Abstract
Taking advantage of the fact that plasmid DNA encoding a single cytotoxic T lymphocyte (CTL) epitope can induce CTLs, we examined the influence of T-cell responses to dominant epitopes on those to a subdominant epitope derived from Listeria monocytogenes. Our data suggest that interaction between T cells against dominant and subdominant epitopes does not operate in the generation of the hierarchy. Furthermore, we found that a single dominant epitope is sufficient for the induction of protective immunity.
Effector CD8+ T-cell responses against infection are restricted to a few epitopes compared to the total number of epitopes potentially available (1, 2, 12, 23). These epitopes are termed immunodominant, and cytotoxic T lymphocyte (CTL) responses to subdominant epitopes can be demonstrated in vivo by the removal of dominant epitopes or the separation of dominant and subdominant epitopes so that they are presented on different cells (9, 18, 21). Various factors have been implicated to determine the position of an epitope in the immunodominant hierarchy: (i) antigen processing efficiency (10, 11, 20), (ii) transporter associated with antigen processing (TAP)-dependent peptide transport (15), (iii) the affinity of the peptide for the major histocompatibility complex (MHC) molecule (6, 24), (iv) the rate of dissociation of the MHC molecule (26, 28), (v) the transport of MHC-peptide complexes to the cell surface (17), and (vi) the response to the T-cell repertoire (5, 7, 8).
Listeria monocytogenes is a gram-positive bacterium that causes life-threatening infections during pregnancy and in immunocompromised individuals (14). L. monocytogenes enters macrophages and hepatocytes and accesses the cytoplasm by secreting listeriolysin O (LLO), resulting in the induction of a vigorous MHC class I-restricted CTL response that enables the rapid clearance of live bacteria (3, 16). Four different L. monocytogenes epitopes are known to be presented to CTLs by the MHC class I H-2Kd molecules (4, 22). These epitopes are derived from bacterial virulent factors, LLO, murein hydrolase p60, and metalloprotease (mpl). Infection of BALB/c mice with a sublethal dose of L. monocytogenes induces dominant CTL responses against LLO 91-99 and p60 217-225 and subdominant responses against p60 449-457 and mpl 84-92. There is no correlation between the amount of epitope that is presented by infected cells and the magnitude of the CTL response (29).
We have previously shown that intramuscular or gene gun immunizations with plasmid DNA encoding a single epitope of L. monocytogenes, LLO 91-99, are able to induce specific CTLs in a CD4+ T-cell-independent manner (19, 27, 31). In the current study, taking advantage of the fact that a single epitope-expressing DNA vaccine can induce CTLs without the influence of the other epitopes, we investigated the influence of dominant T-cell responses on subdominant responses.
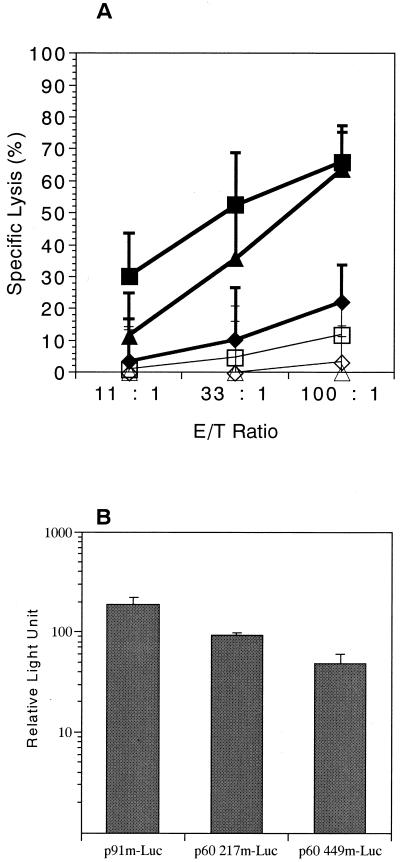
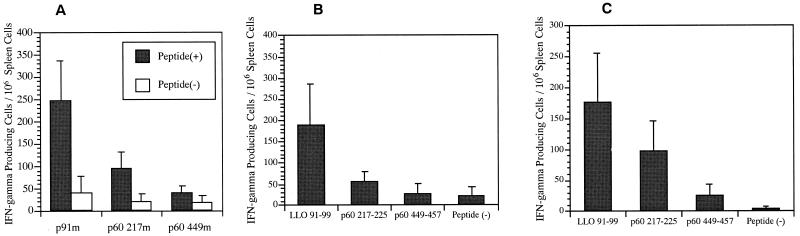
We constructed three plasmids, p91m, p60 217m, and p60 449m, which encode LLO 91-99, p60 217-225, and p60 449-457 of L. monocytogenes, respectively, flanked by ATG start codon and TAA stop codon, under the control of the cytomegalovirus immediate-early enhancer-promoter. The DNA sequences inserted were adapted to the most frequently used codons in murine genes (19, 27). DNA immunization was performed into the shaved abdominal skin of BALB/c mice using the Helios gene gun system (Bio-Rad Laboratories, Hercules, Calif.). Then, 2 μg of plasmid DNA was coated onto 0.5 mg of 1.0-μm gold particles, and the injection was carried out with 0.5 mg of gold/shot. Mice were injected three times with 2 μg of the plasmids weekly. At 2 weeks after the last immunization, immune splenocytes were cultured in 