Abstract
Appendicular skeletal mass is commonly used to assess the loss in muscle mass and Ultra Sound (US) approach represents a valid and reliable method. However, the procedural protocols are still heterogeneous. The aim of this study was to compare the intertransducers validity of thickness, width, and CSA measurements of rectus femoris (RF) muscle. The anteroposterior (AP), (laterolateral) LL and (cross-sectional area) CSA of RF muscle were evaluated with both linear and curve probes in ten healthy subjects and six sarcopenic patients. In the healthy group the mean AP diameters measured with the linear array were significantly higher than those measured with the curved array. AP and CSA were higher in the healthy group compared with the sarcopenic group with both transducers. There was a positive correlation between weight and LL diameter, and a negative correlation between age and muscle AP, measured with the linear probe. Both linear and curved probes represent valid methods in US evaluation of the CSA of the RF muscle. However, in the healthy subjects, the thickness and width of the same muscle, are affected by the type of probe
Key Words: Muscle, quadriceps, ultrasonography, sarcopenia, validity
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Ageing is related to changes in body composition, with a progressive loss of muscle mass.1,2 The intrinsic ability of skeletal muscle to repair after injury-induced damage could be compromised with age and could be associated with reduced force generation, sarcopenia and fibrosis.3 Sarcopenia, affects up to 10% of the general elderly population and represents a condition related to higher risk of morbidity and mortality.4-8 To assess the loss in muscle mass, appendicular skeletal muscle mass measurement is commonly used.9,10 Particularly, it has been demonstrated that the anterior thigh muscle mass assessment may represent a valuable criterion in the early diagnosis of sarcopenia.11
Among the instrumental tools for the assessment of muscle mass, there are the bioelectrical impedance analysis (BIA), dual X-ray absorptiometry (DXA), magnetic magnetic resonance imaging (MRI) and computed tomography (CT).12 Ultra Sound (US) represents the easy, quick, safe, valid and reliable alternative tool to quantify the local muscle mass.13,14 However, the US assessment procedure in the different studies varies and the setting parameters are not always clearly specified. Moreover, as previously demonstrated, the choice of a different transducer (linear or curved) can influence the cross-sectional area (CSA) measurement in rectus femoris (RF) muscle quantitative assessment.15 However, muscle thickness is considered to be the simplest, quickest and most reproducible parameter in evaluating muscle mass, with a good correlation with the gold standard measures.16 Particularly, a strong correlation between quadriceps muscle thickness and isometric voluntary maximum contraction force has been demonstrated.17 Based on these observations the primary aim of the study was to compare the intertransducers validity of thickness, width and CSA measurements of RF muscle in a population of healthy subjects. The second objectives were: (i) to evaluate the intertransducers validity in a small sample of sarcopenic patients, (ii) to compare the results between the two groups and (iii) to evaluate possible correlation between demographical data and RF quantitative parameters. The novelty of our study is represented by the comparison of the two US probes (convex and linear) from different points of view, evaluating their possible differences/similarities in one-dimensional and bi-dimensional measures of the muscle. This evaluation is not clearly provided in literature besides the common use of the US parameters.
Table 1.
Characteristics of the study population.
| Healthy | Sarcopenic | |
|---|---|---|
| Age (years) | 31.45 | 71.33 |
| Female (n/tot) | 6/10 | 4/6 |
| Weight (kg) | 68.27 | 68.17 |
| BMI (kg/m2) | 23.48 | 24.38 |
The results are expressed as means.
Materials and Methods
A representative sample of healthy adult subjects was selected. Inclusion criteria were: age between 18 and 65 years, absence of previous lower extremity muscle trauma, absence of myopathy, neurological and orthopaedic diseases affecting muscles. A sample of sarcopenic patients afferent to our outpatient clinic was also recruited. The diagnosis of sarcopenia was made according to criteria established by EWGSOP2.18 All participants provided written informed consent.
