||
| Mean | SD | FDR corrected p-value | Mean | SD | FDR corrected p-value | ||
| Speech Perception Network | 56.2 | 8.8 | 0.001 | 52.5 | 8.7 | 0.142 | 0.110 |
| Working Memory Network | 54.3 | 8.8 | 0.009 | 52.3 | 7.4 | 0.142 | 0.194 |
| Speech Production Network | 57.7 | 7.1 | 0.000 | 52.6 | 7.3 | 0.141 | 0.024 |
| Perception ∩ Production | 55.5 | 8.6 | 0.002 | 51.6 | 9.4 | 0.248 | 0.122 |
| Working Memory ∩ Production | 53.8 | 8.8 | 0.015 | 51.0 | 7.8 | 0.279 | 0.143 |
| Complement Regions | 55.8 | 8.9 | 0.002 | 53.3 | 7.1 | 0.142 | NA |
| Union of all three NOIs | 56.4 | 7.8 | 0.0005 | 52.2 | 6.5 | 0.142 | NA |
| Intersection of all three NOIs | 55.5 | 8.8 | 0.002 | 51.6 | 9.4 | 0.248 | NA |
To examine whether the differences in decoding accuracy in the speech production NOI were being driven by brain regions common to the speech production and speech perception networks, or by those common to the speech production and working memory networks, we conducted two pairwise t-tests to compare decoding accuracy within these network intersections between ASD and NT. Neither group comparison was significant (Table 3), suggesting that the differences in the speech production network arise within core speech-production specific regions, rather than the overlapping regions between the networks.
To determine whether decoding accuracy within the speech production network was associated with behavioral characteristics in the ASD group, we conducted a series of correlations between decoding accuracy and several behavioral measures (in-scanner nonword repetition accuracy, Social Responsiveness Scale (SRS-2; Constantino and Gruber, 2012, Frazier et al., 2014), Social Communication Questionnaire (SCQ; Rutter et al., 2003), and ADOS Severity Score). Within the autism group, decoding accuracy from the speech production network was not associated with the in-scanner nonword repetition performance (Spearman’s rho < 0.08). Instead, we observed modest negative correlations (Spearman’s rho's > −0.30) between the decoding accuracy of the speech-production network and all three autism-related measures (Supplementary Fig. 1), with lower decoding accuracy correlating with higher autism traits across all measures.
As we did for the univariate analyses, we again conducted targeted follow-up analyses of MVPA decoding accuracy within the speech production network for the two control groups. As seen with the full group, there was significantly higher decoding accuracy for the language-matched NT group vs the language-matched ASD group (t(32) = 1.86, p = 0.036). In contrast, there were no significant differences in decoding accuracy between the RD and ASD group (p = 0.72) or between the RD and NT group (p = 0.123).
4. Discussion
To better understand the brain bases of phonological working memory challenges in autism, we investigated how brain activation during nonword repetition differed in autistic versus neurotypical children across three networks of interest known to support nonword repetition: speech perception, working memory, and speech production. Children with autism exhibited reduced nonword repetition accuracy both in and out of the scanner. Group differences in activation during nonword repetition were found in speech-production regions, the SMA and pre-SMA, which showed greater modulation of activation for longer versus shorter nonwords in NT than in ASD. Multivoxel pattern analysis within the speech production network classified shorter versus longer nonwords less accurately for autistic than neurotypical children. The specificity for the speech production difference in autism was supported by lack of an activation difference in children with RD. Further, these univariate and multivariate findings were replicated in a comparison between NT and ASD children who were matched on language skills, indicating that the brain differences were likely related to the autism phenotype rather than reflecting differences in core language abilities.
Overall, these results suggest that phonological working memory challenges in autism may be due to differential engagement of speech-motor areas, including especially SMA, as seen here during nonword repetition. In contrast, our findings suggest that nonword challenges in autism may not be related to differences in perceptual processing, as activation in superior temporal regions was not atypical in this group. Instead, the results suggest typical phonological representation in autistic children, consistent with some previous neuroimaging and behavioral research (Ceponiene et al., 2003, Pomper et al., 2019).
The univariate analyses revealed that while the ASD and NT groups recruited similar, canonical phonological working memory networks during the nonword repetition task overall, they differed in how this activation was modulated by increased phonological working memory load. Although the ASD group showed typical sensitivity to increased syllable length within bilateral STG, they differed specifically in their recruitment of the pre-SMA and SMA proper – brain regions associated with planning and controlling motor and speech actions (Tremblay and Gracco, 2009, Nachev et al., 2007, Alario et al., 2006). SMA and pre-SMA in particular have been implicated in sequencing and planning of speech (Guenther, 2016). SMA has also exhibited parametric responses for nonword syllable load in purely receptive tasks (Perrachione et al., 2017, Strand et al., 2008), suggesting that engagement of SMA can signal a covert articulatory rehearsal process and does not require explicit speech production.
Beyond the SMA, studies of nonword repetition in neurotypical adults have shown increased load-associated activation in several regions associated with speech production including left precentral gyrus, left inferior frontal gyrus (IFG), and right and left cerebellum (Scott & Perrachione, 2019). A study of nonword repetition in neurotypical adults showed task-specific increases in the functional connections between pre-SMA and left dorsal premotor cortex (Hartwigsen et al., 2013). According to the GODIVA neurocomputational model of speech processing (Bohland et al., 2010), IFG and SMA/pre-SMA subserve parallel phonological buffers, with the former encoding phonemic sequences and the latter representing abstract syllable frames and/or premotor sequencing of speech (Tremblay & Gracco, 2006; Alario et al., 2006). Evidence has also suggested a diverse range of activations in both regions across individual participants (Scott & Perrachione, 2019). In the current study, the differences in SMA, but not in IFG, suggests potential intact phonemic processing during nonword repetition in autistic children, but abnormal motor sequencing, which is independent of the specific phonemic or phonological content of the stimuli.
Not only was there less activation of the SMA in the ASD group, but the ASD group showed a fundamentally different pattern of activation in the SMA. Unlike the NT group who showed greater activation for longer than shorter nonwords, the ASD group showed the reverse pattern with greater activation for shorter than longer nonwords. This suggests there may be less consistent or reliable motor sequencing for longer nonwords in ASD. A diffusion tensor imaging study in autistic adults with average nonverbal IQ revealed a weaker connection in the speech production network between the SMA and ventral premotor cortex compared to neurotypical controls (Peeva et al., 2013). It is possible that SMA functions less efficiently and coherently with other regions in the speech motor system when children with ASD are faced with longer syllable sequences.
The MVPA approach provided evidence supporting altered speech motor function beyond SMA. We used MVPA as an information-based decoding approach, to understand how the decoding accuracy of information content (i.e., voxel-wise brain activity patterns associated with two vs five syllable nonword repetition) varied between ASD and NT children. Above chance decoding accuracy indicates consistent patterns of activation associated with each syllable length, while at-chance decoding suggests an indiscriminate pattern of voxel activation across syllable lengths. Across speech perception, working memory, and speech production networks, only the speech production network had reliably lower decoding accuracy in the ASD group compared to the NT group. Further, lower decoding accuracy within the speech production network was modestly correlated with more severe autism-based symptoms in the ASD group. This functional alteration in the speech motor network remained even when matching language skills across the ASD and NT groups.