12-well plates at a density of 2 × 107/well for 5 days with 2 × 107 syngeneic spleen cells per well that had been treated with 100 μg of mitomycin C per ml and pulsed with 5 μM concentrations of the each of synthetic peptides representing LLO 91-99, p60 217-225, and p60 449-457 for 2 h at 37°C. Each well also received 10 U of human recombinant interleukin-2 (Hoffmann-La Roche, Nutley, N.J.) per ml. Cell-mediated cytotoxicity was assayed against J774 (H-2d) pulsed by incubation with 5 μM concentrations of each peptide using a conventional 51Cr release assay as described previously (27). As shown in Fig. 1A, gene gun immunizations of BALB/c mice with p91m and p60 217m expressing the dominant epitopes LLO 91-99 and p60 217-225, respectively, induced vigorous CTL responses, whereas immunizations with p60 449m encoding a subdominant epitope, p60 449-457, induced only a marginal CTL response. These CTL responses appeared to be the same as that observed with the immunization with live L. monocytogenes (4, 29). The results obtained imply that competitive interaction between CTLs against dominant and subdominant epitopes does not operate in the listerial antigens. It seems unlikely that the relative magnitudes of the CTL responses is attributable to quantitative difference of the three peptides expressed by the plasmids, since a subdominant epitope, p60 449-457, has been reported to be associated with H-2Kd molecules approximately 10 times greater than the dominant epitope p60 217-225 in L. monocytogenes-infected cells (25). Actually, we observed only small differences in the translational efficiencies of these DNA vaccines when we used our readthrough analysis (27), in which the luciferase cDNA was fused to downstream of the adapted LLO 91-99, p60 217-225, and p60 449-457 sequences, resulting in p91m-Luc, p60 217m-Luc, and p60 449m-Luc, respectively, and the conventional luciferase assay was performed with these expression plasmids using BALB/3T3 murine cells (Fig. 1B). However, CTL activities do not always represent the in vivo level of T-cell activities since in vitro restimulation of immunized splenocytes with a relevant peptide is essential to assess CTL activities. It has been demonstrated that T cells with dominant specificities expand optimally to low peptide concentrations, while those specific for subdominant epitopes respond maximally to high peptide concentrations (4). We, therefore, examined the frequencies of T cells specific for LLO 91-99, p60 217-225, and p60 449-457 following gene gun immunization with p91m, p60 217m, and p60 449m, respectively. Figure 2A shows the results of ELISPOT assays of peptide-induced gamma interferon (IFN-γ) production to quantify T-cell frequencies induced by each plasmid DNA; these results indicate that p91m appeared to induce the most dominant frequency, p60 217m induced an intermediate frequency, and p60 449m induced the lowest frequency of specific T cells. In vivo depletion of T-cell subsets with monoclonal antibodies against CD4 or CD8 (27) indicated that the IFN-γ-secreting cells are CD8+ T cells (data not shown). Fensterle et al. (13) reported that primary immunization with a plasmid encoding LLO but not that encoding p60 induced significant specific T-cell responses, whereas booster immunization of the both plasmids after 45 days induced similar specific T-cell frequencies. Their results conflict with ours since, although our three immunizations with an interval of 1 week resulted in a pronounced booster effect (with a 21-fold increase in the LLO 91-99-specific T-cell frequency), we observed the hierarchy of immunodominance in the listerial epitopes. This discrepancy is puzzling at present but may be due to the different DNA vaccines used, i.e., plasmids encoding epitopes versus plasmids encoding whole protein.