B-mode ultrasound was used (Hitachi Avius Hi-vision), using a 14 MHz linear array transducer with a length of 5 cm, and a 5 MHz curved array transducer with a length of 7 cm. Gain (22 dB), depth (40 mm) and focus (20 mm) were kept constant.
Participants were asked to lie in a supine position with extended knee and relaxed muscle. To avoid fluid shifts, subjects respected a 30 minutes rest before the examination.
The measurements were made on both legs for each participant at the union site between the proximal two-thirds and the distal third of a line linking the anterior superior iliac spine and the superior pole of the patellar bone. This point was marked with a dermographic pen (Figure 1). This method was chosen to allow capturing the whole area of RF muscle with both linear and curved probe. After applying a coupling US gel, the probe was placed on the skin along the long axis of the femur with a light pressure to avoid the muscle compression. After identifying the axial section of RF (Figure 2a,2b) three parameters were recorded:
the minimum AP diameter as the distance between the superficial and the deep aponeurosis;
the maximum LL diameter as the width of the muscle;
the cross-sectional area, manually measured by tracing the inner hyperechoic line of the RF aponeurosis.
Student t-test was used to compare the values obtained with the different probes. Results are expressed as means and SD. The validity of AP and LL diameters measurements obtained with the curved array transducer compared with measurements obtained with the linear-array transducer was assessed by Bland-Altman analysis.19 The association between the quantitative measurements and demographical data was evaluated by means of Spearman’s correlation coefficient. Statistical significance was set at p ≤ 0.05.20
Table 2.
Characteristics of the study population.
| Linear | Linear | |||||
|---|---|---|---|---|---|---|
| Parameters | Linear (H) | Curved (H) | vs Curved (H) |
Linear (S) | Curved (S) | vs Curved (S) |
| Right AP diameter | 13.11 ± (1.86) |
12.05 ± (2.21) | p= 0.001* | 6.33 ± (2.19) | 6.28 ± (1.71) | p= 0.92 |
| Left AP diameter | 13.06 ± (2.02) | 12.03 ± (2.05) | p= 0.020* | 6.77 ± (1.91) | 6.20 ± (1.24) | p= 0.92 |
| Right LL diameter | p= 0.048* | p= 0.56 | ||||
| (3.20) | (2.60) | (4.59) | (9.71) | |||
| Left LL diameter | 31.70 ± (3.66) | 33.85 ± (4.01) | p= 0.13 | 32.62 ± (5.51) | 34.42 ± (5.66) | p= 0.21 |
| Right CSA | p= 0.38 | p= 0.55 | ||||
| (52.84) | (66.91) | (75.67) | (92.28) | |||
| Left CSA | p= 0.81 | p= 0.85 | ||||
| (79.28) | (87.90) | (70.45) | (44.01) |
Results are expressed as means ± standard deviations. AP: anteroposterior. LL: laterolateral. CSA: cross sectional area. H: healthy. S: sarcopenic. The AP and LL diameters are reported in mm; the CSA is reported in mm2.
Table 3.
Intergroup comparison.
| LINEAR PROBE | CURVED PROBE | |||||
|---|---|---|---|---|---|---|
| PARAMETERS | H | S | H vs S | H | S | H vs S |
| Right AP diameter | 13.11 ± (1.86) | 6.33 ± (2.19) | p= 0.00004* | 12.05 ± (2.21) | 6.28 ± (1.71) | p= 0.0002* |
| Left AP diameter | 13.06 ± (2.02) | 6.77 ± (1.91) | p= 0.00006* | 12.03 ± (2.05) | 6.20 ± (1.24) | p= 0.0001* |
| Right LL diameter | 32.13 ± (3.20) | 33.07 ± (4.59) | p= 0.658 | 34.24 ± (2.60) | 35.17 ± (9.71) | p= 0.798 |
| Left LL diameter | 31.70 ± (3.66) | 32.62 ± (5.51) | p= 0.714 | 33.85 ± (4.01) | 34.42 ± (5.66) | p= 0.829 |
| Right CSA | 347.90 ± (52.84) | 205.40 ± (75.67) | p= 0.0009* | 359.30 ± (66.91) | 194.00 ± (92.28) | p= 0.0010* |
| Left CSA | 359.50 ± (79.28) | 189.40 ± (70.45) | p= 0.00137* | 364.00 ± (87.90) | 185.60 ± (44.01) | p= 0.0010* |
Results are expressed as means ± standard deviations. AP: anteroposterior. LL: laterolateral. CSA: cross sectional area. H: healthy. S: sarcopenic. The AP and LL diameters are reported in mm; the CSA is reported in mm 2.
