Abstract
The continuous emergence of multidrug-resistant pathogens evoked the development of innovative approaches targeting virulence factors unique to their pathogenic cascade. These approaches aimed to explore anti-virulence or anti-infective therapies. There are evident concerns regarding the bacterial ability to create a superstructure, the biofilm. Biofilm formation is a crucial virulence factor causing difficult-to-treat, localized, and systemic infections. The microenvironments of bacterial biofilm reduce the efficacy of antibiotics and evade the host’s immunity. Producing a biofilm is not limited to a specific group of bacteria; however, Pseudomonas aeruginosa, Acinetobacter baumannii, and Staphylococcus aureus biofilms are exemplary models. This review discusses biofilm formation as a virulence factor and the link to antimicrobial resistance. In addition, it explores insights into innovative multi-targeted approaches and their physiological mechanisms to combat biofilms, including natural compounds, phages, antimicrobial photodynamic therapy (aPDT), CRISPR-Cas gene editing, and nano-mediated techniques.
Keywords: bacterial biofilms, natural compounds, phages, aPDT, CRISPR, nanotechnology
Introduction
Considering the physiological role of bacterial virulence factors when targeting bacteria using conventional antibiotics is promising. Bacterial pathogenesis depends on protein-protein intercommunication, which is surface-bound or secreted to interact with the host-specific molecules or defense system.1 The virulence of microbes is key to enabling them to invade a host, surpass its immune system, and cause an infection. The microbial elements accomplishing this invasion are called “virulence factors”.2 The function of virulence factors is not to instigate the infection; however, it aids the bacteria in surviving hostile environments (ie, the host’s body), resulting in cellular damage.3 This virulence intensifies the ability of the bacterial cell to establish an organized community called biofilm, 10–1000 times more potent than the sessile bacterial cell when treated with conventional antibiotics.4
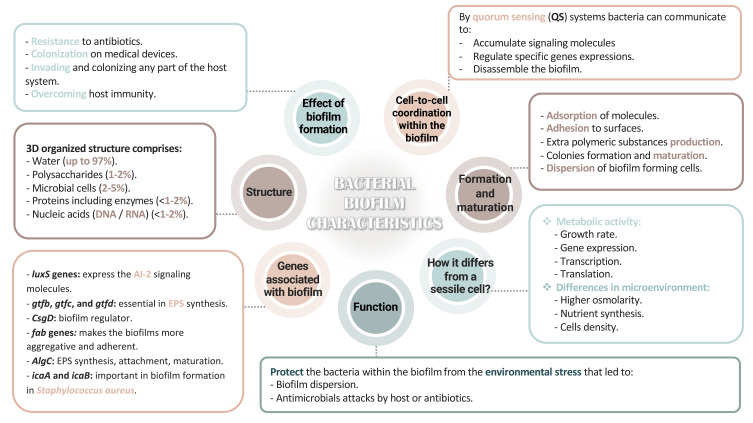
Bacterial cells in the biofilm are contained in a self-produced polymeric extracellular matrix (ECM),2 and its characteristics are summarized in Figure 1.7,8,10,11 The altered phenotypic features in biofilm-forming bacteria include decreasing the transcriptional gene expression and translating proteins essential for bacterial cell metabolism, resulting in reduced metabolic activity.4,5 Biofilm formation increases the genes’ expressing qualities, including adherence, quorum-sensing systems, and competence.6 The secretion of the ECM layer follows successful adherence, which causes colony formation and maturation. The biofilm is dispersed to spread and localize to other sites.7–9 These interactions are controlled through a system known as quorum-sensing (QS), QS signaling molecules accumulate according to cell density.10 These molecules promote biofilm maturation and dispersion and regulate the expression of specific biofilm-related genes and proteins, such as algC, fap, ica operon, gtfBCD, and CsgD.7,11 Consequently, understanding the genetic and phenotypic interactions is essential to comprehend the development of biofilms during infection and to understand the crosstalk linking bacteria within the biofilm and their influence on antibiotic resistance.12
Figure 1.
Bacterial biofilm characteristics.
Abbreviations: 3D, three-dimensional; DNA, deoxyribonucleic acid; RNA, ribonucleic acid; luxS genes, quorum-sensing genes; gtf(b,c,d), glycosyltransferases genes; CsgD, curli subunit gene; fab, fatty acid biosynthetic genes; algC, alginic acid encoding genes; icaAB, intracellular adhesion genes; AI-2, Auto-inducer 2; EPS, extracellular polymeric substances.
Figure 8.
The synergism between aPDT and other antibiotics/antimicrobials.
Figure 10.
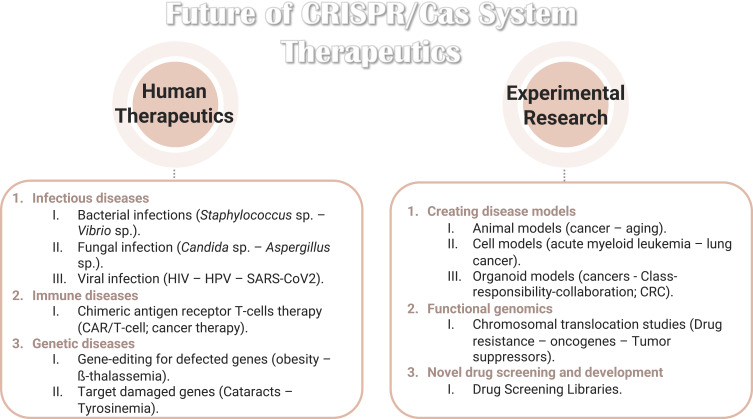
The recent experimental research on CRISPR/Cas system summarized the therapeutic of new approaches targeting bacterial virulence factors by applying nanomedicine to treat specific infectious and metabolic disorders.
Notes: Data from Dubey et al.227
Abbreviations: HIV, human immunodeficiency virus; HPV, human papillomavirus; SARS-CoV2, severe acute respiratory syndrome coronavirus 2; CAR, chimeric antigen receptor T cells; CRC, colorectal cells.
Figure 11.