FIG. 1.
Immunization with DNA vaccine encoding a single dominant or subdominant epitope induces different levels of CTL activity. (A) BALB/c mice were immunized by gene gun with p91m (■, □), p60 217m (▴, ▵), or p60 449m (⧫, ◊) three times. Spleen cells from immunized animals were harvested 2 weeks after the last immunization and stimulated in vitro with LLO 91-99-, p60 217-225-, or p60 449-457-peptide pulsed spleen cells for 5 days. The percentage of specific lysis was determined using J774 cells (H-2d) pulsed with LLO 91-99-peptide (solid symbols) or control medium (open symbols) as target cells. Results are expressed as the mean ± the standard deviation (SD) for six mice. (B) Translational efficiencies of the three plasmid DNA vaccines. Relative luciferase activities were assayed by transient transfections of p91m-Luc, p60 217m-Luc, and p60 449m-Luc in BALB/3T3 mouse fibroblast cells. The relative luciferase activities are normalized to Renilla remiformis luciferase activities by the cotransfected pRL-CMV (Promega, Madison, Wis.). The data are expressed as the mean ± the SD for six samples.
FIG. 2.
In vivo frequencies of T cells specific for each epitope in mice immunized by gene gun with DNA vaccines encoding a dominant or a subdominant epitope. (A) BALB/c mice were immunized with p91m, p60 217m, or p60 449, and the numbers of epitope-specific CD8+ T cells were determined using IFN-γ ELISPOT assays 2 weeks after the last immunization. Immune splenocytes were incubated in the presence of peptide-coated or uncoated P815 cells. Peptide-induced IFN-γ secretion by a single cell was visualized and quantified in triplicates and is expressed as the number of IFN-γ-secreting cells per 106 immune splenocytes. The results are expressed as the mean ± the SD for six mice. The P values are 0.003 for p91m versus p60 217m, 0.002 for p91m versus p60 449m, and 0.0069 for p60 217m versus p60 449m. The Student's t test was used in all statistical analyses. (B) In vivo frequencies of T cells specific for each epitope in mice simultaneously immunized with the three plasmid DNA at non-overlapping sites. BALB/c mice were immunized with the three different plasmid DNAs simultaneously at nonoverlapping sites of the abdominal skin. At 2 weeks after the last immunization, the number of epitope-specific CD8+ T cells was determined by IFN-γ ELISPOT assay. The P values are 0.0071 for LLO 91-99 versus p60 217-225, 0.0023 for LLO 91-99 versus p60 449-457, and 0.0799 for p60 217-225 versus p60 449-457. (C) In vivo frequencies of T cells specific for each epitope in mice immunized with gold particles coated with the mixed three plasmid DNA. The number of epitope-specific CD8+ T cells was determined by IFN-γ ELISPOT assay. The results are expressed as the mean ± the SD for six mice. The P values are 0.0059 for LLO 91-99 versus p60 217-225, 0.0009 for LLO 91-99 versus p60 449-457, and 0.0072 for p60 217-225 versus p60 449-457.