Results
A total of 10 healthy subjects and 6 sarcopenic patients participated to the study. The two groups were homogeneous in mean weight, BMI and gender, while they were different in number of subjects and mean age. Demographical characteristics are presented in Table 1.
In the healthy group the mean AP diameters of both right and left side measured with the linear array were significantly higher than those measured with the curved array (respectively p= 0.001 and p= 0.02 for the right and left side). The values of the LL diameter were significantly higher in the curved array measurement only for the right side (p= 0.048). Differences in CSA of rectus femoris recorded with the two transducers were not significant in both healthy and sarcopenic groups. Results are presented in Table 2. Figure 3a-d shows the agreement between the evaluations of muscle size with the different probes.
In the healthy group significant higher values in both right and left AP diameters (p= 0.00004 and p= 0.00006, respectively) were observed with the linear array transducer. Higher mean CSA of both right and left RF (p= 0.0009 and p= 0.0014, respectively) was also observed. Similarly, the curved array transducer measurements showed higher mean AP diameter values in the healthy group for both right (p= 0.0002) and left side (p= 0.0001). The CSA of right (p= 0.0023) and left (p= 0.001) RF muscle revealed higher values in healthy participants too. The remaining parameters were not significant. Data are presented in Table 3. Figure 3 shows the level of agreement between linear and curve probes’ assessment of the AP and LL diameters of RF muscle.
A significant positive correlation between weight and LL diameter of right (r= 0.576, p= 0.02) and left (r= 0.614, p= 0.01) side measured with the linear array transducer was observed. Moreover, there was a significant negative correlation between age and right AP diameter (r= -0.612, p= 0.012) and between age and left AP diameter (r= -0.590, p= 0.016), both measured with the linear probe. A significant negative correlation between age and right and left AP diameters was observed also with the curved array transducer measurement (respectively: r= -0.556, p= 0.025; r= -0.548, p= 0.028). Results are shown in Table 4.
Table 4.
Correlation coefficients (r) between demographical and ultrasonographic data.
| Linear | |||||||
|---|---|---|---|---|---|---|---|
| Right AP | Right LL | Right CSA | Left AP | Left LL | Left CSA | ||
| Weight | r | 0.016 | 0.576* | 0.148 | 0.183 | 0.614* | 0.313 |
| p | 0.952 | 0.020 | 0.585 | 0.497 | 0.011 | 0.238 | |
| Age | r | -0.612* | 0,239 | -0.559* | -0.590* | 0.109 | -0.545* |
| p | 0.012 | 0.373 | 0.024 | 0.016 | 0.689 | 0.029 | |
| Curved | |||||||
| Right AP | Right LL | Right CSA | Left AP | Left LL | Left CSA | ||
| Weight | r | 0.086 | 0.192 | 0.232 | 0.175 | 0.356 | 0.353 |
| p | 0.750 | 0.477 | 0.388 | 0.517 | 0.176 | 0.179 | |
| Age | r | -0.556* | -0.055 | -0.454 | -0.548* | 0.058 | -0.557* |
| p | 0.025 | 0.841 | 0.077 | 0.028 | 0.830 | 0.025 | |
AP: anteroposterior. Significance was set at p<0.05. LL: latero-lateral. CSA: cross sectional area.