Together, these complementary findings support a speech-motor atypicality account for reduced nonword repetition performance in autism. However, the differences in SMA and speech production network do not imply that differences only occured at the last temporal phase of nonword repetition. The fMRI task, involving immediate repetition after listening, does not allow differentiation of temporal components of the behavior: speech perception, maintenance, and speech production. In addition, our fMRI nonword repetition task does not differentiate between verbal short-term recall and working memory, two associated cognitive processes captured by the same paradigm (Oberauer et al., 2012, Archibald and Gathercole, 2007). Future research investigating the brain bases of phonological working memory should include additional behavioral tasks to validate these findings (e.g., serial recall of lists of monosyllabic nonwords).
Our findings add to a growing literature showing broad motor system differences in ASD, including motor differences related to speech and language. In fact, motor differences in autism have been found to be more closely linked to the diagnostic features of ASD, social communication and restricted and repetitive behaviors, than cognitive ability (Ketcheson et al., 2021). Related to speech and language, better fine motor skills in autistic children are associated with stronger receptive and expressive language skills (Mody et al., 2017). Visuomotor, general coordination, and fine motor skills have been shown to predict language delays in autistic children (Bhat, 2022). Similarly, oromotor, manual motor skills, and fine motor skills have been shown to be strong indicators of speech development (Gernsbacher et al., 2008, Bal et al., 2019). Notably, Krishnan et al. (2013) demonstrated that NT children’s ability to imitate and reproduce nonlinguistic oromotor sequences predicted their performance in a nonword repetition task. This relationship is independent of language skills and general cognitive abilities, suggesting an involvement of domain-general motor skills and planning underlying phonological working memory, which is consistent with the present findings across autistic children with and without reduced concurrent language skills. These findings, together with the neuroimaging evidence from the current study, suggest that atypical motor function in autism might not be a byproduct of concurrent language challenges, but a possible indicator for further risks in language development due to its association with reduced phonological working memory.
Atypical activation of SMA occurred in the ASD group but not in an RD group that had similar behavioral impairment in nonword repetition. ASD and RD, however, may share some brain differences in relation to nonword repetition, such as atypical structural connectivity (Lu et al., 2016). Perhaps atypical functional organization in speech perception (Boets et al., 2011) and working memory networks (Hachmann et al., 2020, Jeffries and Everatt, 2004, Smith-Spark and Fisk, 2007) may better explain the challenges of RDs in nonword repetition. Although SMA activation in RD was similar to NT during nonword repetition, future research with a larger sample size is necessary to investigate other potential mechanisms related to speech production in RD, such as phonemic/phonological representation as opposed to sequencing, and whether they are related to nonword repetition deficits given the known difficulties in phonological processing in reading disabilities (Kovelman et al., 2012, Van Bergen et al., 2014). Behavioral research has documented some evidence of speech production difficulties in RD, including reduced speech rate and increased speech errors (e.g., Catts, 1989; Carroll et al., 2014, Lambrecht Smith et al., 2010). Some researchers connect speech errors with phonological awareness (Cabbage et al., 2018), while others suggest a complex relationship between speech sound disorder, reading disability, and language impairment Hayiou-Thomas et al. (2017). Therefore, research in other language disorders will provide greater clarification for the specific and shared neural substrates underlying a common behavioral hallmark (cf. Pigdon et al., 2020, Liégeois et al., 2011).
The present findings should be considered within the context of several methodological limitations. Due to the scarcity of pediatric neuroimaging studies, the networks of interest for our analyses were based on meta-analyses from primarily adult participants. However, there is evidence that the core speech perception, speech production, and cognition networks are also implicated and selective in children during development (Hiersche et al., 2022). Despite this, we demonstrated a substantial overlap between the Neurosynth networks of interest and the activation patterns during nonword repetition observed in our pediatric sample (Supplementary Fig. 2). An alternative approach for future studies is to generated task-specific individual-specific functional ROIs using a separate speech production task. These individualized ROIs would be developmentally more appropriate and might improve decoding accuracy across all participant groups.
Secondly, a replication study with a larger sample size is warranted. Due to the heterogeneity of the populations with autism and reading disabilities, the current study is limited in its power to detect more subtle group differences that may exist in other brain networks or brain regions. In fact, none of the networks of interest in either the ASD group or the RD group reliably classified syllable length above chance. Therefore, the MVPA results may also reveal a global neural inconsistency phenomenon that is neither unique to autism nor unique to the speech production network (e.g., Centanni et al., 2021). In addition, a larger sample size is necessary to uncover reliable brain-behavior correlations. Despite the small sample size, however, within the context of this paper, the targeted investigation with rigorous control for multiple comparisons allowed us to test the specificity of the brain and behavioral differences to autism.
Thirdly, there was a wide age range of participants in each group. Groups were carefully matched on data quality and age. However, because we used one standard MNI template across all groups, the degree of spatial warping for younger brains was greater than older brains during normalization, which might lead to additional age-related confound during preprocessing. Although our main neuroimaging findings do not appear to be associated with age (Supplementary Table 6), a larger sample size in future studies will allow us to examine the developmental changes of motor speech functions in age-specific anatomical spaces. Different developmental trajectories might still exist between autistic and neurotypical groups.
5. Conclusions and future directions
Autistic children – even those with core language skills matched to NT children – may perform and function differently during nonword repetition tasks due to atypical engagement of the speech production network and especially the SMA. The vulnerability in phonological working memory even for language-matched autistic children could lead to downstream language and language-learning challenges (Baddeley et al., 1998), which will require future longitudinal samples to verify and characterize. Speech production, and its underlying neural mechanisms, is an important part of phonological working memory that has been largely neglected in previous phonological working memory research. Future research should investigate how motor functions in both the verbal and nonverbal domains predict language learning outcomes in not only autistic children, but also children with other communication disorders who have concomitant motor and working memory difficulties.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank the participating families for their contribution to our study; Satrajit Ghosh and Anila D’Mello for advice on data analysis; Michelle Han, Sara Beach, Abigail Cyr, Clara Baron-Hyppolite, Colleen Buckless, Katalina Sher, Calvin Goetz, and Mengyuan Xu for help on data collection; and Atsushi Takahashi, Sheeba Arnold Anteraper, and Steven Shannon at the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research, Massachusetts Institute of Technology, for technical support.
Funding
This work was funded by National Institutes of Health Grant No. R01 DC011339 (to JDEG, HTF, and KW); TKP was supported by NIH grant R03 DC014045 and a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation; AMO was funded by NIH Training Grant: T32 DC000038 and the Hock E. Tan and K. Lisa Yang Center for Autism Research. ZQ was supported by NIH grant R21DC017576.
Footnotes
Many autistic people prefer identity-first language (“autistic person”) to person-first language (“person with autism”), while some members within the community prefer person-first language (Lei et al., 2021). These preferences guide our semantic language choices; we use identity-first and person-first language interchangeably throughout the manuscript.
Supplementary data for this article can be found online at https://doi.org/10.1016/j.nicl.2022.103299.
Appendix A.
Two-, three-, four, and five-syllable nonword stimuli. Nonword pronunciation is shown in parentheses using International Phonetic Alphabet transcription).
2-Syllable Nonwords.