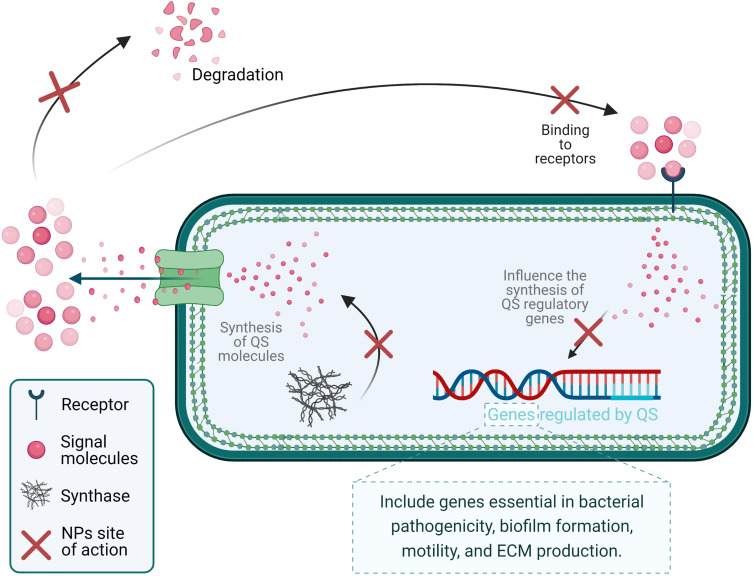
Schematic illustration of anti-QS mechanisms of nanoparticles.
Notes: Data from Qais et al.252
Abbreviations: QS, quorum-sensing systems; NPs, nanoparticles; ECM, extracellular matrix.
Targeting bacterial virulence includes targeting the bacterial attachment and invasion, biofilm formation, type 2 secretion system (toxin secretions), type 3 secretion system (protein transformation), response systems (two-component response systems; TCRs), and quorum-sensing systems.13 This approach is a specific method that differs according to the bacteria. It is a valuable tactic that can:
Promote the creation of new antibacterial agents with new targets.
Decrease future resistance by avoiding pressuring the bacteria to evolve, due to selective pressure.14
This review summarizes and clarifies why biofilms are medically critical. First, we discuss numerous cited studies that exemplify how these clusters of bacteria grow in microbial communities due to forming a complex three-dimensional structure (biofilms). Secondly, we discussed the functions associated with the pathophysiology of biofilm-associated virulence factors in bacteria causing biofilm-associated infections. Lastly, we discuss innovative biofilm-eradication strategies using novel antibiofilm agents and approaches.
Biofilm-Forming Bacteria
The biofilm-forming bacteria can be 10 to 1000-fold more tolerant to antibiotics than sessile cells.15 The morphological changes in biofilm-forming bacteria promote surface adherence, hydrophobicity, low-level motility, and auto-aggregation. Variations between sessile cells and biofilm-associated cells aid the survival of biofilms in hostile environments.5,16 Biofilm-associated infections are clinically challenging at many levels. They require multiple antibiotics to treat the disease, raising the likelihood of drug toxicity.17 However, combined antibiotic therapy causes antibiotic tolerance or resistance, resulting in persistent and difficult-to-treat infections.18 In addition, the lack of universal diagnostic protocols in Medical settings leads to delayed or improper diagnoses.19
Antibiotics tolerance is acquired due to two features in biofilm-forming bacteria:
The matrix formation alters the structural phenotype of the bacteria.
The enlarged populations of bacterial persister cells (cells with decreased metabolism rate).6
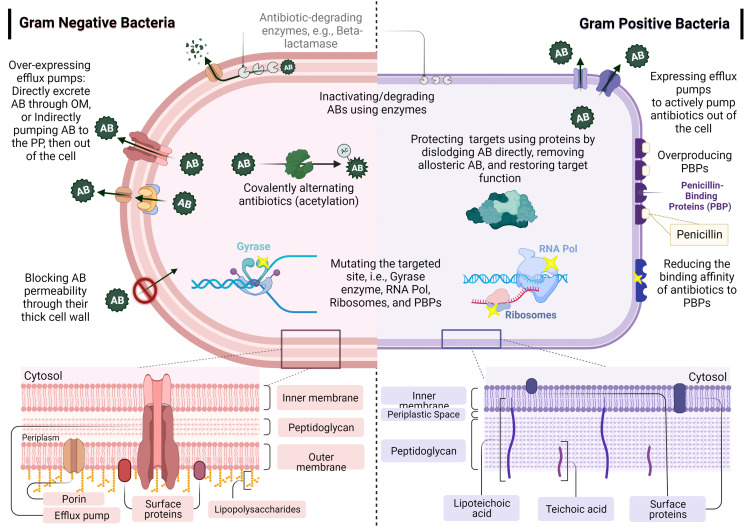
As shown in Figure 2, the resistance mechanisms of antibiotics include efflux pumps (extruding the antibiotics to the bacterial periplasmic space), changing the expression of the membrane proteins (porins), decreasing the cellular membrane’s permeability, and reducing small inhibitory molecules diffusion.20,21 These mechanisms might be similar in Gram-negative and Gram-positive bacteria, including overexpressing efflux pumps, inactivating/degrading antibiotics using enzymes (eg, β-lactamase), and mutating the targeted sites (yellow stars in Figure 2 highlight mutation sites). Other mechanisms differ according to the bacteria, such as blocking the permeability of antibiotics, covalently alternating antibiotics, overproducing PBPs, reducing antibiotic binding affinity, and protecting the targets of antibiotics using proteins.22–24
Figure 2.
Bacterial biofilm resistance with different cellular mechanisms in Gram-positive and Gram-negative bacteria.
Notes: Data from these studies.22,25
Abbreviations: AB/Abs, antibiotic; AC, acetylation; OM, outer membrane; PP, periplasm; PBPs, penicillin-binding proteins; RNA Pol, RNA polymerase.
Diagnosing biofilm-caused infections are dependent on laborious and expensive procedures. In addition, the antibiotic sensitivity assays measure the bacterial susceptibility in its planktonic form, not in the biofilm form, which may result in prescribing the wrong antibiotics or dosage.19 Researching antibiofilm drugs with specific, rapid, and cost-efficient diagnostic tools will advance the management of biofilm-caused infections.26 Scientists are exploring simple and fast procedures to evaluate the susceptibility of bacterial biofilm and not solely depend on the susceptibility of planktonic cells.27,28
Pseudomonas aeruginosa, Acinetobacter baumannii, and Staphylococcus aureus are known pathogens in the medical setting. Their ability to exert variable virulence factors, eg, biofilm formation, is essential during the disease they cause.29,30 We will discuss the current knowledge related to the role of biofilm formation in terms of their prevalence and medical significance.