Fig 1.
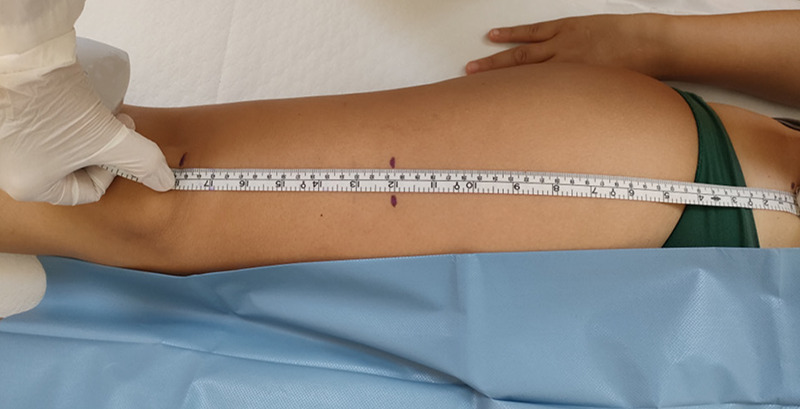
Identification of the measurement point.
A significant positive correlation between weight and LL diameter of right (r= 0.576, p= 0.02) and left (r= 0.614, p= 0.01) side measured with the linear array transducer was observed. Moreover, there was a significant negative correlation between age and right AP diameter (r= -0.612, p= 0.012) and between age and left AP diameter (r= -0.590, p= 0.016), both measured with the linear probe. A significant negative correlation between age and right and left AP diameters was observed also with the curved array transducer measurement (respectively: r= -0.556, p= 0.025; r= -0.548, p= 0.028). Results are shown in Table 4.
Discussion
Three major findings emerge from this study. First, although the linear and the curved probes provide similar results in estimating the CSA of the RF, in the healthy subjects the thickness (AP diameter) and width (LL diameter) values are affected by the type of probe used. Probably, the difference in the diameters are significant but small and they cannot necessarily imply a significant variation in CSA. However, the ultrasonographic intertransducer comparison for the assessment of the thickness of RF muscle, showed significant higher mean values for the linear-array transducer compared to the curved one. This finding could be explained by the different propagation of the sound waves from the two transducers into the tissues. Indeed, while the scan lines originated from a linear probe propagate parallel to each other, the scan lines produced by a curved probe propagate with an arc shape, thus creating a distorted image.21 This assumption could be confirmed by the observation of higher mean values of the LL diameters of RF muscle (corresponding to the muscle width) with the curved array measurements, although significant only for the right side. The result is relevant because, in some studies, the assessment of AP diameter is used for muscle evaluation. According to the EWGSOP, sarcopenia diagnosis is based on three criteria: i) low muscle strength, ii) small muscle quantity or quality, and iii) low physical performance.18,22 US represents a valid and reliable method to estimate the peripheral muscle size, thus making it an useful diagnostic tool for sarcopenia.4,16 Although the main parameters for evaluating muscle mass (muscle thickness, CSA and pennation angle) have been identified in previous studies, less is known about the complete US procedure. Particularly, although the selection of the transducer represent an important part of the US examination, only one study investigated the intertransducer validity between different transducers in assessing RF muscle.15 However, the cited study only assesses the differences in CSA, not including the thickness measurement’s comparison, that is considered to be the simplest, quickest and most reproducible parameter for muscle mass, which correlates well with the gold standard measures.16,23 The second finding of the study is that the intergroup comparison showed that both linear and curved array transducers provided significant