To investigate the effect of the in vivo interactions of T cells induced by the three different plasmid DNAs, p91m, p60 217m, and p60 449m, we immunized mice with the three different plasmid DNAs simultaneously at nonoverlapping sites of the abdominal skin and then evaluated the frequencies of T cells specific for LLO 91-99, p60 217-225, and p60 449-457 using ELISPOT (Fig. 2B). This method was designed to have different antigen-presenting cells present different epitopes to T cells in order to avoid the possibility of epitope competitions for binding to the same H-2Kd molecules. The data obtained are essentially the same the results we observed with immunization with a single plasmid DNA (Fig. 2A), suggesting that competitive interaction between CTLs against dominant and subdominant epitopes does not operate in the listerial epitopes. As shown in Fig. 2C, essentially the same results were obtained when mice were inoculated with gold particles coated with the mixed three plasmids (1 μg each). These data imply that the hierarchy of immunodominace in the listerial epitopes is readily determined by interaction between responder T cells and CTL epitopes presented by MHC class I molecules and that competitive interaction between responder T cells may not operate in the generation of the hierarchy of the listerial epitopes. An attempt has been performed to delete the two dominant epitopes, LLO 91-99 and p60 217-225, from L. monocytogenes by anchor residue mutagenesis to study the influence in vivo of dominant T-cell response on the subdominant response during the listerial infection (30). Consistent with our data, the experiment demonstrated that the loss of these two dominant T-cell responses does not enhance T-cell responses to the subdominant epitopes (p60 449-457 and mpl 84-92).
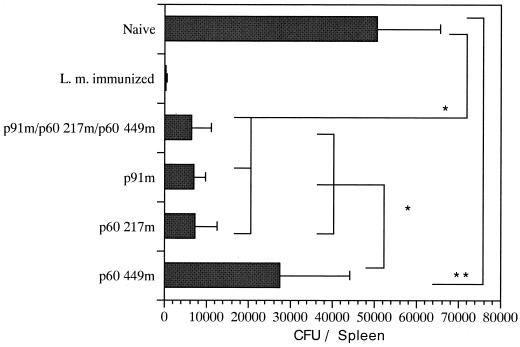
Given that the three plasmid DNAs induced different levels of specific lysis, we wanted to determine the biologic effect of the plasmids against bacterial challenge. At 72 h after sublethal listerial challenge (2 × 104 CFU), mice from each group were sacrificed, and the CFU from the spleens were counted. Consistent with the ELISPOT data, both p91m and p60 217m, but not p60 449m, induced remarkable protective immunities against listerial infection (Fig. 3). Of particular interest is that simultaneous inoculation of these three plasmids failed to surpass a single plasmid expressing a dominant epitope, LLO 91-99 or p60 217-225, in the protection, suggesting that a single dominant epitope may be sufficient for the induction of CTL-mediated protective immunity. These data have implications for the development of vaccines designed to emphasize CTL-mediated immunity.
FIG. 3.
Protective immunity induced by immunization with plasmid DNA encoding a dominant or subdominant epitope and by simultaneous immunization with the three plasmids. Mice were immunized with p91m, p60 217m, or p60 449m or were simultaneously immunized with these plasmids three times and, 2 weeks later after the last immunization, were challenged with 2 × 104 CFU of L. monocytogenes intravenously. Bacterial titers of spleens were determined 72 h after the challenge infection by plating 10-fold dilutions of tissue homogenates on Trypticase soy agar plates. The results are expressed as the mean ± the SD for six mice. ∗, P < 0.01; ∗∗, P = 0.02.
Acknowledgments
This work was supported in part by grants-in Aid from the Ministry of Education, Science, Sports, and Culture of Japan.
We thank Tetsumichi Matsuo for excellent experimental assistance.