Plever/pever (ˈplɛvɚ/ˈpɛvɚ), pencid/pecid (ˈpɛnsɪd/ˈpɛsɪd), tector/tetor (ˈtɛktɚ/ˈtɛtɚ), mubler/muber (ˈmublɚ/ˈmubɚ), sufting/sufing (ˈsʌftɪŋ/ˈsʌfɪŋ), bicket/bippet (ˈbɪkət/ˈbɪpət), blimpet/blimset (ˈblɪmpət/ˈblɪmsət), nadle/naidle (ˈneɪdəl/ˈnaɪdəl), shoken/shopen (ˈʃoʊkən/ˈʃoʊpən), sploiter/spleiter (ˈsploɪtɚ/ˈspleɪtɚ), cranscow/cranksow (ˈkrænskaʊ/ˈkrænksaʊ), troglem/trolgem (ˈtragləm/ˈtralgəm), kolite/kilote (ˈkoʊlaɪt/ˈkaɪloʊt), preport/preprot (ˈpripɔrt/ˈpriprot), riftoon/rooftin (ˈrɪftun/ˈruftɪn), priote (ˈpraɪoʊt), kabant (ˈkæbənt), ballom (ˈbaləm), kather (ˈkæðɚ), gammert (ˈgæmɚt), shable (ˈʃeɪbəl), morant (ˈmɔrænt), neaving (ˈnivɪŋ), klamic (ˈklæmɪk), ablit (ˈæblɪt), blekking (ˈblɛkɪŋ), dorson (ˈdɔrsən), quipid (ˈkwɪpɪd), yantel (ˈyæntəl), promin (ˈproʊmən).
3-Syllable Nonwords.
esconter (ˌɛsˈkantɚ)/thiprisant (ˈθɪpˌrɪsænt)/femotal (ˌfəˈmotl̩)/breekerment (ˈbriˌkɚmənt)/bindacy (ˈbɪnˌdəsi)/candronish (ˌkænˈdronɪʃ)/seblantic (ˌsəˈblæntɪk)/kalandly (ˈkæˌlændli)/saplitize (ˈsæˌplɪtɑɪz)/mesedine (ˈmɛsəˌdɑɪn)/rekipance (ˌriˈkɪpəns)/siprisom (ˈsɪˌprɪsom)/prothader (ˈproˌðeθɚ)/gronify (ˈgroˌnɪfɑɪ)/minerack (ˈmɪnɚˌræk)/accrevate or accrebate (ˈækrɪˌvet or ˈækrɪˌbet)/taggrement (ˈtægɚˌmənt)/premonish (ˌprimaˈnɪʃ)/guppering (ˈgʌˌpɚɪŋ)/restandent (ˌrɪˈstændənt)/misinter (ˈmɪzɪnˌtɚ)/jandersing (ˈdʒændɚˌsɪŋ)/vagalish (ˈvegəˌlɪʃ)/trabalize (ˈtrebl̩ˌɑɪz).
4-Syllable Nonwords.
icnidator/icidator (ˈɪknɪˌdeɪtɚ/ˈɪkɪˌdeɪtɚ), metretory/meretory (ˈmɛtrəˌtɔrɪ/ˈmɛrəˌtɔrɪ), astragular/atragular (ˌæˈstrægjulɚ/ˌæˈtrægjulɚ), hibernatist/hiberatist (haɪˈbɝnəˌtɪst/haɪˈbɝəˌtɪst), gasprodoxy/gasrodoxy (ˈgæsprəsˌdaksɪ/ˈgæsrəˌdaksɪ), decepoment/decegoment (ˌdɪˈsipomənt/ˌdɪˈsigomənt), exvomition/exvotition (ˌɛksvoʊˈmɪʃən/ˌɛksvoʊˈtɪʃən), challopism/chollopism (ˈʧæloˌpɪzəm/ˈʧoʊloˌpɪzəm), trallocistic/trallopistic (ˌtræloˈsɪstɪk/ˌtræloˈpɪstɪk), canastocize/canistocize (kæˈnæsto ˌsaɪz/kæˈnɪstoˌsaɪz), crimipism/cripimism (ˈkrɪmɪˌpɪzəm/ˈkrɪpɪˌmɪzəm), matastrocy/mastatrocy (məˈtæstroˌsɪ/məˈstætroˌsɪ), candalopy/canladopy (kænˈdæloˌpɪ/kænˈlædoˌpɪ), besepalment/bepesalment (biˈsipəlmənt/biˈpisəlmənt), reaquisment/reasquiment (ˌriˈækwɪzmənt/ˌriˈæzkwɪmənt), hemostify (ˌhiˈmoʊstəfaɪ), allotastry (ˈæloʊˌtæstrɪ), shagonazle (ˈʃægoˌnæzəl), kamasticize (kəˈmæstəˌsaɪz), rendoristat (rɛnˈdɔrɪˌstæt), posidriate (ˌpoˈsɪdrieɪt), benopify (ˌbiˈnopɪfaɪ), athandanate (ˌæˈθændəneɪt), nuplarative (ˌnuˈplɛrətɪv), fandosity (ˌfænˈdoʊsɪtɪ), masadolyte (mæˈsædoˌlaɪt), kinimerate (ˌkɪˈnɪmɚeɪt), cavanator (ˈkævəˌneɪtɚ), reostify (ˌriˈoʊstɪfaɪ), illostratic (ˌɪloˈstrætɪk).
5-Syllable Nonwords.
ionificate (ɑɪoˈnɪfɪˌket)/uninagable (ˌʌnɪnˈægəbl̩)/mallerogasty (ˌməlɛrəˈgæsti)/osacreatic (oˈzakriˌætɪk)/androchiable (ænˈdrokiˌəbl̩)/veronicity (ˌvəraˈnɪsɪti)/kadastaline (kəˈdæstˌl̩ɑɪn)/kiliometric (ˌkɪlioˈmɛtrɪk)/apristiation (əˌprɪstiˈeʃən)/kredomiotic (ˌkredomɑɪˈatɪk)/cyoblisity (ˌsɑɪəˈblɪsəti)/aglomantacy (ˌɛgloˈmæntəsi)/aniobity (ˌæniˈobɪti or ˌæniˈobɪdɪ)/reancidity (ˌriænˈsɪdɪti)/hieromaly (ˌhɑɪɚraˈməli)/amerondable (ˌæmɚˈandəbl̩)/hereomatic (ˌhirioˈmætɪk)/lamonicify (ˈlæˌmanɪsɪfɑɪ)/thidiometry (ˌθidiˈamətri or ˌvidiˈamətri)/cabernacity (ˌkæbɚˈnæsɪti)/trandoriable (ˌtrænˈdoriəbl̩)/remastography (riˌmæˈstagrəfi)/etocrastical (itoˈkræˌstɪkl̩)/antriovacy (ˈænˌtriovəsi).
Appendix B. Supplementary data
The following are the Supplementary data to this article:
Data availability
Preprocessed fMRI data in the form of z-maps are available on Github (https://github.com/Amandaobrien8/ynicl_103299/).
References
- Acheson D.J., Hamidi M., Binder J.R., Postle B.R. A Common Neural Substrate for Language Production and Verbal Working Memory. Journal of Cognitive Neuroscience (Vol. 2011;23(6):1358–1367. doi: 10.1162/jocn.2010.21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A.M., Gathercole S.E. Phonological Working Memory and Speech Production in Preschool Children. Journal of Speech, Language, and Hearing Research. 1996;38(2):403–414. doi: 10.1044/jshr.3802.403. [DOI] [PubMed] [Google Scholar]
- Adams, L. (1998). Oral-Motor and Motor-Speech Characteristics of Children with Autism. In Focus on Autism and Other Developmental Disabilities (Vol. 13, Issue 2, pp. 108–112). 10.1177/108835769801300207.