higher values in muscle thickness (AP diameter) and CSA in healthy subject compared to the sarcopenic patients. This result confirms what is previously demonstrated, since ageing leads to loss in muscle mass and increase in muscle fatty infiltration.24,25 The age-related reduction in skeletal muscle mass does not occur at the same time in all anatomic regions but is greater in lower limbs compared to the upper limbs, particularly at the anterior tight muscles.11 Moreover, the functional correlates of quantitative muscle ultrasonographic parameters demonstrated that variations of muscle mass are good predictors of changes in muscle function (26). Therefore, the muscle US evaluation in sarcopenic patients could be useful not only in the diagnostic process, but also in the rehabilitation phase, helping the clinician to adapt and customize the rehabilitation program to the individual subject. Indeed, due to the plasticity of muscle architecture, it has been demonstrated that a prolonged eccentric resistance training exercise program could lead to an increase in fiber length of trained muscles, measured by ultrasonography, in both younger and older subjects, while a fascicle shortening has been reported after gastrocnemius recession in spastic diplegia patients.27–30
Third, the comparison between anthropometric and ultrasonographic data, showed a positive correlation between body weight and muscle width (LL diameter) and a negative correlation between age and muscle thickness and CSA. These results are similar to those of previous studies and can be explained by the decrease in total muscle mass in ageing.11,16,31 This phenomenon can be, at least in part, explained by reorganization in the neuromuscular system and the Central Nervous System, with a progressive loss of spinal motor neurons and a consequent decline in muscle fiber number and size.32 The loss in muscle mass dramatically increases after the age of 60 and is associated to leads to an increased risk of physical disability,33 cognitive decline, 34 metabolic disorders and mortality.35 Although DXA has been widely used for the assessment of skeletal muscle mass, particularly for the determination of appendicular lean tissue mass, in older adults, this technique is costly and exposes participants to radiation.11 On the other hand, ultrasound has been demonstrated to represent a reliable and valid tool for the assessment of muscle size in older adults,14 and the results of the current study are in accordance with the literature. Based on the data of our study, we want to propose an US procedure for the evaluation of RF by underlying characteristics that differentiate it from current guideline indications:
Fig 2.
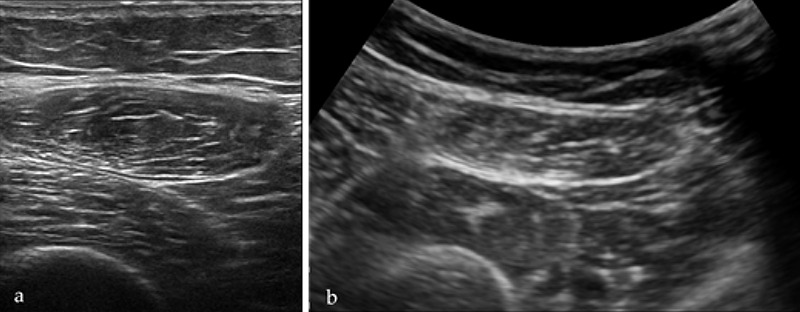
Ultrasonographic axial view of the RF muscle with linear (a) and curved (b) array transducers.
Fig 3.
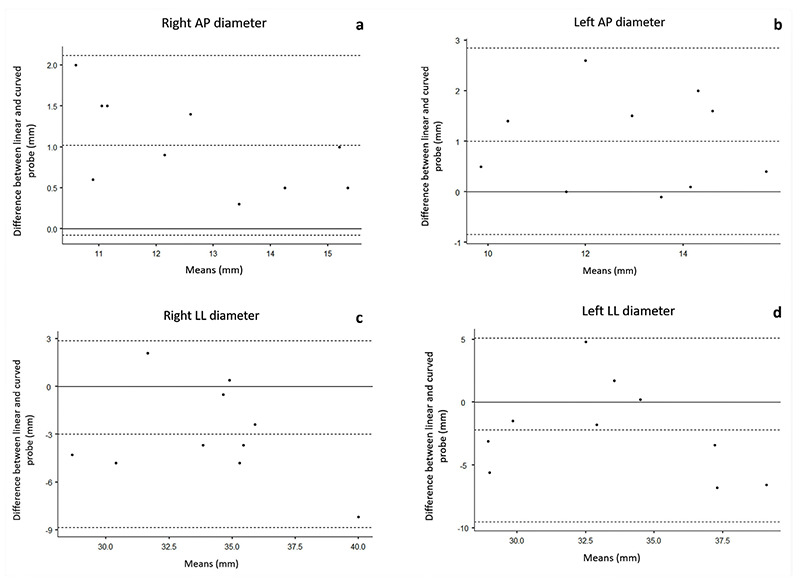
Bland-Altman plots illustrating the agreement between linear and curved array transducers for the right AP diameter (a), left AP diameter (b), right LL diameter (c) and left LL diameter (d). AP: antero-posterior. LL: laterolateral.