REFERENCES
- 1.Adorini L, Appella E, Doria G, Nagy Z A. Mechanisms influencing the immunodominance of T cell determinants. J Exp Med. 1988;168:2091–2104. doi: 10.1084/jem.168.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber L D, Parham P. The essence of epitopes. J Exp Med. 1994;180:1191–1194. doi: 10.1084/jem.180.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bielecki J, Youngman P, Connelly P, Portnoy D A. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990;345:175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- 4.Busch D H, Pamer E G. MHC class I/peptide stability: implications for immunodominance, in vitro proliferation, and diversity of responding CTL. J Immunol. 1998;160:4441–4448. [PubMed] [Google Scholar]
- 5.Cao W, Myers-Powell B A, Braciale T J. The weak CD8+ CTL response to an influenza hemagglutinin epitope reflects limited T cell availability. J Immunol. 1996;157:505–511. [PubMed] [Google Scholar]
- 6.Chen W, Khilko S, Fecondo J, Margulies D H, McCluskey J. Determinant selection of major histocompatibility complex class I-restricted antigenic peptides is explained by class I-peptide affinity and is strongly influenced by nondominant anchor residues. J Exp Med. 1994;180:1471–1483. doi: 10.1084/jem.180.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connolly J M. The peptide p2Ca is immunodominant in allorecognition of Ld by beta chain variable region V beta 8+ but not V beta 8− strains. Proc Natl Acad Sci USA. 1994;91:11482–11486. doi: 10.1073/pnas.91.24.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly K, Nguyen P, Woodland D L, Blackman M A. Immunodominance of major histocompatibility complex class I-restricted influenza virus epitopes can be influenced by the T-cell receptor repertoire. J Virol. 1995;69:7416–7422. doi: 10.1128/jvi.69.12.7416-7422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng Y, Yewdell J W, Eisenlohr L C, Bennink J R. MHC affinity, peptide liberation, T cell repertoire, and immunodominance all contribute to the paucity of MHC class I-restricted peptides recognized by antiviral CTL. J Immunol. 1997;158:1507–1515. [PubMed] [Google Scholar]
- 10.Dick L R, Aldrich C, Jameson S C, Moomaw C R, Pramanik B C, Doyle C K, DeMartino G N, Bevan M J, Forman J M, Slaughter C A. Proteolytic processing of ovalbumin and beta-galactosidase by the proteasome to a yield antigenic peptides. J Immunol. 1994;152:3884–3894. [PMC free article] [PubMed] [Google Scholar]
- 11.Eggers M, Boes-Fabian B, Ruppert T, Kloetzel P M, Koszinowski U H. The cleavage preference of the proteasome governs the yield of antigenic peptides. J Exp Med. 1995;182:1865–1870. doi: 10.1084/jem.182.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairchild P J, Wraith D C. Lowering the tone: mechanisms of immunodominance among epitopes with low affinity for MHC. Immunol Today. 1996;17:80–85. doi: 10.1016/0167-5699(96)80584-6. [DOI] [PubMed] [Google Scholar]
- 13.Fensterle J, Grode L, Hess J, Kaufmann S H E. Effective DNA vaccination against listeriosis by prime/boost inoculation with the gene gun. J Immunol. 1999;163:4510–4518. [PubMed] [Google Scholar]
- 14.Gellin B G, Broome C V. Listeriosis. JAMA. 1989;261:1313–1320. [PubMed] [Google Scholar]
- 15.Heemels M T, Ploegh H. Generation, translocation, and presentation of MHC class I-restricted peptides. Annu Rev Biochem. 1995;64:463–491. doi: 10.1146/annurev.bi.64.070195.002335. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann S H, Rodewald H R, Hug E, De Libero G. Cloned Listeria monocytogenes specific non-MHC-restricted Lyt-2+ T cells with cytolytic and protective activity. J Immunol. 1988;140:3173–3179. [PubMed] [Google Scholar]
