Abstract
BACKGROUND
Ganglioneuromas are rare peripheral nervous system tumors of neural crest origin. Most are often asymptomatic and incidentally found, but large tumors can cause mass effect. Herein, the authors report a case of a giant ganglioneuroma that arose from the lumbar foramina into the retroperitoneal and thoracic cavities.
OBSERVATIONS
A 62-year-old female presented with low back pain, left lower extremity swelling, and increased sensation of an abdominal mass. Surgical treatment options were reviewed with the patient and coordinated care was planned by surgical oncological specialists. The patient opted for multistage exploratory laparotomy for abdominal mobilization, diaphragm resection, and en bloc resection with neuromonitoring. After surgery, the patient experienced significant improvement in symptoms.
LESSONS
A combined surgical exposure involving gastrointestinal, thoracic, and neurological surgeons can be important in the safe resection of ganglioneuromas that span multiple body cavities. Hence, a thorough preoperative assessment could help plan surgery accordingly.
Keywords: ganglioneuroma, spinal, resection, laparotomy, giant
ABBREVIATIONS: CT = computed tomography, MRI = magnetic resonance imaging
Ganglioneuromas are rare tumors that develop from neural crest cells.1,2 Often, these tumors originate within the retroperitoneal space (51%) or the posterior mediastinum (40%).1,3 Nevertheless, they can originate from the sympathetic chain adjacent to the cervical or lumbar spine.2 Retroperitoneal ganglioneuromas usually develop in younger children and adolescents, contrary to the patient described in our report.4,5 The incidence rate of ganglioneuromas is equal in both sexes.3 Nonetheless, higher incidence rates are associated with familial syndromes such as Turner syndrome, multiple endocrine neoplasia II, and neurofibromatosis type 1.6–8 Ganglioneuromas are usually asymptomatic unless they reach a substantial size or compress a vital structure, at times causing radiculopathy. Additionally, some reports indicated that these tumors may express some vasoactive peptides or hormones (e.g., catecholamines, vasoactive peptides).9,10 Malignant ganglioneuromas are extremely rare and may transform secondary to radiation therapy.11,12
Illustrative Case
Clinical Presentation
A 62-year-old female presented with low back pain, and investigative imaging showed evidence of a retroperitoneal lesion (Fig. 1). The patient experienced progressive back pain and left lower extremity swelling for over 1 year, along with an increased sensation of a left-sided abdominal mass. There was no radicular pain, focal weakness, or bowel and bladder incontinence. These symptoms were mechanical in onset, and the patient began to use a cane for support. The neurological examination finding was that the patient was intact, except for tenderness from the midthorax extending into the low back and a pain-limited antalgic gait.
FIG. 1.
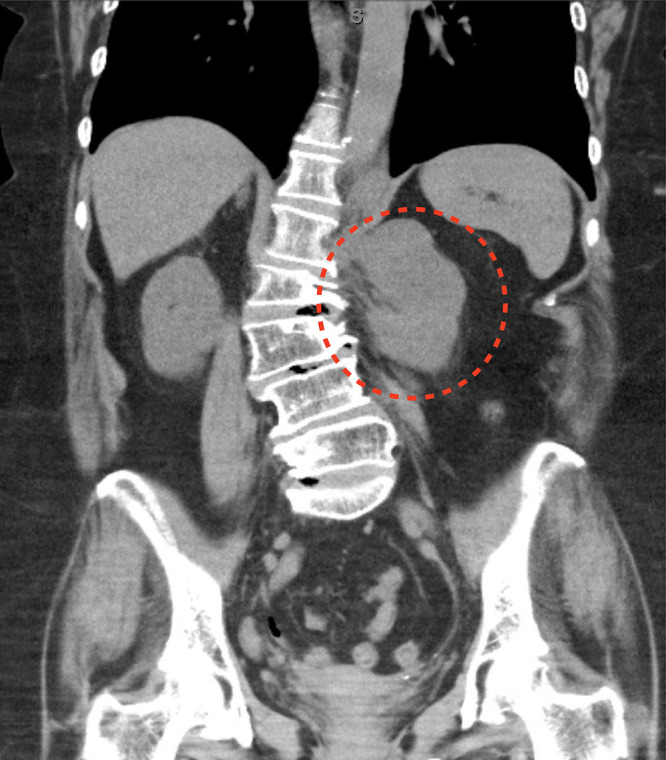
Coronal thoracoabdominal CT showing a large left paraspinal mass (dotted red line) at the level of the L1 neural foramen and associated dextroscoliosis of the lumbar spine.