Point of measurement placed at 2/3 of the distance between the antero-superior iliac spine and the superior patellar pole. Indeed, at this point it is possible to analyze the entire muscle belly with both the curved and the linear array transducers in all subjects.
Detection of AP diameter (thickness) and CSA as main quantitative parameters that are valid, reliable, and repeatable also with different probes, compared, for example, to qualitative parameters, such as echogenicity, that are difficult to objectively quantify, or other quantitative measures, such as pennation angle, not detectable with curved probes.
Evaluation of LL diameter, not included among the standard parameters, but used in clinical practice for the dynamic study of muscle contraction.
Single measurement of each parameter by a single experienced clinician instead of considering the mean of three values, to speed up the execution in daily clinical practice.
This study has some limitations. First, the small sample size that makes it difficult to extend the results to the general population. Moreover, since the main findings are observed in the healthy subjects’ group, the considerations cannot be extended to individuals with obesity or peripheral edema. Third, the lack of inter-operator comparison and the detection of only one value per parameter could lead to a possible underestimation of the inter-operator variability.
In conclusion, data from the current study confirm that both linear and the curved probe represent valid methods in US evaluation of the CSA of the RF muscle. However, in the healthy subjects, the thickness and width measurements of the same muscle, are affected by the type of probe used. Based on these considerations, it is necessary to specify the type of probe to facilitate standardization of intra- and inter-operator methodology.
Acknowledgments
The authors thank the persons and patients who volunteered for the study.
List of acronyms
- AP
anteroposterior
- BIA
bioelectrical impedance analysis
- BMI
Body Mass Index
- B-mode
Brightness Mode
- CSA
cross-sectional area
- CT
computed tomography
- DXA
dual X-ray absorptiometry
- EWGSOP2
European Working Group on Sarcopenia in Older People
- LL
laterolateral
- MRI
magnetic resonance imaging
- RF
rectus femoris
- SD
standard deviation
- US
ultrasonography3
Funding Statement
Funding: The authors received no specific funding.
Contributor Information
Daniele Coraci, Email: daniele.coraci@unipd.it.
Giacomo Farì, Email: dr.giacomofari@gmail.com.
Valeria Vallenari, Email: valeria.vallenari@gmail.com.
Stefano Masiero, Email: stef.masiero@unipd.it.