- 17.Levitsky V, Zhang Q J, Levitskaya J, Kurilla M G, Masucci M G. Natural variants of the immunodominant HLA A11-restricted CTL epitope of the EBV nuclear antigen-4 are nonimmunogenic due to intracellular dissociation from MHC class I:peptide complexes. J Immunol. 1997;159:5383–5390. [PubMed] [Google Scholar]
- 18.Mylin L M, Bonneau R H, Lippolis J D, Tevethia S S. Hierarchy among multiple H-2b-restricted cytotoxic T-lymphocyte epitopes within simian virus 40 T antigen. J Virol. 1995;69:6665–6677. doi: 10.1128/jvi.69.11.6665-6677.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagata T, Uchijima M, Yoshida A, Kawashima M, Koide Y. Codon optimization effect on translational efficiency of DNA vaccine in mammalian cells: analysis of plasmid DNA encoding a CTL epitope derived from microorganisms. Biochem Biophys Res Commun. 1999;261:445–451. doi: 10.1006/bbrc.1999.1050. [DOI] [PubMed] [Google Scholar]
- 20.Niedermann G, King G, Butz S, Birsner U, Grimm R, Shabanowitz J, Hunt D F, Eichmann K. The proteolytic fragments generated by vertebrate proteasomes: structural relationships to major histocompatibility complex class I binding peptides. Proc Natl Acad Sci USA. 1996;93:8572–8577. doi: 10.1073/pnas.93.16.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oukka M, Riche N, Kosmatopoulos K. A nonimmunodominant nucleoprotein-derived peptide is presented by influenza A virus-infected H-2b cells. J Immunol. 1994;152:4843–4851. [PubMed] [Google Scholar]
- 22.Pamer E G, Harty J T, Bevan M J. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature. 1991;353:852–855. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sercarz E E, Lehmann P V, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 24.Sette A, Vitiello A, Reherman B, Fowler P, Nayersina R, Kast W M, Melief C J, Oseroff C, Yuan L, Ruppert J, et al. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol. 1994;153:5586–5592. [PubMed] [Google Scholar]
- 25.Sijts A J A M, Neisig A, Neefjes J, Pamer E G. Two Listeria monocytogenes CTL epitopes are processed from the same antigen with different efficiencies. J Immunol. 1996;156:685–692. [PubMed] [Google Scholar]
- 26.Sijts A J, Pamer E G. Enhanced intracellular dissociation of major histocompatibility complex class I-associated peptides: a mechanism for optimizing the spectrum of cell surface-presented cytotoxic T lymphocyte epitopes. J Exp Med. 1997;185:1403–1411. doi: 10.1084/jem.185.8.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uchijima M, Yoshida A, Nagata T, Koide Y. Optimization of codon usage of plasmid DNA vaccine is required for the effective MHC class I-restricted T cell responses against an intracellular bacterium. J Immunol. 1998;161:5594–5599. [PubMed] [Google Scholar]
- 28.van der Burg S H, Visseren M J, Brandt R M, Kast W M, Melief C J. Immunogenicity of peptides bound to MHC class I molecules depends on the MHC-peptide complex stability. J Immunol. 1996;156:3308–3314. [PubMed] [Google Scholar]
- 29.Vijh S, Pamer E G. Immunodominant and subdominant CTL responses to Listeria monocytogenes infection. J Immunol. 1997;158:3366–3371. [PubMed] [Google Scholar]
- 30.Vijh S, Pilip I M, Pamer E G. Noncompetitive expansion of cytotoxic T lymphocytes specific for different antigens during bacterial infection. Infect Immun. 1999;67:1303–1309. doi: 10.1128/iai.67.3.1303-1309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida A, Nagata T, Uchijima M, Higashi T, Koide Y. Advantage of gene gun-mediated over intramuscular inoculation of plasmid DNA vaccine in reproducible induction of specific immune responses. Vaccine. 2000;18:1725–1729. doi: 10.1016/s0264-410x(99)00432-6. [DOI] [PubMed] [Google Scholar]