- Alario F.X., Chainay H., Lehericy S., Cohen L. The role of the supplementary motor area (SMA) in word production. Brain Research. 2006;1076(1):129–143. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Archibald Working memory and language learning: A review. Child Language Teaching and Therapy. 2017;33(1):5–17. doi: 10.1177/0265659016654206. [DOI] [Google Scholar]
- Archibald L., Gathercole S. Nonword repetition and serial recall: Equivalent measures of verbal short-term memory? Applied Psycholinguistics. 2007;28(4):587–606. doi: 10.1017/S0142716407070324. [DOI] [Google Scholar]
- Archibald L.M.D., Joanisse M.F. On the sensitivity and specificity of nonword repetition and sentence recall to language and memory impairments in children. Journal of Speech, Language, and Hearing Research: JSLHR. 2009;52(4):899–914. doi: 10.1044/1092-4388(2009/08-0099). [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory and language: an overview. Journal of Communication Disorders. 2003;36(3):189–208. doi: 10.1016/s0021-9924(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Baddeley, A., & Hitch, G. (1974). Working Memory. In Psychology of Learning and Motivation (pp. 47–89). 10.1016/s0079-7421(08)60452-1.
- Baddeley A., Gathercole S., Papagno C. The phonological loop as a language learning device. Psychological Review. 1998;105(1):158–173. doi: 10.1037/0033-295x.105.1.158. [DOI] [PubMed] [Google Scholar]
- Bae G.-Y., Leonard C.J., Hahn B., Gold J.M., Luck S.J. Assessing the information content of ERP signals in schizophrenia using multivariate decoding methods. In NeuroImage: Clinical. 2020;25:p. 102179). doi: 10.1016/j.nicl.2020.102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird G., Slonims V., Simonoff E., Dworzynski K. Impairment in non-word repetition: a marker for language impairment or reading impairment? Developmental medicine and child neurology. 2011;53(8):711–716. doi: 10.1111/j.1469-8749.2011.03936.x. [DOI] [PubMed] [Google Scholar]
- Bal V.H., Kim S.-H., Fok M., Lord C. Autism spectrum disorder symptoms from ages 2 to 19 years: Implications for diagnosing adolescents and young adults. In Autism Research (Vol. 2019;12(1):89–99. doi: 10.1002/aur.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendse E.M., Hendriks M.P., Jansen J.F., Backes W.H., Hofman P.A., Thoonen G., Kessels R.P., Aldenkamp A.P. Working memory deficits in high-functioning adolescents with autism spectrum disorders: neuropsychological and neuroimaging correlates. Journal of neurodevelopmental disorders. 2013;5(1):14. doi: 10.1186/1866-1955-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford R., Pickles A., Lord C. Early gross motor skills predict the subsequent development of language in children with autism spectrum disorder. Autism Research. 2016;9(9):993–1001. doi: 10.1002/aur.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat A. Motor Impairment Increases in Children With Autism Spectrum Disorder as a Function of Social Communication, Cognitive and Functional Impairment, Repetitive Behavior Severity, and Comorbid Diagnoses: A SPARK Study Report. Autism Research. 2021;14(1):202–219. doi: 10.1002/aur.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat A. Multidimensional motor performance in children with autism mostly remains stable with age and predicts social communication delay, language delay, functional delay, and repetitive behavior severity after accounting for intellectual disability or cognitive delay: A SPARK dataset analysis. Autism Research. 2022 doi: 10.1002/aur.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat A.N., Landa R.J., Galloway J.C. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Physical Therapy. 2011;91(7):1116–1129. doi: 10.2522/ptj.20100294. [DOI] [PubMed] [Google Scholar]
- Bloom . One word at a time ; the use of single word utterances before syntax. Mouton; L., 1973. [Google Scholar]
- Boersma P. Praat, a system for doing phonetics by computer. Glot International. 2001;5:341–345. [Google Scholar]
- Boets B., Vandermosten M., Poelmans H., Luts H., Wouters J., Ghesquière P. Preschool impairments in auditory processing and speech perception uniquely predict future reading problems. Research in Developmental Disabilities. 2011;32(2):560–570. doi: 10.1016/j.ridd.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Bohland J., Bullock D., Guenther F.H. Neural Representations and Mechanisms for the Performance of Simple Speech Sequences. Journal of Cognitive Neuroscience. 2010;22(7):1504–1529. doi: 10.1162/jocn.2009.21306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S. Applications of multivariate pattern classification analyses in developmental neuroimaging of healthy and clinical populations. In. Frontiers in Human Neuroscience. 2009;3 doi: 10.3389/neuro.09.032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno R.M., Walker S.C. Comprehensive Test of Phonological Processing (CTOPP) In Diagnostique (Vol. 1999;24(1–4):69–82. doi: 10.1177/153450849902401-408. [DOI] [Google Scholar]
- Buchsbaum B., D’Esposito M. A sensorimotor view of verbal working memory. Cortex; a journal devoted to the study of the nervous system and behavior. 2019;112:134–148. doi: 10.1016/j.cortex.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Cabbage F.K., Iuzzini-Seigel J., Zuk J., Hogan T.P. Exploring the Overlap Between Dyslexia and Speech Sound Production Deficits. Language, Speech & Hearing Services in Schools. 2018;49(4):774–786. doi: 10.1044/2018_LSHSS-DYSLC-18-0008. [DOI] [PubMed] [Google Scholar]
- Carroll J.M., Mundy I.R., Cunningham A.J. The roles of family history of dyslexia, language, speech production and phonological processing in predicting literacy progress. Developmental science. 2014;17(5):727–742. doi: 10.1111/desc.12153. [DOI] [PubMed] [Google Scholar]
- Catts H.W. Speech production deficits in developmental dyslexia. The Journal of speech and hearing disorders. 1989;54(3):422–428. doi: 10.1044/jshd.5403.422. [DOI] [PubMed] [Google Scholar]
- Centanni T., Beach S.D., Ozernov-Palchik O., May S., Pantazis D., Gabrieli J.D.E. Categorical perception and influence of attention on neural consistency in response to speech sounds in adults with dyslexia. Annals of Dyslexia. 2021 doi: 10.1007/s11881-021-00241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceponiene R., Lepisto T., Shestakova A., Vanhala R., Alku P., Naatanen R., Yaguchi K. Speech-Sound-Selective Auditory Impairment in Children with Autism: They Can Perceive but Do Not Attend. Proceedings of the National Academy of Sciences - PNAS. 2003;100(9):5567–5572. doi: 10.1073/pnas.0835631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant B.K.E., Chang E.F. Speech map in the human ventral sensory-motor cortex. Current Opinion in Neurobiology. 2013;24(1):63–67. doi: 10.1016/j.conb.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J.N., Gruber C.P. Western Psychological Services; Torrance, CA: 2012. Social Responsiveness Scale-Second Edition (SRS-2) [Google Scholar]
- Craig F., Crippa A., Ruggiero M., Rizzato V., Russo L., Fanizza I., Trabacca A. Characterization of Autism Spectrum Disorder (ASD) subtypes based on the relationship between motor skills and social communication abilities. Human Movement Science. 2021;77 doi: 10.1016/j.humov.2021.102802. [DOI] [PubMed] [Google Scholar]
- Cui J., Gao D., Chen Y., Zou X., Wang Y. Working Memory in Early-School-Age Children with Asperger’s Syndrome. In Journal of Autism and Developmental Disorders (Vol. 2010;40(8):958–967. doi: 10.1007/s10803-010-0943-9. [DOI] [PubMed] [Google Scholar]
- Dollaghan C., Campbell T.F. Nonword Repetition and Child Language Impairment. Journal of Speech, Language, and Hearing Research. 1998;41(5):1136–1146. doi: 10.1044/jslhr.4105.1136. [DOI] [PubMed] [Google Scholar]
- Ehrhorn A.M., Adlof S.M., Fogerty D., Laing S. Probing Phonological Processing Differences in Nonword Repetition for Children with Separate or Co-Occurring Dyslexia and Developmental Language Disorder. Scientific Studies of Reading. 2021;25(6):486–503. doi: 10.1080/10888438.2020.1849223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O., Markiewicz C.J., Blair R.W., Moodie C.A., Isik A.I., Erramuzpe A., Kent J.D., Goncalves M., DuPre E., Snyder M., Oya H., Ghosh S.S., Wright J., Durnez J., Poldrack R.A., Gorgolewski K.J. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nature Methods. 2019;16(1):111–116. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes K.G., Evans J.L., Else-Quest N.M. Differences in the Nonword Repetition Performance of Children With and Without Specific Language Impairment: A Meta-Analysis. In Journal of Speech, Language, and Hearing Research (Vol. 2007;50(1):177–195. doi: 10.1044/1092-4388(2007/015). [DOI] [PubMed] [Google Scholar]
- Fiez, J. A. (2016). Neural Basis of Phonological Short-Term Memory. In Neurobiology of Language (pp. 855–862). 10.1016/b978-0-12-407794-2.00068-7.