Pseudomonas aeruginosa
Pseudomonas aeruginosa is ubiquitous in diverse environments and notoriously resist antibiotics.31 Human infections caused by P. aeruginosa are prevalent; it is a primary opportunistic pathogen causing nosocomial infections using its hierarchical QS signaling pathways, regulates multiple virulence factors, and produces biofilm.32 Carbapenem-resistant Enterobacteriaceae P. aeruginosa (CRPA) is the leading cause of various healthcare-associated infections (HAI) in the United States, with more than 50,000 cases annually. This results from the presence of resistance plasmids with diverse virulence factors and metabolic pathways.33
Pseudomonas aeruginosa is of the most prevailing Gram-negative biofilm-forming bacteria in medical settings.34 It is responsible for 10%-20% of all HAI, which is challenging to treat if it colonizes the host tissue, specifically in cystic fibrosis (CF) patients. The biofilm matrix, known as the ECM layer, comprises of DNA, exopolysaccharides, and proteins. In addition, mucoid strains isolated from CF patients produce a plethora of exopolysaccharide alginic acid.34 The proteinaceous constituents essential in biofilm formation are lectins- and carbohydrates-binding proteins. P. aeruginosa makes two lectins, LecA and LecB; both are important in biofilm formation and instigating infections.35 Observing clinical Pseudomonas aeruginosa biofilm, versatile factors are expressed in response to environmental stressors and are activated to adapt to these stressors.35 The essential elements in P. aeruginosa biofilm formation and their genetics are listed in Table 1. These elements include extracellular DNA, exopolysaccharide (ie, alginate, Pel, Psl), and motility apparatus such as flagella and pili. Lipopolysaccharides (LPS) are potent constituents of Pseudomonas biofilm, comprising mainly O antigen (O-polysaccharide), oligosaccharides, and Lipid.36 The LPS layer affects the host’s immunity by suppressing the host’s defenses and inducing hyperinflammatory reactions. This contributes to the chronic nature of the biofilm-associated infections in P. aeruginosa by producing key virulence factors, ie, biofilm formation and toxin (Lipid A) secretion.36,37 Comprehending the dynamics underpinning biofilm formation in P. aeruginosa is vital to develop appropriate antibiofilm agents, as these factors can be used as targets for new approaches.
Table 1.
Summary of Required Components for Biofilm Formation and Maturation in P. aeruginosa
| Factor | Identity or Chemistry | Responsible Gene/Operon | Function | Refs |
|---|---|---|---|---|
| Alginate | Exopolysaccharide/ O-acetylated 1–4 linked D-mannuronic acid and variable proportions of its five epimer L-guluronic acid | The alginate operon (algD, alg8, alg44, algk, alagE, alaG, algX, algL, algl, algJ, algf, and alqA and alqC) | Biofilm matrix formation in mucoid strains, Antibiotics tolerance and protection from host immunity, Aminoglycosides resistance |
[32,38–40] |
| eDNA | Nucleic acid (extracellular DNA) | Cell Lysis | Virulence and biofilm matrix dynamics Antibiotic tolerance Nutrient source |
[32,38,40,41] |
| Flagella | Multiprotein complex | At least 41 genes clustered in three regions of the genome encode flagellin structural and regulatory components | Irreversible adhesion and biofilm development | [32,42] |
| Pel | Exopolysaccharide/ partially acetylated (1–4) glycosidic linkage of N-acetylgactosamine and N-acetylglucosamine | The pelA-G operon GDP-mannuronic acid |
Biofilm formation on solid surfaces Biofilm matrix development and maintenance Virulence Aminoglycosides resistance |
[32,38,40,43] |
| Psl | Exopolysaccharide/Repeating pentasaccharides containing D-mannose, D-glucose, and L-rhamnose | The psIA-O operon | Biofilm matrix formation, Antibiotic protection | [32,38] |
| Type 4 pili | Multiprotein complex/ Type 4a Pill |
The piliM/N/P/Q and the fimU-pilVWXY1Y2E operons |
Early attachment, adhesion, and biofilm development | [32,42] |
Quorum-sensing expresses more than 300 genes, including genes fundamental in biofilm formation and the virulence in P. aeruginosa.44,45 The QS-dependent virulence factors produced for the pathophysiology of P. aeruginosa are listed in Table 2. In Pseudomonas, virulence factors and QS-molecules are synthesized differently during biofilm formation, adhesion, formation, maturation, and dispersion. This contributes to surface attachment, stability, nutrition, cellular arrangement, and protection against antibiotics and host immunity.46
Table 2.
Representation of QS-Dependent Virulence Factors in P. aeruginosa
| Virulence Factor | Class or Chemistry | Encoding Gene | Secretion | Function | Refs |
|---|---|---|---|---|---|
| Alkaline Protease (aeruginolysin) | Extracellular enzymes-aided invasion/ M10 peptidase family/ zinc-metalloendopeptidase | aprA | Type 1 secretion system (T1SS) | Wide protease activity, tissue damage, immune system evasion, iron accessibility | [32,47] |
| Exotoxin A | Toxin/ PE belongs to the two-component AB toxin family/ NAD+- diphthamide-ADP- ribosyltransferase | toxA | Type 2 secretion system (T2SS) | Toxicity, immunosuppression, modifying the elongation factor 2 in eukaryotic cells, tissue damage, and death | [32,47] |
| Hydrogen cyanide (HCN) | Toxic secondary metabolite | hcnABC operon | Diffusible | Highly toxic/potent inhibitor of cytochrome c oxidase and other metalloenzymes, cytotoxicity, colonization | [32,47] |
| LasB Elastase | Extracellular enzymes-aided invasion/ M4 thermolysin peptidase family/ zinc metalloprotease | lasA | The Sec pathway and Type 2 secretion system (T2SS) | Protease and elastolytic activity, cleaving a wide range of glycine-containing proteins, tissue damage, immune system evasion, and invasion | [32,47] |
| LasB Elastase | Extracellular enzymes-aided invasion/ Β-lytic zinc metalloendopeptidase (staphylolytic)/ serine protease | lasB | The Sec pathway and Type 2 secretion system (T2SS) | Protease and elastolytic activity, invasion, infection, tissue damage, iron accessibility | [32,47] |
| Lectin A | Toxic secondary metabolite/ Tetrameric protein | lecA (or pa1 L) | Intracellular; only a small fraction present on the cell surface | Galactophilic, adhesive, colonization, infection | [32,47] |
| PlcB | Extracellular enzymes-aided invasion/ Phospholipases C | plc B | The Sec pathway and Type 2 secretion system (T2SS) | Hydrolyzing phosphatidylcholine and phosphatidylethanolamine, invasion, programmed tissue death, iron accessibility | [32,47] |
| Pyocyanin | Toxic secondary metabolite/ tricyclic phenazine | phzA1-G1 and phzA2-G2 | Type 2 secretion system (T2SS) | Redox-active, immunity evasion, colonization, toxicity | [32,47] |