Magnetic resonance imaging (MRI) of the abdomen revealed a lobulated mass within the left retroperitoneal space displacing the left psoas muscle anteriorly, traversing into the left retrocrural space of the esophagus, and measuring 8.2 × 5.2 × 6.3 cm (Fig. 2). The mass appeared to originate from the left L1 foramen with associated spinal dextroscoliosis. The lesion showed areas of fat signals on T2-weighted images. No significant enhancement was noted in the postcontrast imaging studies. No other involvement of any other abdominal organs was noted. Computed tomography (CT) image-guided biopsy confirmed the pathological diagnosis of ganglioneuroma.
FIG. 2.
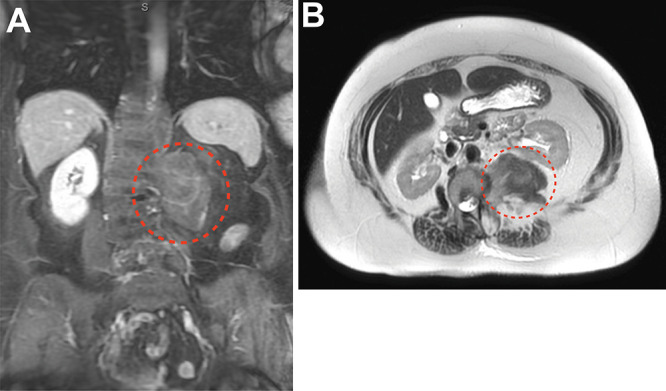
Coronal (A) and axial (B) MRI with contrast showing a retroperitoneal and retrocrural mass with minimal enhancement (dotted red line).
Operative Technique
The patient underwent a joint approach for tumor exposure and en bloc resection with surgical oncology, thoracic surgery, and neurosurgery. The patient was placed supine in a right decubitus position on the operating table with general endotracheal anesthesia. Laparotomy was performed with an incision made from the xiphoid to the pubis, and the gastrocolic ligament was opened. Subsequently, complete medial mobilization (Cattell-Braasch maneuver) was performed by mobilizing the spleen, pancreas, kidney, and left colon/splenic flexure from lateral to medial to expose the left retroperitoneum. The left colon and sigmoid colon were moved medially off of the retroperitoneum to expose the tumor and the psoas muscle down to the bifurcation of the left iliac artery. The phrenicosplenic ligament and the posterior aspects of the spleen, pancreas, and left kidney were dissected and mobilized out of the retroperitoneum to expose the psoas and the tumor. The tumor was exposed from the quadratus lumborum. It extended along the psoas muscle, and dissection was performed from the psoas muscle at the level of the iliac vessel bifurcation to the lumbar spine.
From the abdominal exposure, the mass was visualized extending up into the thoracic cavity. A left bronchial blocker was placed by the thoracic surgery team, then the bronchial balloon was inflated. A portion of the diaphragm not in the vicinity of the phrenic nerve was then resected. The pleura posterior to the aorta was dissected off the aorta using pledgeted dissectors and LigaSure. The tumor was further dissected from the posterior mediastinum, and the last of the visible attachments were released in the thoracic cavity. The mass was firmly attached to the L1 foramen, along with substantial bands that connected the tumor to the lateral aspects of the lumbar vertebral body. The left femoral nerve was identified and stimulated at 2 mA using the monopolar stimulator as a positive control. No functional nerve was identified going into this tumor. The fibrotic tumor capsule and attachments were safely resected using bipolar and microsurgical techniques, and continuous stimulation was used for up to 10 mA. The bilobed 8 × 5–cm lesion was removed en bloc (Fig. 3).
FIG. 3.