- Frazier T.W., Ratliff K.R., Gruber C., Zhang Y., Law P.A., Constantino J.N. Confirmatory factor analytic structure and measurement invariance of quantitative autistic traits measured by the Social Responsiveness Scale-2. Autism. 2014;18(1):31–44. doi: 10.1177/1362361313500382. [DOI] [PubMed] [Google Scholar]
- Frith U. Blackwell; Oxford: 1989. Autism: Explaining the enigma. [Google Scholar]
- Gaab N., Gabrieli J.D.E., Glover G.H. Assessing the influence of scanner background noise on auditory processing. II. An fMRI study comparing auditory processing in the absence and presence of recorded scanner noise using a sparse design. Human Brain Mapping. 2007;28(8):721–732. doi: 10.1002/hbm.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab N., Gabrieli J.D.E., Glover G.H. Resting in peace or noise: Scanner background noise suppresses default-mode network. Human Brain Mapping. 2008;29(7):858–867. doi: 10.1002/hbm.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabig C.S. Verbal working memory and story retelling in school-age children with autism. Language, Speech, and Hearing Services in Schools. 2008;39(4):498–511. doi: 10.1044/0161-1461(2008/07-0023). [DOI] [PubMed] [Google Scholar]
- Gathercole S.E. Nonword repetition: More than just a phonological output task.Cognitive. Neuropsychology. 1995;12(8):857–861. doi: 10.1080/02643299508251405. [DOI] [Google Scholar]
- Gathercole S.E. Nonword repetition and word learning: The nature of the relationship. Applied Psycholinguistics. 2006;27(4):513–543. [Google Scholar]
- Gathercole S.E., Willis C.S., Baddeley A.D., Emslie H. The children’s test of nonword repetition: A test of phonological working memory. In Memory. 1994;2(2):103–127. doi: 10.1080/09658219408258940. [DOI] [PubMed] [Google Scholar]
- Gathercole S.E., Hitch G.J., Service E., Martin A.J. Phonological short-term memory and new word learning in children. Developmental Psychology. 1997;33(6):966–979. doi: 10.1037//0012-1649.33.6.966. [DOI] [PubMed] [Google Scholar]
- Gernsbacher M.A., Sauer E.A., Geye H.M., Schweigert E.K., Hill Goldsmith H. Infant and toddler oral- and manual-motor skills predict later speech fluency in autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2008;49(1):43–50. doi: 10.1111/j.1469-7610.2007.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K.J., Alfaro-Almagro F., Auer T., Bellec P., Capotă M., Chakravarty M.M., Churchill N.W., Cohen A.L., Craddock R.C., Devenyi G.A., Eklund A., Esteban O., Flandin G., Ghosh S.S., Guntupalli J.S., Jenkinson M., Keshavan A., Kiar G., Liem F., Poldrack R.A. BIDS apps: Improving ease of use, accessibility, and reproducibility of neuroimaging data analysis methods. PLoS Computational Biology. 2017;13(3):e1005209. doi: 10.1371/journal.pcbi.1005209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K., Risi S., Pickles A., Lord C. The Autism Diagnostic Observation Schedule (ADOS) Journal of Autism and Developmental Disorders. 2006 doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- Gray S., Fox A.B., Green S., Alt M., Hogan T.P., Petscher Y., Cowan N. Working Memory Profiles of Children with Dyslexia, Developmental Language Disorder, or Both. Journal of Speech, Language, and Hearing Research. 2019;62(6):1839–1858. doi: 10.1044/2019_JSLHR-L-18-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther . The MIT Press; 2016. Neural Control of Speech. [Google Scholar]
- Habib, A., Harris, L., Pollick, F., & Melville, C. (2019). A meta-analysis of working memory in individuals with autism spectrum disorders. In PLOS ONE (Vol. 14, Issue 4, p. e0216198). 10.1371/journal.pone.0216198. [DOI] [PMC free article] [PubMed]
- Hachmann W.M., Cashdollar N., Postiglione F., Job R. The relationship of domain-general serial order memory and reading ability in school children with and without dyslexia. Journal of experimental child psychology. 2020;193 doi: 10.1016/j.jecp.2019.104789. [DOI] [PubMed] [Google Scholar]
- Hanke, M. (2009). PyMVPA: a unifying approach to the analysis of neuroscientific data. In Frontiers in Neuroinformatics (Vol. 3). 10.3389/neuro.11.003.2009. [DOI] [PMC free article] [PubMed]
- Hansen B.B., Klopfer S.O. Optimal full matching and related designs via network flows. In. Journal of Computational and Graphical Statistics. 2006;5(3):609–627. [Google Scholar]
- Happe F., Frith U. The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G., Saur D., Price C.J., Baumgaertner A., Ulmer S., Siebner H.R. Increased facilitatory connectivity from the pre-SMA to the left dorsal premotor cortex during pseudoword repetition. Journal of Cognitive Neuroscience. 2013;25(4):580–594. doi: 10.1162/jocn_a_00342. [DOI] [PubMed] [Google Scholar]
- Hayiou-Thomas M.E., Carroll J.M., Leavett R., Hulme C., Snowling M.J. When does speech sound disorder matter for literacy? The role of disordered speech errors, co-occurring language impairment and family risk of dyslexia. Journal of Child Psychology and Psychiatry. 2017;58(2):197–205. doi: 10.1111/jcpp.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J.-D., Rees G. Decoding mental states from brain activity in humans. Nature Reviews. Neuroscience. 2006;7(7):523–534. doi: 10.1038/nrn1931. [DOI] [PubMed] [Google Scholar]
- Hiersche, K. J., Schettini, E., Li, J., & Saygin, Z. M. (2022). The language network is selective and distinct from other cognition in both function and connectivity in early childhood. bioRxiv. 10.1101/2022.08.11.503597.