| Pyoverdine | Iron acquisition/ Pyoverdines/ dihydroquinoline-type chromophore linked to a peptide | Large multi-modular enzymes/ non-ribosomal peptide synthetases (NRPSs) | PvdRT-opmQ Efflux pump and MexAB-OprM efflux pumps |
High affinity to Fe (III) /iron acquisition/fluorescent, tissue invasion, biofilm development | [32,47] |
| Rhamnolipids | Toxic secondary metabolite/Rhamnose-containing glycolipid compounds | rhlAB operon and rhiC | - | Biosurfactant, detergent, structure, hemolytic activity, biofilm dispersal, toxicity | [32,47] |
Earlier studies noted the effect of biofilm formation by P. aeruginosa on infection and antibiotic resistance.40,48–50 A prospective study that monitored the HAI incidence in Rome for 24 months, reported a prevalence of Pseudomonas infections of 25%.51 A meta-analysis of studies between 2000 and 2010 reported P. aeruginosa as the most frequently found wound pathogen.52 This prevalence urged Researchers to study the effectiveness of new approaches to combat this pathogen. For instance, anti-virulence drugs proved effective by disarming the bacteria rather than targeting their replication and growth. As a result, the infection rate will eventually slow down, allowing the immune system to attack the invading pathogen.53 A recent study by Rezzoagli and researchers demonstrated a novel procedure by combining antibiotics and anti-virulence drugs, Gallium (siderophore-binding molecule) and furanone C-30 (QS-inhibitor), to treat P. aeruginosa in vitro.54 Their approach proved effective against the bacteria in a concentration-dependent manner. They observed a promising synergy at intermediate drug concentrations for specific drug pairs.54
Acinetobacter baumannii
Acinetobacter baumannii represents a unique species. It is possible to isolate A. baumannii from Healthcare facilities, food, water, and soil. Literature highlighted their clinical significance due to the increase in carbapenem-resistant strains in hospitals and communities.55 Infections caused by A. baumannii are increasingly challenging for healthcare professionals globally, particularly in intensive care units. The multi-drug resistance (MDR) rate has increased significantly in recent years, with a tolerance to last-line antibiotics, such as colistin.56
The World Health Organization (WHO) labelled A. baumannii a “critical” pathogen, constituting a significant global human health risk.57 This opportunistic pathogen mediates pathophysiology mechanisms, including bacterial virulence factors (eg, biofilm formation), genes (eg, resistance), and host immune responses (eg, invasion)55 (Table 3). Biofilm formation is a characteristic that results in the survival of A. baumannii in the presence of antibiotics and stressors. A. baumannii causing wound infections produces virulence factors that lengthen the treatment course, especially in burn patients, elevating the mortality and morbidity rates.58
Table 3.
Summary of Virulence Factors, Genes, and Pathogenesis of A. baumannii
| Virulence Factors or Genes | Role in Pathogenesis | Model | Refs |
|---|---|---|---|
| Porin (OmpA, Omp33-36, Omp22, CarO, OprD-like) | Adherence and invasion, Induction of apoptosis, serum resistance, Biofilm formation, persistence | In vitro studies and induced tracheal aspiration pneumonia by AB 5075 strain in C57BL\6 mice; Growth rate and cell cytotoxicity of clinical isolate compared to ATCC 19606; intraperitoneally challenged C57BL/6 mice with Omp33-36 knockout in AB 17978 compared to wild type | [59–71] |
| Capsular polysaccharide | Growth in serum, survival in tissue infection, biofilm formation | C57BL/6 mice intraperitoneally challenged with stimulated AB ATCC 17978 to form capsule compared to capsule-mutated strains | [59,60,72–75] |
| Lipopolysaccharide (LPS) genes | Serum resistance, survival in tissue infection, evasion of the host immune response | BALB/c mice intraperitoneally challenged with knockouts in AB ATCC 19606 compared to wild types. | [59,60,76–81] |
| Phospholipase (PLC and PLD) LipA (lipase) | Serum resistance, invasion, in vivo survival | Neutropenic tail vein infection of DBA mice infected with LipA knockout in A. baumannii ATCC 17978 and compared to wild type | [59,60,82–86] |
| Outer membrane vesicle (OMV) | Delivery of virulence factors, horizontal transfer of antibiotic resistance gene | A. nosocomialis ATCC 17903 purified from OMVs and administered in vitro to cells and BALB/c mice model | [59,60,87–93] |
| Iron acquisition system –siderophore (Acinetobactin and NfuA) | In vivo survival, persistence, the killing of host cells | Galleria and C57BL/6 mice models with intraperitoneally infected with acinetobactin knockouts AB ATCC 19606 and compared to the wild type | [59,60,85,94–99] |
| Zinc acquisition system (ZnuABC and ZigA) | In vivo survival | C57BL/6 mice challenged with intranasal infection by A. baumannii ATCC 17978 or a ZigA knockout strain | [59,60,94,95] |
| Manganese acquisition system (MumC and MumT) | In vivo survival | — | [59,100] |
| Type 2 protein secretion system (T2SS); gspD | In vivo survival and an intermediate effect on bacterial population density | C57BL/6 mice received nasal inoculation of a gspD-knockout strain of A. nosocomialis M2 and compared to the wild type | [59,60,86,101,102] |
| Type 6 protein secretion system (T6SS) | The killing of competing bacteria, host colonization | T6SS was analyzed in AB ATCC 17978, AB DSM30011 non-clinical isolates, and three clinical isolates | [59,60,103–106] |
| Type 5 protein secretion system (T5SS) | Biofilm Formation, adherence | — | [59,107] |
| Penicillin-binding protein 7/8/ and β-LactamasePER-1 | Serum resistance, in vivo survival, adherence | — | [59,108,109] |
| CipA | Serum resistance, invasion | — | [59,110] |
| Tuf | Serum resistance | — | [59,111] |
| RecA | In vivo survival and repairing damaged DNA | CD1 mice with intraperitoneal infection using A. baumannii ATCC 17978 after RecA knockout compared to wild type | [59,60,112] |
| SurA1 | Serum resistance, in vivo survival, growth rate | Chicks infected with Knockout of SurA1 A. baumannii CCGGD201101 | [59,60,113,114] |
| GigABCD | In vivo survival, the killing of host cells | — | [59] |
| Universal stress protein A (UspA) | In vivo survival, the killing of host cells, pathogenesis of sepsis and pneumonia infections | C57BL/6 mice were challenged intranasally and intraperitoneally using the UspA knockout strain of AB ATCC 17978 and compared to the wild type | [59,60,113,115] |
| Sensor kinas (GacS) and catabolic pathway of phenylacetic acid (PaaE) | Neutrophil influx | DBA mice challenged with intraperitoneal infection of knockout in AB ATCC 17978 and compared to wild type | [59,60,113,116,117] |
| Pili | Adherence, biofilm formation | — | [59,118,119] |