References
- 1.Anton SD, Woods AJ, Ashizawa T, Barb D, Buford TW, Carter CS, Clark DJ, Cohen RA, Corbett DB, Cruz-Almeida Y, Dotson V, Ebner N, Efron PA, Fillingim RB, Foster TC, Gundermann DM, Joseph AM, Karabetian C, Leeuwenburgh C, Manini TM, Marsiske M, Mankowski RT, Mutchie HL, Perri MG, Ranka S, Rashidi P, Sandesara B, Scarpace PJ, Sibille KT, Solberg LM, Someya S, Uphold C, Wohlgemuth S, Wu SS, Pahor M. Successful aging: Advancing the science of physical independence in older adults. Ageing Res Rev. 2015. Nov;24(Pt B):304-27. doi: 10.1016/j.arr.2015.09.005. Epub 2015 Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003. Oct;58(10):M911-6. doi: 10.1093/gerona/58. 10.m911.. [DOI] [PubMed] [Google Scholar]
- 3.Coletti D, Daou N, Hassani M, Li Z, Parlakian A. Serum Response Factor in Muscle Tissues: From Development to Ageing. Eur J Transl Myol. 2016. Jun 22;26(2):6008. doi: 10.4081/ejtm.2016.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JC, Wu WT, Chang KV, Chen LR, Chi SY, Kara M ÖL. Ultrasound Imaging for the Diagnosis and Evaluation of Sarcopenia: An Umbrella Review. Life. 2021;12(1):9. doi: 10.3390/ life12010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellmanns K., McBean K. Magnetic Resonance Imaging in the measurement of whole body muscle mass: A comparison of interval gap methods. Radiography. 2015;21:e35–e39. doi: 10.1016/ j.radi.2014.09.009. [Google Scholar]
- 6.Cao Q., Xiong Y., Zhong Z., Ye Q. Computed Tomography-Assessed Sarcopenia Indexes Predict Major Complications following Surgery for Hepatopancreatobiliary Malignancy: A Meta-Analysis. Ann Nutr Metab. 2019;74:24–34. doi: 10.1159/000494887. [DOI] [PubMed] [Google Scholar]
- 7.Buckinx F., Landi F., Cesari M., Fielding R.A., Visser M., Engelke K., Maggi S., Dennison E., Al-Daghri N.M., Allepaerts S., Al E. Pitfalls in the measurement of muscle mass: A need for a reference standard. J Cachexia Sarcopenia Muscle. 2018;9:269–278. doi: 10.1002/jcsm.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam F.M.H., Su Y., Lu Z.H., Yu R., Leung J.C.S., Kwok TC. Cumulative and Incremental Value of Sarcopenia Components on Predicting Adverse Outcomes. J Am Med Dir Assoc. 2020;21:1481–9. doi: 10.1016/j.jamda.2020.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol Rev. 2019;99(1):427–511. doi:10.1152/ physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kara M, Kaymak B, Frontera W, Ata AM, Ricci V, Ekiz T, Chang KV, Han DS, Michail X, Quittan M, Lim JY, Bean JF, Franchignoni F, Özçakar L. Diagnosing sarcopenia: Functional perspectives and a new algorithm from ISarcoPRM. J Rehabil Med. 2021;53(6). doi: 10.2340/16501977-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abe T, Patterson KM, Stover CD, Geddam DA, Tribby AC, Lajza DG, Young KC. Site-specific thigh muscle loss as an independent phenomenon for age-related muscle loss in middle-aged and older men and women. Age (Omaha). 2014;36(3):1353–8. doi: 10.1007/s11357-014-9634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dehghan M, Merchant A. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr J. 2008;7(26). doi: 10.1186/1475-2891-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger J, Bunout D, Barrera G, de la Maza MP, Henriquez S, Leiva L, Hirsch S. Rectus femoris (RF) ultrasound for the assessment of muscle mass in older people. Arch Gerontol Geriatr. 2015;61(1):33–8. doi: 10.1016/j.archger.2015.03. 006. [DOI] [PubMed] [Google Scholar]
- 14.Nijholt W, Scafoglieri A, Jager-Wittenaar H, Hobbelen JSM, van der Schans CP. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle. 2017;8(5):702–12. doi: 10.1002/jcsm.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond K, Mampilly J, Laghi FA, Goyal A, Collins EG, McBurney C, Jubran A, Tobin MJ. Validity and reliability of rectus femoris ultrasound measurements: Comparison of curved-array and linear-array transducers. J Rehabil Res Dev. 2014;51(7):1155–64. doi: 10.1682/JRRD.2013.08.0187. [DOI] [PubMed] [Google Scholar]