- Heller Murray E., Segawa J., Karahanoglu F.I., Tocci C., Tourville J.A., Nieto-Castanon A., Tager-Flusberg H., Manoach D.S., Guenther F.H. Increased intra-subject variability of neural activity during speech production in people with autism spectrum disorder. Research in Autism Spectrum Disorders. 2022;94:101955. doi: 10.1016/j.rasd.2022.101955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. The functional neuroanatomy of language. Physics of life reviews. 2009;6(3):121–143. doi: 10.1016/j.plrev.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nature Reviews. Neuroscience. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hitch G.J., Baddeley A.D. Verbal Reasoning and Working Memory. In. Quarterly Journal of Experimental Psychology. 1976;28(4):603–621. doi: 10.1080/14640747608400587. [DOI] [Google Scholar]
- Ho D., Imai K., King G., Stuart E.A. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. Journal of Statistical Software. 2011;42(8):1–28. [Google Scholar]
- Hus V., Lord C. The Autism Diagnostic Observation Schedule, Module 4: Revised Algorithm and Standardized Severity Scores. Journal of Autism and Developmental Disorders. 2014;1–17 doi: 10.1007/s10803-014-2080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P., Levelt W.J.M. The spatial and temporal signatures of word production components. Cognition. 2004;92(1–2):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jacquemot C., Scott S.K. What is the relationship between phonological short-term memory and speech processing? Trends in Cognitive Sciences. 2006;10(11):480–486. doi: 10.1016/j.tics.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Järvinen-Pasley A., Wallace G.L., Ramus F., Happé F., Heaton P. Enhanced perceptual processing of speech in autism. Developmental Science. 2008;11(1):109–121. doi: 10.1111/j.1467-7687.2007.00644.x. [DOI] [PubMed] [Google Scholar]
- Jeffries S., Everatt J. Working memory: its role in dyslexia and other specific learning difficulties. Dyslexia (Chichester, England) 2004;10(3):196–214. doi: 10.1002/dys.278. [DOI] [PubMed] [Google Scholar]
- Joanisse M., Manis F.R., Keating P., Seidenberg M.S. Language Deficits in Dyslexic Children: Speech Perception, Phonology, and Morphology. Journal of Experimental Child Psychology. 2000;77(1):30–60. doi: 10.1006/jecp.1999.2553. [DOI] [PubMed] [Google Scholar]
- Joseph R.M., Tager-Flusberg H. The relationship of theory of mind and executive functions to symptom type and severity in children with autism. Development and psychopathology. 2004;16(1):137–155. doi: 10.1017/s095457940404444x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitani Y., Tong F. Decoding the visual and subjective contents of the human brain. Nature Neuroscience. 2005;8(5):679–685. doi: 10.1038/nn1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A.S., Kaufman N.L. Manual; 2004. Kaufman Brief Intelligence Test. KBIT 2; [Google Scholar]
- Ketcheson L.R., Pitchford E.A., Wentz C.F. The Relationship Between Developmental Coordination Disorder and Concurrent Deficits in Social Communication and Repetitive Behaviors Among Children with Autism Spectrum Disorder. Autism Research: Official Journal of the International Society for Autism Research. 2021;14(4):804–816. doi: 10.1002/aur.2469. [DOI] [PubMed] [Google Scholar]
- Kjelgaard M.M., Tager-Flusberg H. An Investigation of Language Impairment in Autism: Implications for Genetic Subgroups. Language and Cognitive Processes. 2001;16(2–3):287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino, H., Carpenter, P. A., Minshew, N. J., Cherkassky, V. L., Keller, T. A., & Just, M. A. (2005). Functional connectivity in an fMRI working memory task in high-functioning autism. In NeuroImage (Vol. 24, Issue 3, pp. 810–821). 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed]
- Koster-Hale, J., Saxe, R., Dungan, J., & Young, L. L. (2013). Decoding moral judgments from neural representations of intentions. In Proceedings of the National Academy of Sciences (Vol. 110, Issue 14, pp. 5648–5653). 10.1073/pnas.1207992110. [DOI] [PMC free article] [PubMed]
- Kovelman I., Norton E.S., Christodoulou J.A., Gaab N., Lieberman D.A., Triantafyllou C., Gabrieli J.D. Brain basis of phonological awareness for spoken language in children and its disruption in dyslexia. Cerebral Cortex. 2012;22(4):754–764. doi: 10.1093/cercor/bhr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kover S.T., Atwood A.K. Establishing equivalence: methodological progress in group-matching design and analysis. American Journal of Intellectual Developmental Disabilities. 2013;118(1):3–15. doi: 10.1352/1944-7558-118.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S., Alcock K.J., Mercure E., Leech R., Barker E., Karmiloff-Smith A., Dick F. Articulating novel words: children's oromotor skills predict nonword repetition abilities. Journal of Speech, Language, and Hearing Research. 2013;56(6):1800–1812. doi: 10.1044/1092-4388(2013/12-0206). [DOI] [PubMed] [Google Scholar]
- Lambrecht Smith S., Roberts J.A., Locke J.L., Tozer R. An Exploratory Study of the Development of Early Syllable Structure in Reading-Impaired Children. Journal of Learning Disabilities. 2010;43(4):294–307. doi: 10.1177/0022219410369094. [DOI] [PubMed] [Google Scholar]
- Leung R.C., Vogan V.M., Powell T.L., Anagnostou E., Taylor M.J. The role of executive functions in social impairment in Autism Spectrum Disorder. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence. 2016;22(3):336–344. doi: 10.1080/09297049.2015.1005066. [DOI] [PubMed] [Google Scholar]
- Liégeois M.A., Connelly A., Vargha-Khadem F. Endophenotypes of FOXP2: Dysfunction within the human articulatory network. European Journal of Paediatric Neurology. 2011;15(4):283–288. doi: 10.1016/j.ejpn.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Long, Katlowitz, K., Svirsky, M., Clary, R., Byun, T., Majaj, N., Oya, H., Howard, M., & Greenlee, J. . (2016). Functional Segregation of Cortical Regions Underlying Speech Timing and Articulation. Neuron (Cambridge, Mass.), 89(6), 1187–1193. 10.1016/j.neuron.2016.01.032. [DOI] [PMC free article] [PubMed]
- Lord C., Risi S., Lambrecht L., Cook E.H., Jr, Leventhal B.L., DiLavore P.C., Pickles A., Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of autism and developmental disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C., DiLavore P.C., Gotham K., Guthrie W., Luyster R., Risi S., Rutter M. (2nd Edition). Western Psychological Services; 2012. Autism diagnostic observation schedule: ADOS-2. [Google Scholar]
- Lu C., Qi Z., Harris A., Weil L.W., Han M., Halverson K., Perrachione T.K., Kjelgaard M., Wexler K., Tager-Flusberg H., Gabrieli J.D.E. Shared neuroanatomical substrates of impaired phonological working memory across reading disability and autism. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging. 2016;1(2):169–177. doi: 10.1016/j.bpsc.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn A., Luna B., O’Hearn K. Visual working memory performance is intact across development in autism spectrum disorder. Autism Research. 2022;15(5):881–891. doi: 10.1002/aur.2683. [DOI] [PubMed] [Google Scholar]