| OmpR/EnvZ | The killing of host cells | — | [59,120] |
| FepA (enterobactin receptor) | Competitive growth rates | CBA/J mice challenged with intravenous infection by A. baumannii ATCC 17978 with a mutant transposon library | [59,60,121] |
| AbeD | The killing of host cells | — | [59,122] |
| gacA and gacS (regulator genes) | — | Zebrafish embryos challenged with AB ATCC 17978 and knockouts | [60,117] |
| Biofilm gene (LH92_11085) | Biofilm formation | Identification of gene expression level and biofilm formation in A. baumannii MAR002 | [60,123] |
In a systematic review and meta-analysis, scientists investigated the association between the ability to form biofilm in A. baumannii in patients suffering from burns, and the antibiotic resistance trend. Results indicate that more than 90% of A. baumannii strains produced biofilms, contributing to antibiotic resistance in the burn units.58
Altınok and others compared virulence genes and A. baumannii’s ability to form a biofilm and revealed that the biofilm formation was mostly related to genes encoding curli fiber (csgA), the chaperone-usher fimbria (csuE), and the outer membrane (ompA).124 Different virulence genes such as csgA, csuE, fimH, ompA, and blaPER-1 were investigated, and it was determined that 60.3% of the isolates formed biofilm. The frequency of csgA, csuE, ompA, fimH and blaPER-1 genes of all isolates were 71.2%, 32.1%, 21.8%, 7.1%, and 3.2% respectively. The frequency of csgA, ompA, bap, csuE, and fimH virulence genes of the biofilm-producing isolates was 41.5%, 24.5%, 20.2%, and 5.3%, respectively. All the genes studied were abundant in the isolates with a strong and medium-positive biofilm production. This demonstrates that, except for the fimH gene, the csgA, csuE, and ompA genes contributed to the biofilm formation in invasive A. baumannii isolates.124
Another review by Colquhoun and Rather investigated the genetic regulation mechanism of biofilm formation in A. baumannii.125 The research divided the known 132 up-regulated biofilm genes in the outer membrane proteins, attachment/motility, metabolism, transcription, translation, and hypothetical proteins. The top categories were metabolism (49 genes), translation (30 genes), and outer membrane proteins (29 genes). This indicates that the transition and maintenance of the biofilm environment require alterations in the metabolic pathways and configuration of the outer membrane, assisted mainly through the translation machinery required for the production of nascent proteins.125 The seriousness of A. baumannii infections is due to its resistance to the latest line of antibiotics, colistin, which mandates researching new treatment approaches. Srisakul and others reported novel synergistic activity between colistin and sulbactam against clinical isolates of colistin-resistant A. baumannii. This combination was tested in mouse models and in vitro; combinatorial therapy may provide a new option to treat this critically alarming pathogen.126
Staphylococcus aureus
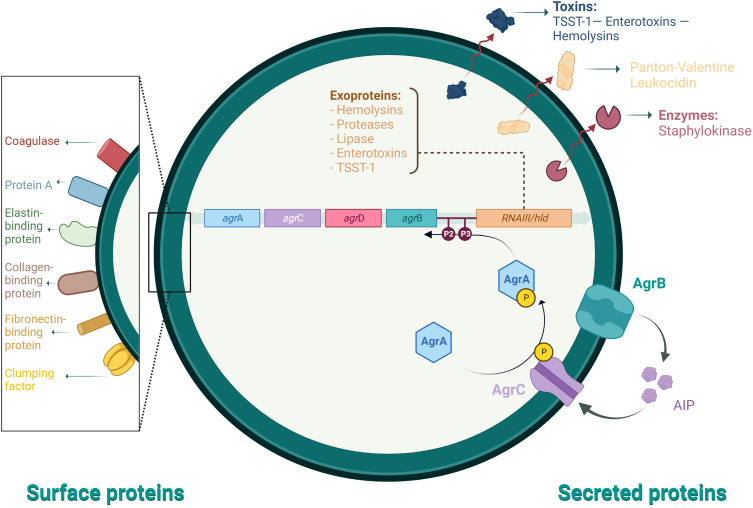
Staphylococcus aureus is of the most ubiquitous and dangerous facultative intracellular human pathogens due to its virulence and ability to develop antibiotic resistance.127 Methicillin-resistant Staphylococcus aureus (MRSA) transmission occurs from infected patients, healthcare personnel, or items colonized with MRSA due to contact, known as hospital and community transmission of MRSA, respectively.128 S. aureus produces extracellular enzymes important for the pathophysiological mechanisms and regulates novel virulence factors. Multiple environmental factors, including nutrients, antibacterial agents, pH, shearing force, and temperature influence the stages of biofilm formation. These stages are attachment, maturation, detachment, and development.129 They significantly influence several virulence determinants that evoke the host’s immune response to the bacteria. Biofilm-associated infections are correlated with intravascular catheters or attachment to medical implants and host tissue.129 Some Staphylococcal diseases are toxin-mediated because many strains produce exotoxins, such as toxic shock syndrome (TSS toxin 1), scalded skin syndrome (exfoliative toxin), and food poisoning (enterotoxin).130 Surface and secreted proteins are essential for the mechanisms of pathophysiology and regulation of novel virulence factors for S. aureus (Figure 3).
Figure 3.
Staphylococcus aureus pathophysiology mechanisms and regulation of virulence determinants.
Notes: Data from these studies.129,131
Abbreviations: TSST-1, toxic shock syndrome toxin 1; P1 and P2, promoters; agrABCD, accessory gene regulator genes operon and the precursors for AgrABC proteins; RNAIII, RNAIII transcript yields RNA as the primary effector; AIP, autoinducing peptide.
One of the features of S. aureus biofilm infections is its ability to survive on biotic and abiotic surfaces and its tendency to resist drugs.132 If we compare MRSA infections with all other infections caused by MDR Gram-negative bacteria, it is 10 times more prevalent. The WHO included MRSA as one of the 12 urgent pathogens jeopardizing human health.132 Notably, the genotypic variation of S. aureus strains may play a role in biofilm production, but evidence is still insufficient to support these associations.133
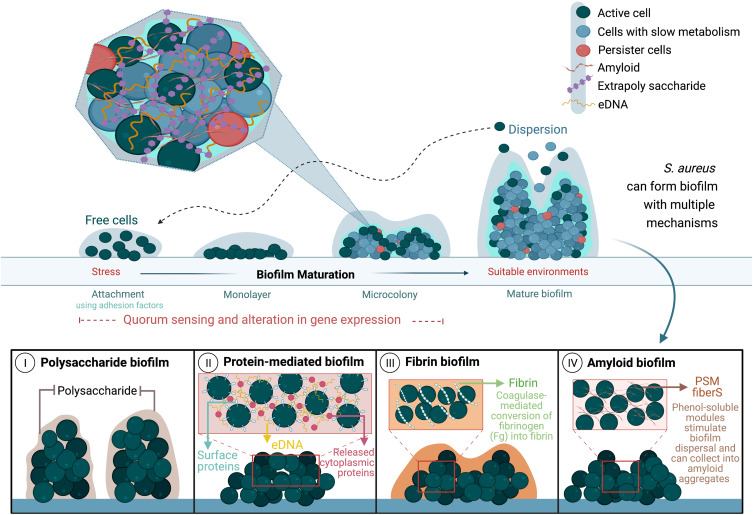
S. aureus formulate four types of biofilms: polysaccharide biofilm, protein-mediated biofilm, fibrin biofilm, and amyloid biofilm.134 The polysaccharide intercellular adhesin (Pia) protein, involved in intercellular adhesion and biofilm formation, is a documented virulence factor in the S. aureus cell wall.135 This element is also the first identified mediator of biofilm formation in Staphylococcus epidermidis, which has formed the foundation of other biofilm mechanisms in S. epidermidis and S. aureus.134 Studies indicate that S. aureus produces biofilm using proteins instead of polysaccharides (protein-mediated biofilms) in an ica-independent manner136 (Figure 4). Numerous surface adhesins are involved in protein-mediated biofilm production, including Bap, Spa, FnBPA, FnBPB, and SasG. These proteins are referred to as “cell wall-anchored proteins.” They enable S. aureus to resist polysaccharide-degrading enzymes, such as dispersin B, and survive harsh environments.137–139 Fibrin biofilm forms mostly during blood infection when staphylococcal coagulase attaches to the host’s prothrombin forming a complex, which causes the conversion of fibrinogen in the blood to fibrin, shielding the biofilm from its surroundings.134 Phenol-soluble modulins produce amyloid biofilm after accumulating in the amyloid fibers that improve biofilm formation (Figure 4).134
Figure 4.