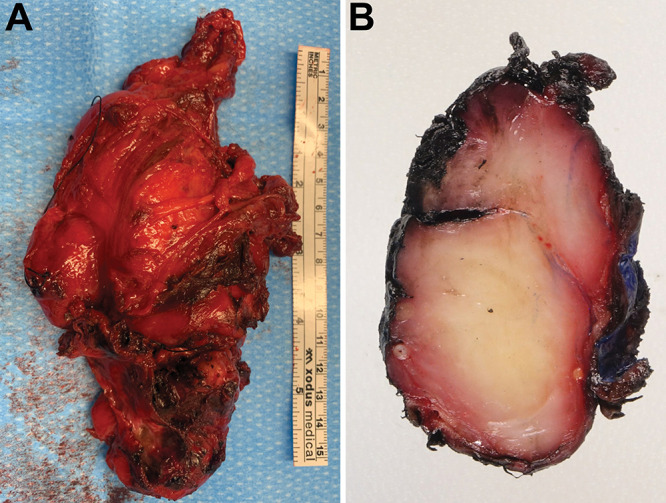
A: Photograph of the excised mass showing irregular, red-brown, ragged, firm tumor tissue. B: A cut section of the excised tissue demonstrating the firm homogeneous tissue architecture and areas of focal hemorrhage.
Neuromonitoring after resection was at baseline. No cerebrospinal fluid was encountered intraoperatively. Complex reconstruction of the diaphragm was performed with a 9 × 5–cm GORE-TEX patch by the thoracic surgery team, followed by the placement of a chest tube. The chest cavity was completely closed off, and the left lung was reinflated. Final hemostasis in the abdomen was obtained, followed by the placement of a retroperitoneal drain and closure. Postoperative imaging demonstrated gross total resection of the mass (Fig. 4).
FIG. 4.
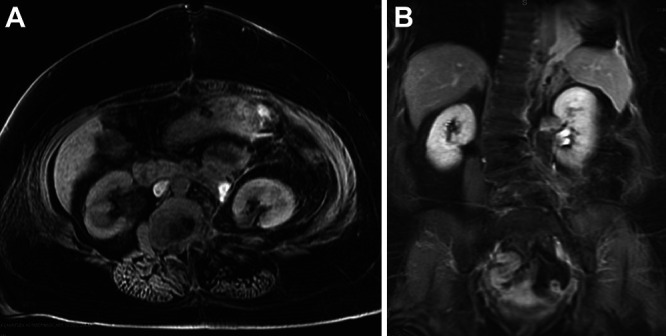
Postoperative axial (A) and coronal (B) MRI showing the gross total resection of the tumor mass.
Discussion
Observations
Ganglioneuromas are often discovered incidentally while investigating other or nonspecific symptoms. Ultrasonographic assessment will reveal hypoechogenic, homogeneous, and well-circumscribed lesions.13 CT findings include a well-demarcated, lobular, encapsulated iso- to hypoattenuating mass when compared with muscle signal.14,15 MRI will reveal homogeneous low or intermediate signal on T1-weighted images, whereas T2-weighted images will reveal heterogeneous intermediate or high signal.14,16 However, scintigraphic imaging usually cannot differentiate between ganglioneuromas, neuroblastomas, and ganglioneuroblastomas.17 The standard of care in management is resection. Laparoscopic resection is preferred for smaller tumors, especially adrenal ganglioneuromas.18,19 Wider-exposure resection is resorted to with larger tumors, considering the location, the pathological features, and whether the tumor secretes a vasoactive substance.20–22 Postoperatively, adjuvant therapies (chemotherapy, radiation therapy) are usually not needed, and the prognosis is mostly favorable because these tumors lack the ability to metastasize.23,24
In this case, the giant ganglioneuroma had extensive involvement of the abdominal and thoracic compartments, the resection of which required a combined approach with several surgical disciplines. There have not been prior reports of clinical presentation and operative resection of a similar ganglioneuroma. A single or minimally invasive exposure for this patient would not have allowed en bloc resection, nor would it provide the direct visualization of the loculated mass that was necessary for stimulation, mobilization, and safe resection, because it coursed near structures that included the femoral nerve, diaphragm, and descending aorta. In the present case, there were dense adhesions of the tumor along the foraminal portions that were left as small residuals during dissection. Ganglioneuromas in general have a favorable prognosis, given their low metastatic potential.