- Macizo P., Soriano M.F., Paredes N. Phonological and Visuospatial Working Memory in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2016;46(9):2956–2967. doi: 10.1007/s10803-016-2835-0. [DOI] [PubMed] [Google Scholar]
- Majerus S. Language repetition and short-term memory: An integrative framework. Frontiers in Human Neuroscience. 2013;7, Article 357 doi: 10.3389/fnhum.2013.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis F., Mcbride-Chang C., Seidenberg M.S., Keating P., Doi L.M., Munson B., Petersen A. Are Speech Perception Deficits Associated with Developmental Dyslexia? Journal of Experimental Child Psychology. 1997;66(2):211–235. doi: 10.1006/jecp.1997.2383. [DOI] [PubMed] [Google Scholar]
- Melby-Lervag M., Lervag A. Oral Language Skills Moderate Nonword Repetition Skills in Children With Dyslexia: A Meta-Analysis of the Role of Nonword Repetition Skills in Dyslexia. Scientific Studies of Reading. 2012;16(1):1–34. doi: 10.1080/10888438.2010.537715. [DOI] [Google Scholar]
- Mody M., Shui A.M., Nowinski L.A., Golas S.B., Ferrone C., O’Rourke J.A., McDougle C.J. Communication Deficits and the Motor System: Exploring Patterns of Associations in Autism Spectrum Disorder (ASD) Journal of Autism and Developmental Disorders. 2017;47(1):155–162. doi: 10.1007/s10803-016-2934-y. [DOI] [PubMed] [Google Scholar]
- Mottron L., Burack J.A. In: The Development of Autism: Perspectives From Theory and Research. Burack J.A., Charman T., Yirmiya N., Zelazo P.R., editors. Lawrence Erlbaum Associates Publishers; 2001. Enhanced perceptual functioning in the development of autism; pp. 131–148. [Google Scholar]
- Nachev P., Wydell H., O'neill K., Husain M., Kennard C. The role of the pre-supplementary motor area in the control of action. NeuroImage. 2007;36(Suppl 2(3–3)):T155. doi: 10.1016/j.neuroimage.2007.03.034. T163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadig A., Mulligan A. Intact non-word repetition and similar error patterns in language-matched children with autism spectrum disorders: A pilot study. Journal of Communication Disorders. 2017;66:13–21. doi: 10.1016/j.jcomdis.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberauer K., Lewandowsky S., Farrell S., et al. Modeling working memory: An interference model of complex span. Psychon Bull Rev. 2012;19:779–819. doi: 10.3758/s13423-012-0272-4. [DOI] [PubMed] [Google Scholar]
- Ozonoff S., Rogers S.J., Pennington B.F. Asperger's syndrome: evidence of an empirical distinction from high-functioning autism. Journal of child psychology and psychiatry, and allied disciplines. 1991;32(7):1107–1122. doi: 10.1111/j.1469-7610.1991.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Pang E.W., Valica T., MacDonald M.J., Taylor M.J., Brian J., Lerch J.P., Anagnostou E. Abnormal Brain Dynamics Underlie Speech Production in Children with Autism Spectrum Disorder. Autism Research: Official Journal of the International Society for Autism Research. 2016;9(2):249–261. doi: 10.1002/aur.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Carp J., Hebrank A., Park D.C., Polk T.A. Neural specificity predicts fluid processing ability in older adults. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(27):9253–9259. doi: 10.1523/JNEUROSCI.0853-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle J.E. Methodological challenges and solutions in auditory functional magnetic resonance imaging. Frontiers in neuroscience. 2014;8:253. doi: 10.3389/fnins.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeva T.J.A., Agam Y., Holland B., Manoach D.S., Guenther F.H. White matter impairment in the speech network of individuals with autism spectrum disorder. NeuroImage Clinical. 2013;3(C):234–241. doi: 10.1016/j.nicl.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce J.W., Gray J.R., Simpson S., MacAskill M.R., Höchenberger R., Sogo H., Kastman E., Lindeløv J. PsychoPy2: experiments in behavior made easy. Behavior Research Methods. 2019 doi: 10.3758/s13428-018-01193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano E. The development of executive function in autism. Autism research and treatment. 2012;2012 doi: 10.1155/2012/146132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano E., Burr D. When the world becomes 'too real': a Bayesian explanation of autistic perception. Trends in cognitive sciences. 2012;16(10):504–510. doi: 10.1016/j.tics.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Perrachione T.K., Ghosh S.S. Optimized design and analysis of sparse-sampling FMRI experiments. Frontiers in Neuroscience. 2013;7:55. doi: 10.3389/fnins.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrachione T.K., Ghosh S.S., Ostrovskaya I., Gabrieli J.D.E., Kovelman I. Phonological Working Memory for Words and Nonwords in Cerebral Cortex. Journal of Speech, Language, and Hearing Research: JSLHR. 2017;60(7):1959–1979. doi: 10.1044/2017_JSLHR-L-15-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter B., Raskind W.H., Matsushita M., Lisowski M., Vu T., Berninger V.W.…Brkanac Z. Replication of CNTNAP2 association with nonword repetition and support for FOXP2 association with timed reading and motor activi- ties in a dyslexia family sample. Journal of Neurodevelopmental Disorders. 2011;3:39–49. doi: 10.1007/s11689-010-9065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigdon L., Willmott C., Reilly S., Conti-Ramsden G., Liegeois F., Connelly A., Morgan A.T. The neural basis of nonword repetition in children with developmental speech or language disorder: An fMRI study. Neuropsychologia. 2020;138 doi: 10.1016/j.neuropsychologia.2019.107312. [DOI] [PubMed] [Google Scholar]
- Poeppel, D. (2015). Speech Perception. In Brain Mapping (pp. 429–434). 10.1016/b978-0-12-397025-1.00264-5.
- Pomper R., Ellis Weismer S., Saffran J., Edwards J. Specificity of Phonological Representations for Children with Autism Spectrum Disorder. Journal of autism and developmental disorders. 2019;49(8):3351–3363. doi: 10.1007/s10803-019-04054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posar A., Visconti P. Early Motor Signs in Autism Spectrum Disorder. Children (Basel, Switzerland) 2022;9(2):294. doi: 10.3390/children9020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiee A., Vasaghi-Gharamaleki B., Samadi S.A., Amiri-Shavaki Y., Alaghband-Rad J., Seyedin S., Hosseini S. Impaired nonverbal working memory in high-functioning autism spectrum disorder. Medical journal of the Islamic Republic of. Iran. 2018;32:107. doi: 10.14196/mjiri.32.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J., Wagner A.D. Distributed representations in memory: insights from functional brain imaging. Annual Review of Psychology. 2012;63:101–128. doi: 10.1146/annurev-psych-120710-100344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M., Bailey A., Lord C. Western Psychological Services; Los Angeles, CA: 2003. The social communication questionnaire. [Google Scholar]
- Sachse M., Schlitt S., Hainz D., et al. Executive and Visuo-motor Function in Adolescents and Adults with Autism Spectrum Disorder. J Autism Dev Disord. 2013;43:1222–1235. doi: 10.1007/s10803-012-1668-8. [DOI] [PubMed] [Google Scholar]
- Sajjadtorabian. (2018, August 10). sajjadtorabian/PyMVPA: PyMVPA BIDS App version v1.0.0 (Version v1.0.0). Zenodo. http://doi.org/10.5281/zenodo.1343531.