Stages of S. aureus biofilm production and its different formation mechanisms.
Notes: Data from these studies.134,140
Abbreviations: eDNA, extracellular DNA; Fg, fibrinogen, PSM fibers, phenol-soluble modulins fibers.
Biofilm formation impacts the patient experience by affecting their chances of being readmitted within 90 days of discharge or decreasing their 90-day mortality rate.133 Luther et al, 2018, studied the connection between strong and weak biofilm formation in S. aureus and its ramification on hospitalized patients. Results showed links between the strong biofilm production in MRSA and agr genes deficiency, pigmentation, and administration of immunosuppressants or chemotherapy within the last 90 days.133 Research related to the effect of the genotypic variations in S. aureus strains, including MRSA, and its magnitude on biofilm formation is urgently required.
To summarize, many bacteria can produce a structured ecosystem called the “biofilm.” Gram-positive and -negative bacteria possess varying morphology, genetics, and physiologies, but several biofilm-formation dynamics are comparable between the two types of bacteria.141 However, researchers suggest exploring how these dynamics might change in polymicrobial biofilms, comprising the two types of bacteria or other species, and their contribution during infection. Pseudomonas aeruginosa, Acinetobacter baumannii, and Staphylococcus aureus are associated with most human infections and known for their inherent tolerance to antimicrobials and the host’s immune responses.142–144 These medically prevailing pathogens pose a global burden, primarily in hospitals and communal areas, that adversely influence the patient experience and are costly to treat.Biofilm-associated infections challenge the current therapeutics and diagnostics. More surveys of the incidence of infection must be done to provide evidence for the public and scientific community to encourage a hygienic lifestyle and urge researchers to investigate novel control approaches. Table 4 summarizes medically important Gram-positive and negative biofilm-forming bacteria, associated infections, and numerous innovative research and treatment approaches.
Table 4.
Biofilm-Associated Infections and Innovative Treatment Approach
| Bacteria | Biofilm Infection | Approach | Refs |
|---|---|---|---|
| Gram-negative, obligate Aerobic | |||
| Escherichia coli | Urinary tract infections (UTI) Neonatal sepsis Meningitis Catheter-associated urinary tract infections (CAUTI) Crohn’s disease and enteric syndromes associated infections Enteric hemorrhage Veterinary infectious diseases, including mastitis |
Anti-adhesion agents Phage therapy Antimicrobial peptides Natural compounds Nanocarriers Combinatory therapy, ie, CRI-dots nanocomplex (CRISPR-Cas and Carbon quantum dots) |
[145,146] |
| Pseudomonas aeruginosa | Nosocomial infection Chronic infections in immunocompromised patients Chronic lung infection Chronic wound infection Chronic rhinosinusitis |
Anti-biofilm strategies (eg, Fimbriae or pili assembly blockers, OmpA inhibitors) Antiquorum-sensing (QS) molecules (eg, AHL, abaR receptors) Extracellular polymeric substances (EPS) inhibitors Antibacterial agents (iron-chelators) Antimicrobial peptides Advanced nanocarriers |
[46,147] |
| Acinetobacter baumannii | Nosocomial infections Ventilator-associated pneumonia Urinary tract infections Wound infections Bacteremia Endocarditis Meningitis Severe community-associated infections |
Antibiofilm peptides, inhibitors of natural and synthetic sources, anti-QS molecules, degrading enzymes, combinatorial therapy of essential oils and nanocarriers (eg, polymer, metal, and silica-based nanoparticles) Phage therapy Photodynamic therapy |
[148–151] |
| Salmonella sp, S. Enterica serovar Typhi, S. typhimurium | Typhoid fever bowel perforation septicemia; meningitis; Catheters-associated infections Non-typhoidal salmonellae diseases, ie, chronic gallbladder infection Recalcitrant typhoid fever leading to hepatobiliary cancers |
EPS production inhibitors Antimicrobial Peptides Curli amyloid fibers inhibitor by a human monoclonal antibody Combinatory therapy, ie, ultrasound and disinfectants, halogenated furanones and antibiotics or disinfectants, and nano- and micro-emulsion |
[152–157] |
| Klebsiella pneumonia | Community-acquired and nosocomial infections Medical devices-associated infections Urinary and biliary tract infections Osteomyelitis Bacteremia Chronic endometritis-associated infections Mastitis Prostatitis |
Natural compounds (eg, 3-methyl-2(5H)-furanone and 2´-hydroxycinnamic acid) Phage therapy Biofilm matrix-degrading enzymes Histidine Functionalized Silver Nanoparticles Combinatory therapy, ie, non-ionic surfactants and phages; carboxypterin, methylene blue, and antimicrobial photodynamic therapy (aPDT); nanotechnology aPDT, and antimicrobial photothermal therapy (aPTT). |
[152,158–164] |
| Gram-positive, facultative Anaerobic | |||
| Staphylococcus aureus | Mastitis Chronic Endometritis-associated infections Laryngitis Pharyngitis Catheters-associated infections (eg, intravenous catheters, urinary catheters, dialysis catheters) Infections associated with implanted medical devices (eg, pacemakers, joint prostheses, and fluid shunts) |
Antibiofilm surfaces: Anti-adhesion agents (eg, polyethylene glycol (PEG) coating Antifoulants (coatings using paints containing metal nanoparticles) aPDT Laser Shock waves (LSW) Antimicrobial peptides Enzymes Phage therapy Combinatory therapy, ie, ultrasound and disinfectants, using multiple antibiotics. |
[152,154,165,166] |
| S. epidermidis | Medically implanted devices infections Skin and soft tissue infections Bone and muscle infections Lung infections Bloodstream infections |
QS-inhibitors (eg, thiophenones and furanones) EPS dispersion agents (eg, ß-glucosidase, N-acetylcysteine; NAC) Antibiofilm surfaces: Anti-adhesion agents (PEG coating) Antifoulants (metal nanoparticles-containing paints) Combinatory therapy, eg, nanotechnology and aPDT, antimicrobial photothermal therapy (aPTT) |
[157,159,161–163,165,167,168] |
| Streptococcus pneumoniae | Colonization of the nasopharynx leads to otitis media Chronic Endometritis associated infections Rhinosinusitis Laryngitis Pharyngitis |
Nanotechnology (eg, Zinc oxide nanoparticles) Inhibition by small molecules (eg, Quercetin, DNA adenine methyltransferase Inhibitor, and Pyrimidinedione) |
[152,169–171] |
Innovative Approaches to Curb Biofilm Formation
Biofilm is present in more than 90% of bacteria and impacts bacterial infection significantly.172 The increasing incidence of multi-drug-resistant bacteria warrant the development of novel interventions. Bacterial virulence management is an interesting viewpoint compared to antibiotic therapy, which provides methods to prevent pathogenesis without adding stress on the targeted bacteria.13,173 This review explores innovative techniques that might affect the control and defense against biofilm formation by targeting bacterial virulence factors. These approaches include phytochemicals, antimicrobial photodynamic therapy (aPDT), bacteriophages, gene editing by CRISPR-CAS, and nano-mediated techniques.