Lessons
A giant lumbar ganglioneuroma was successfully treated through a stagewise combined surgical exposure. This is the first report to date to use medial abdominal mobilization and partial diaphragmatic resection to obtain circumferential exposure in the retroperitoneum and thoracic cavity. The procedure can be safely performed and should be discussed with patients preoperatively.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Deng, Zhang, McCarthy, Zinn. Acquisition of data: Habib, Andrews, McCarthy, Dhupar, Choudry, Zinn. Analysis and interpretation of data: Habib, Wei, Zinn. Drafting the article: Deng, Habib, Zhang, Choudry, Zinn. Critically revising the article: Deng, Zhang, Dhupar, Choudry, Zinn. Reviewed submitted version of manuscript: Habib, Zhang, Zinn. Statistical analysis: Zinn. Administrative/technical/material support: Andrews, Zinn. Study supervision: Deng, Zinn.
References
- 1. Dages KN, Kohlenberg JD, Young WF, Jr, et al. Presentation and outcomes of adrenal ganglioneuromas: a cohort study and a systematic review of literature. Clin Endocrinol (Oxf) 2021;95(1):47–57. doi: 10.1111/cen.14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sarnat HB, Yu W. Ganglion cell maturation in peripheral neuroblastic tumours of children. Clin Neuropathol. 2022;41(3):101–113. doi: 10.5414/NP301450. [DOI] [PubMed] [Google Scholar]
- 3. Fliedner SMJ, Winkelmann PER, Wesley R, Vonthein R, Lehnert H. Ganglioneuromas across age groups: systematic review of individual patient data. Clin Endocrinol (Oxf) 2021;94(1):12–23. doi: 10.1111/cen.14297. [DOI] [PubMed] [Google Scholar]
- 4. Farzan A, Ahmadi P, Tasdighi E, Zinatzadeh MR, Pourbakhtyaran E. Primary spinal tumors and masses in children. Iran J Child Neurol. 2022;16(2):129–135. doi: 10.22037/ijcn.v16i2.30614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Emre Ş, Özcan R, Bakır AC, et al. Adrenal masses in children: imaging, surgical treatment and outcome. Asian J Surg. 2020;43(1):207–212. doi: 10.1016/j.asjsur.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 6. Tan CY, Liu JW, Lin Y, et al. Bilateral and symmetric C1-C2 dumbbell ganglioneuromas associated with neurofibromatosis type 1: a case report. World J Clin Cases. 2019;7(1):109–115. doi: 10.12998/wjcc.v7.i1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bacci C, Sestini R, Ammannati F, et al. Multiple spinal ganglioneuromas in a patient harboring a pathogenic NF1 mutation. Clin Genet. 2010;77(3):293–297. doi: 10.1111/j.1399-0004.2009.01292.x. [DOI] [PubMed] [Google Scholar]
- 8. Lora MS, Waguespack SG, Moley JF, Walvoord EC. Adrenal ganglioneuromas in children with multiple endocrine neoplasia type 2: a report of two cases. J Clin Endocrinol Metab. 2005;90(7):4383–4387. doi: 10.1210/jc.2004-2526. [DOI] [PubMed] [Google Scholar]
- 9. Ishihara H, Kikuno N, Hayakawa N, Ryoji O, Tanabe K. Retroperitoneal catecholamine-producing ganglioneuroma with a birth history of monozygotic twins who both suffered from neuroblastoma during their childhoods: a case report with genome analysis. J Neurol Sci. 2015;357(1-2):329–331. doi: 10.1016/j.jns.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 10. Schmid KW, Dockhorn-Dworniczak B, Fahrenkamp A, et al. Chromogranin A, secretogranin II and vasoactive intestinal peptide in phaeochromocytomas and ganglioneuromas. Histopathology. 1993;22(6):527–533. doi: 10.1111/j.1365-2559.1993.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 11. Rakici SY, Koksal V, Bedir R, Goksel S. An urban legend: malignant transformation caused by radiotherapy in patients with presacral ganglioneuroma. The necessity and first-time administration of radiotherapy. Case report and literature review. J Cancer Res Ther. 2021;17(1):248–254. doi: 10.4103/jcrt.JCRT_380_18. [DOI] [PubMed] [Google Scholar]