- Samuel A.G. Speech Perception. Annual Review of Psychology. 2011;62(1):49–72. doi: 10.1146/annurev.psych.121208.131643. [DOI] [PubMed] [Google Scholar]
- Scott T.L., Perrachione T.K. Common cortical architectures for phonological working memory identified in individual brains. NeuroImage. 2019;202 doi: 10.1016/j.neuroimage.2019.116096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E., Wiig E.H., Secord W.A. The Psychological Corporation/A Harcourt Assessment Company; Toronto, Canada: 2004. Clinical evaluation of language fundamentals, fourth edition—Screening test (CELF-4 screening test) [Google Scholar]
- Smith-Spark J.H., Fisk J.E. Working memory functioning in developmental dyslexia. Memory. 2007;15(1):34–56. doi: 10.1080/09658210601043384. [DOI] [PubMed] [Google Scholar]
- Smith-Spark J.H., Henry L.A., Messer D.J., Edvardsdottir E., Zięcik A.P. Executive functions in adults with developmental dyslexia. Research in developmental disabilities. 2016;53-54:323–341. doi: 10.1016/j.ridd.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Soulières I., Mottron L., Saumier D., Larochelle S. Atypical categorical perception in autism: autonomy of discrimination? Journal of Autism and Developmental Disorders. 2007;37(3):481–490. doi: 10.1007/s10803-006-0172-4. [DOI] [PubMed] [Google Scholar]
- Strand F., Forssberg H., Klingberg T., Norrelgen F. Phonological working memory with auditory presentation of pseudo-words — An event related fMRI Study. Brain Research. 2008;1212(30 May):48–54. doi: 10.1016/j.brainres.2008.02.097. [DOI] [PubMed] [Google Scholar]
- Tarar, J. M., Meisinger, E. B., & Dickens, R. H. (2015). Test Review: Test of Word Reading Efficiency–Second Edition (TOWRE-2) by Torgesen, J. K., Wagner, R. K., & Rashotte, C. A. In Canadian Journal of School Psychology (Vol. 30, Issue 4, pp. 320–326). 10.1177/0829573515594334.
- Torgesen, J. K., Wagner, R., & Rashotte, C. (2012). Test of Word Reading Efficiency: (TOWRE-2).
- Tourville J.A., Guenther F.H. The DIVA model: A neural theory of speech acquisition and production. Language and Cognitive Processes. 2011;26(7):952–981. doi: 10.1080/01690960903498424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay P., Gracco V.L. Contribution of the pre-SMA to the production of words and non-speech oral motor gestures, as revealed by repetitive transcranial magnetic stimulation (rTMS) Brain Res. 2009;1(1268):112–124. doi: 10.1016/j.brainres.2009.02.076. Epub 2009 Mar 12 PMID: 19285972. [DOI] [PubMed] [Google Scholar]
- Tryfon A., Foster N.E.V., Sharda M., Hyde K.L. Speech perception in autism spectrum disorder: An activation likelihood estimation meta-analysis. Behavioural Brain Research. 2018;338:118–127. doi: 10.1016/j.bbr.2017.10.025. [DOI] [PubMed] [Google Scholar]
- Van Bergen E., van der Leij A., de Jong P. The intergenerational multiple deficit model and the case of dyslexia. Frontiers in Human Neuroscience. 2014;8:346. doi: 10.3389/fnhum.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogan V.M., Francis K.E., Morgan B.R., Smith M.L., Taylor M.J. Load matters: neural correlates of verbal working memory in children with autism spectrum disorder. Journal of Neurodevelopmental Disorders. 2018;10(1):19. doi: 10.1186/s11689-018-9236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang Y.b., Liu L.l., et al. A Meta-Analysis of Working Memory Impairments in Autism Spectrum Disorders. Neuropsychol Rev. 2017;27:46–61. doi: 10.1007/s11065-016-9336-y. [DOI] [PubMed] [Google Scholar]
- Weismer S.E., Bruce Tomblin J., Zhang X., Buckwalter P., Chynoweth J.G., Jones M. Nonword Repetition Performance in School-Age Children With and Without Language Impairment. In Journal of Speech, Language, and Hearing Research (Vol. 2000;43(4):865–878. doi: 10.1044/jslhr.4304.865. [DOI] [PubMed] [Google Scholar]
- West K.L. Infant motor development in autism spectrum disorder: A synthesis and meta-analysis. Child development. 2019;90(6):2053–2070. doi: 10.1111/cdev.13086. [DOI] [PubMed] [Google Scholar]
- Whitehouse Barry J.G., Bishop D.V. Further defining the language impairment of autism: Is there a specific language impairment subtype? Journal of Communication Disorders. 2008;41(4):319–336. doi: 10.1016/j.jcomdis.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Whyatt C., Craig C. Sensory-motor problems in Autism. Frontiers in integrative neuroscience. 2013;7:51. doi: 10.3389/fnint.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.L., Goldstein G., Carpenter P.A., Minshew N.J. Verbal and spatial working memory in autism. Journal of Autism and Developmental Disorders. 2005;35(6):747–756. doi: 10.1007/s10803-005-0021-x. [DOI] [PubMed] [Google Scholar]
- Williams D.L., Goldstein G., Minshew N.J. The profile of memory function in children with autism. In Neuropsychology. 2006;20(1):21–29. doi: 10.1037/0894-4105.20.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D., Payne H., Marshall C. Non-word repetition impairment in autism and specific language impairment: evidence for distinct underlying cognitive causes. Journal of Autism and Developmental Disorders. 2013;43(2):404–417. doi: 10.1007/s10803-012-1579-8. [DOI] [PubMed] [Google Scholar]
- Woodcock R.W. third edition. Pearson; 2011. Woodcock reading mastery tests. [Google Scholar]
- Wynn C.J., Josephson E.R., Borrie S.A. An Examination of Articulatory Precision in Autistic Children and Adults. Journal of Speech, Language, and Hearing Research. 2022;65(4):1416–1425. doi: 10.1044/2021_JSLHR-21-00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T., Markiewicz C.J., de la Vega A., Gorgolewski K.J., Salo T., Halchenko Y.O., McNamara Q., DeStasio K., Poline J.-B., Petrov D., Hayot-Sasson V., Nielson D.M., Carlin J., Kiar G., Whitaker K., DuPre E., Wagner A., Tirrell L.S., Jas M., Blair R. PyBIDS: Python tools for BIDS datasets. Journal of Open Source Software. 2019;4(40) doi: 10.21105/joss.01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung M.K., Lee T.L., Chan A.S. Right-lateralized frontal activation underlies successful updating of verbal working memory in adolescents with high-functioning autism spectrum disorder. Biological Psychology. 2019;148 doi: 10.1016/j.biopsycho.2019.107743. [DOI] [PubMed] [Google Scholar]
- Zampella C.J., Wang L.A.L., Haley M., Hutchinson A.G., de Marchena A. Motor Skill Differences in Autism Spectrum Disorder: a Clinically Focused Review. Current Psychiatry Reports. 2021;23(10):64. doi: 10.1007/s11920-021-01280-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Pre-processed z-maps have been uploaded to GitHub as an anonymized data set (https://github.com/Amandaobrien8/ynicl_103299/). Additionally, one anonymized participant's data has been uploaded to GitHub with an accompanying tutorial to reproduce our MVPA results at a single-subject level.
Preprocessed fMRI data in the form of z-maps are available on Github (https://github.com/Amandaobrien8/ynicl_103299/).