Natural Products (Phytochemicals)
New methods widely explored phytochemicals extracted from natural sources to prevent biofilm formation and quorum-sensing.174,175 Phytochemicals, alone or in combination, have been used to repurpose old antibiotics or reduce the dose of antibiotics.176,177 Human cultures have been using herbal remedies for centuries, and some of these natural products are useful in treatment and prevention.172 Traditional Chinese medicinal herbs, for instance, were widely used in treating bacterial infections and prevention, and their antibacterial capacity was demonstrated in some herbs, such as Scutellaria, Taraxacum, and Tussilago. Plant extracts have also recently been described to control biofilm development and inhibit quorum-sensing (QS) in bacteria.172
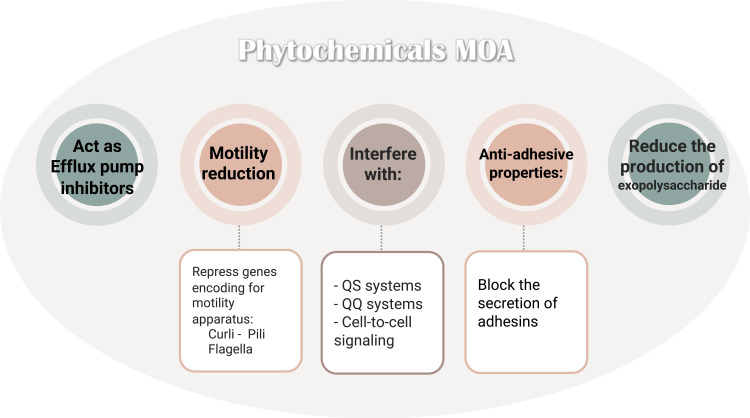
Many natural anti-biofilm compounds, such as phytochemicals, biosurfactants, and antimicrobial peptides, are effective against a broad range of microbial biofilms with different modes of action.178 Novel natural antibiofilm agents such as phytochemicals broadly comprise numerous natural compounds with anti-biofilm properties: phenolics, terpenoids, lectins, alkaloids, polypeptides, and polyacetylenes. These natural phytocompounds, with their mechanisms of action (Figure 5), can regulate QS and quorum-quenching (QQ) systems, crucial players in bacterial biofilm formation, virulence factors and antibiotic production in Gram-negative and positive bacterial infections.178
Figure 5.
Presents the numerous modes of action of phytochemicals on various biofilms.
Notes: Data from Mishra et al.178
Abbreviations: QQ, quorum-quenching; QS, quorum-sensing.
The bacterial QS systems received increasing research focus. Targeting the bacterial QS system is an efficient strategy to prevent biofilm formation. Evidence shows that QS inhibitors obtained from natural compounds, such as 1,2-benzene-dicarboxylic acid, diisooctyl ester play a significant role in inhibiting bacterial adhesion and suppressing biofilm-related genes.179 Singh and others suggested that phytochemicals down-regulate biofilm formation genes by competing with the QS-molecules, such as N-Acyl homoserine lactones (AHL) and autoinducers (AIs) and preventing their binding to these genes.179 Scientists are exploring the therapeutic value of traditional medical practices by using natural products and their effect on QS systems. Research focuses on studying natural QS-controlling substances and their mechanisms, to treat microbial-related diseases and impede antibiotic resistance.180–182 Literature suggests using green methods to manage biofilm, including enzymes, phages, and bioregulation in the food industry.183,184 Using detergents from enzymes as bio-cleaners, also called green chemicals, will support solving the issue of biofilm in the dietary industry.185
Reen et al reviewed the potentiality of using coumarins to inhabit biofilm and QS. Coumarins are a vast family of naturally derived fused benzene and a-pyrone rings. They are predominantly present in plants with many pharmacological properties. Examples of coumarin compounds with antibiofilm activity include ellagic acid, warfarin, nodakenetin, and fraxin.180 Girennavar and others explored the anti-biofilm and anti-QS ability of a constituent containing joined coumarins and furan molecules known as furocoumarins.186 This constituent (extracted from grapefruit) showed similar activity to coumarins and inhibited 95% of the autoinducer-1 (AI-1) and autoinducer-2 (AI-2) signaling systems in the biosensor strain Vibrio harveyi. It inhibited biofilm formation in Gram-negative bacteria, ie, Escherichia coli, Salmonella typhimurium, and P. aeruginosa.186,187
Other researchers tested seven structurally related coumarin compounds against P. aeruginosa and Chromobacterium violaceum (biosensor strain).188 The results indicated that these compounds, largely the compounds retaining a hydroxyl group, affect QS and biofilm formation in bacteria. This phytochemical impacted biofilm formation in P. aeruginosa but not bacterial growth, suggesting that this inhibitory action targeted the QS-signaling system in the bacteria.188
Many natural compounds, medicinal plants and phytochemicals, contributed to the research and discovery of antibiofilm agents. For example, Allium subhirsutum L. (hairy garlic) proved to have antibiofilm, anti-quorum-sensing, and antibacterial activity against different bacterial and fungal isolates.189 Eruca sativa Miller was tested against numerous food-borne pathogens for their antibacterial and antibiofilm activity and demonstrated reduced bacterial density within the biofilm community and a disturbed integrity of the biofilms by interfering with exopolysaccharides synthesis.190 Also, the molecular analysis found that E. sativa phytochemicals fused with multiple adhesion proteins associated with biofilm formation, causing compromised biofilm formation.190 Advanced spectroscopic analysis showed that different bioactive compounds (eg, flavonoids, phenols, terpenes, and tannins) contribute to the versatile biological action of natural constituents, including the antibiofilm effect.189–192
In conclusion, bacteria alter their gene expression by sensing the levels of molecules depending on the external environment, thus affecting biofilm production and other virulence factors.193 Phytochemicals can regulate QS and, as a result, control biofilm-caused infections by controlling bacterial gene expression. The primary challenge of applying phytochemicals in antibiofilm therapy is the insufficiency of animal studies and clinical trials. Advancing research to these studies will accelerate their applicability in medicine.