- 12. Chen PY, Chen WY, Ho DM, Pan CC. Malignant ganglioneuroma arising from mediastinal mixed germ cell tumor. J Chin Med Assoc. 2007;70(2):76–79. doi: 10.1016/S1726-4901(09)70306-5. [DOI] [PubMed] [Google Scholar]
- 13. Linos D, Tsirlis T, Kapralou A, Kiriakopoulos A, Tsakayannis D, Papaioannou D. Adrenal ganglioneuromas: incidentalomas with misleading clinical and imaging features. Surgery. 2011;149(1):99–105. doi: 10.1016/j.surg.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 14. Scherer A, Niehues T, Engelbrecht V, Mödder U. Ganglioneuromas in childhood: CT and MRI characteristics. Rofo. 2000;172(5):482–486. doi: 10.1055/s-2000-668. Article in German. [DOI] [PubMed] [Google Scholar]
- 15. Armstrong EA, Harwood-Nash DC, Ritz CR, Chuang SH, Pettersson H, Martin DJ. CT of neuroblastomas and ganglioneuromas in children. AJR Am J Roentgenol. 1982;139(3):571–576. doi: 10.2214/ajr.139.3.571. [DOI] [PubMed] [Google Scholar]
- 16. Serra AD, Rafal RB, Markisz JA. MRI characteristics of two cases of adrenal ganglioneuromas. Clin Imaging. 1992;16(1):37–39. doi: 10.1016/0899-7071(92)90088-q. [DOI] [PubMed] [Google Scholar]
- 17. Geoerger B, Hero B, Harms D, Grebe J, Scheidhauer K, Berthold F. Metabolic activity and clinical features of primary ganglioneuromas. Cancer. 2001;91(10):1905–1913. doi: 10.1002/1097-0142(20010515)91:10<1905::aid-cncr1213>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 18. Sapalidis K, Mandalovas S, Kesisoglou I. Laparoscopic excision of an adrenal ganglioneuroma presented as an incindentaloma of the retro peritoneum. Curr Health Sci J. 2018;44(1):71–75. doi: 10.12865/CHSJ.44.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zografos GN, Kothonidis K, Ageli C, et al. Laparoscopic resection of large adrenal ganglioneuroma. JSLS. 2007;11(4):487–492. [PMC free article] [PubMed] [Google Scholar]
- 20. Tavares WM, de Franca SA, Vasconcelos AS, Parra DSL, Araújo SRR, Teixeira MJ. Robotic and standard surgical intervention as adjunct therapies for retroperitoneal ganglioneuroma resection: a case report. BMC Surg. 2021;21(1):143. doi: 10.1186/s12893-021-01146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lawrie K, Whitley A, Rokošný S, Bafrnec J, Gürlich R, Baláž P. Oncovascular resection of a ganglioneuroma involving the coeliac trunk and hepatic artery – a case report. Vasc Endovascular Surg. 2021;55(5):519–523. doi: 10.1177/1538574421994414. [DOI] [PubMed] [Google Scholar]
- 22. Kirchweger P, Wundsam HV, Fischer I, et al. Total resection of a giant retroperitoneal and mediastinal ganglioneuroma – case report and systematic review of the literature. World J Surg Oncol. 2020;18(1):248. doi: 10.1186/s12957-020-02016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu Z, Zeng Q, Zhang X, Tu S, Zhang F. Ganglioneuroma in unusual sites: clinical, radiologic and pathological features. Int J Clin Exp Pathol. 2018;11(10):4862–4866. [PMC free article] [PubMed] [Google Scholar]
- 24. Lee JH, Chai YJ, Kim TH, et al. Clinicopathological features of ganglioneuroma originating from the adrenal glands. World J Surg. 2016;40(12):2970–2975. doi: 10.1007/s00268-016-3630-y. [DOI] [PubMed] [Google Scholar]