- 16.Ticinesi A, Meschi T, Narici M V., Lauretani F, Maggio M. Muscle Ultrasound and Sarcopenia in Older Individuals: A Clinical Perspective. J Am Med Dir Assoc [Internet]. 2017;18(4):290–300. doi: 10.1016/j.jamda.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Strasser EM, Draskovits T, Praschak M, Quittan M, Graf A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age (Omaha). 2013;35(6):2377–88. doi: 10.1007/s11357-013-9517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coraci D, Giovannini S, Loreti C, Ruggeri F PL. The hyperchoic rim of the normal nerve in ultrasound: how significant is it? Neurol Sci. 2020;41(10):2985–7. doi: 10.1007/s10072-020-04405-6. [DOI] [PubMed] [Google Scholar]
- 20.Adamec C. Pouzití Spearmanova koeficientu korelace poradí pri validaci [The use of Spearman's correlation order coefficient in validation]. Cesk Zdrav. 1966. Feb;14(2):100-2. Czech. [PubMed] [Google Scholar]
- 21.Ozcakar L, De Muynck Martine. Musculoskeletal Ultrasound in Physical and Rehabilitation Medicine. 1st Ed. Edi Ermes. 2014. p. 11–15. [Google Scholar]
- 22.Gonzalez A, Valero-Breton M, Huerta-Salgado C, Achiardi O, Simon F C-VC. Impact of exercise training on the sarcopenia criteria in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Transl Myol. 2021;31(1):9630. doi: 10.4081/ejtm.2021.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perkisas S, Bastijns S, Sanchez-Rodriguez D, Piotrowicz K, De Cock AM; full SARCUS working group. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update. Eur Geriatr Med [Internet]. 2021;12(1):45–59. doi: 10.1007/s41999-021-00462-y. [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–48. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Won CW. Sex-different changes of body composition in aging: a systemic review. Arch Gerontol Geriatr [Internet]. 2022;102 (May): 104711. doi: 10.1016/j.archger.2022.104711. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell W, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;(July):1–18. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blazevich AJ, Cannavan D, Coleman DR, Horne S. Influence of concentric and eccentric resistance training on architectural adaptation in human quadriceps muscles. J Appl Physiol. 2007;103(5):1565–75. doi: 10.1152/japplphysiol. 00578.2007 [DOI] [PubMed] [Google Scholar]
- 28.Alegre LM, Jiménez F, Gonzalo-Orden JM, Martín-Acero R AX. Effects of dynamic resistance training on fascicle length and isometric strength. J Sport Sci. 2006;24(5):501–8. doi: 10.1080/ 02640410500189322. [DOI] [PubMed] [Google Scholar]
- 29.Reeves ND, Narici MV MC. In vivo human muscle structure and function: adaptations to resistance training in old age. Exp Physiol. 2004;89(6):675–89. doi: 10.1113/expphysiol.2004. 027797. [DOI] [PubMed] [Google Scholar]
- 30.Seynnes OR, de Boer M NM. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J Appl Physiol. 2007;102(1):368–73. doi: 10.1152/japplphysiol.00789.2006. [DOI] [PubMed] [Google Scholar]
- 31.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Eur Geriatr Med [Internet]. 2021;12(1):1–13. doi: 10.1093/ ageing/afz046. [Google Scholar]
- 32.Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjær M. Role of the nervous system in sarcopenia and muscle atrophy with aging: Strength training as a countermeasure. Scand J Med Sci Sport. 2010;20(1):49–64. doi: 10.1111/j.1600-0838.2009 .01084.x. [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ LR. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998; 147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 34.Burns JM, Johnson DK, Watts A, Swerdlow RH BW. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch Neurol. 2010;67(4):428–33. doi: 10.1001/archneurol.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, Harris TB, Kritchevsky S, Tylavsky FA, Nevitt M, Cho YW NA. Health, Aging, and Body Composition Study. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32(11):1993–7. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]