Bacteriophages
Phages were introduced as a potential treatment for multi-drug resistant (MDR) pathogens, and several laboratories and research centers globally perform intensive bacteriophage research. Bacteriophages, in short phages, are bacterial viruses that can infect and kill or incapacitate specific kinds of bacteria but leave other bacteria and human cells unharmed.194–196 Phages are the most abundant biological entities in the biosphere, and they have been used therapeutically in some parts of the world since the 1920s.197 Phages are under renewed consideration globally as tools for fighting the growing crisis of antibiotic-resistant bacteria.198 The phage biology makes them potential candidates for phage therapy applications and other activities against multidrug-resistant (MDR) pathogens in general. Phage biology includes target selection where the phages, which have specificity in their cell infection, can infect the bacterial cell, propagate in the cell, and then lyse vast numbers of the cell.199 Towards the end of the infection cycle, phage lytic enzymes destroy their bacterial host cell wall.200 As a phage-based therapeutic option, phage enzymes are exploited to eradicate bacterial virulence symptoms, such as biofilms.201 Using these enzymes to treat bacterial infections associated with biofilms was suggested by destroying the biofilm matrix.202 Phages can penetrate the biofilm layers through pores and channels and subsequently destruct the biofilm matrix. Phages encode various lysis enzymes, such as depolymerase, holins, and endolysins, which can degrade bacterial polysaccharides and rapidly destroy the integrity of biofilms to facilitate phage penetration of the cells within the inner biofilm layers.203,204 Depolymerases have been identified in 143 phages and classified in two classes: hydrolases and lyases, which recognize, bind, and digest EPSs to disturb biofilm structure.205 Endolysins are peptidoglycan hydrolases produced at the end of the infection cycle and cleave the peptidoglycans in the cell wall. Using phages and their derivatives against biofilms and MDR infections associated with biofilms align with developing safe and effective therapeutic strategies against biofilm-associated diseases.206–208
Pei and Lamas-Samanamud engineered a T7 phage that overcame the limitation of affecting a host’s biofilm and disrupt polymicrobial biofilms.209 This phage cleaves the biofilm signaling molecules in an approach known as quorum-quenching. Consequently, it aids in treating multiple hosts in a mixed biofilm infection.209 Silpe and Bassler reported that the V. cholera phage altered its response, lysis or lysogenic, based on the quorum-sensing molecules produced by the host (bacteria) and symbolized it as the phage having the power to “listen in” to the bacteria.210 These QS signals are necessary for virulence and biofilm production. They suggested we could engineer phages to induce bacterial killing in the future, which can be advantageous in medicine, agriculture, and industry.210
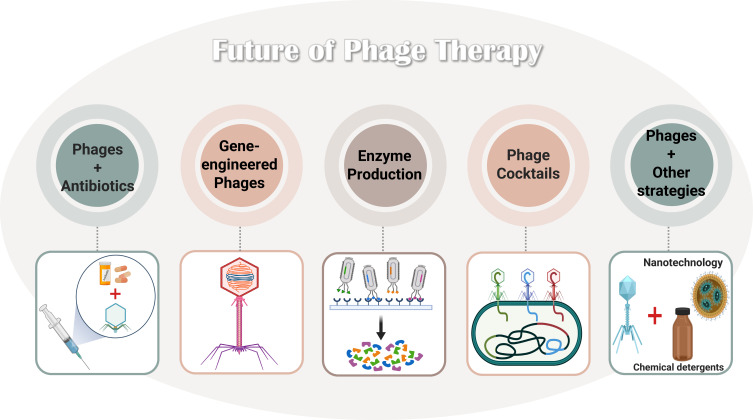
Several phage-based therapeutic options are available to prevent pathogenic bacterial biofilm formation. Figure 6 summarizes a few examples of potential applications of phages, eg, combining phages and antibiotics (eg, the sublethal concentration of ciprofloxacin and phage), engineering phages genetically (eg, knocKing-out virulence-encoding genes), using phage-derived enzymes (eg, endolysins), and utilizing phage cocktail therapy (mixture of phages).211,212
Figure 6.
Future of phage therapy and the prospects of applying it to treat and prevent bacteria and their biofilm. Data from Liu et al.211
Promoting phages in maintaining human health is imperative to developing novel, safe, and efficient treatments for antibiotic-resistant biofilm-mediated infections.211 The current limitations facing phage therapy include the shortage of local phage therapy Centers that specialize in isolating, identifying, and applying them to treat patients with acute MDR infections. In addition, resistance to phages is a rising issue that requires further exploration to provide better treatment.
Bacteriophages and Nanotechnology
Combining phages and nanotechnology enhances phage infectivity, stability, and delivery. Esteban and others introduced Bacteriophage-K in an oil-in-water nano-emulsion formula to improve its stability and infectivity, which increased the bactericidal effect against S. aureus.213 Interestingly, the phage-nano emulsion formula lowered the electrostatic repulsion between the phage and bacteria, both negatively charged, thus boosting the phage-bacterial interaction and improving antibacterial activity.214 Magnetic phage-nanocomposite conjugates (PNCs) were used to disturb the biofilm’s inner layers and to eradicate the bacterial biofilms. Small-sized PNCs removed both dual and multi-species bacterial biofilm with 98% and 92% elimination efficiency rates.215 Yu et al estimated that this activity followed the equal dispersal of phages into the bottom layer of the biofilm.215 In another study by Liu et al, phages were locally isolated, purified, and conjugated with chitosan film as a biocompatible agent to control bacterial infections and subsequent biofilm formation in medical implants.216 The conjugated phage-chitosan film reduced bacterial growth and stimulated neural tissue regeneration in vitro.216 Compared to the free phage, the antibacterial activity was less effective in the phage-chitosan conjugate, which is likely due to the controlled motion of the phage when conjugated. Still, it was estimated that the biofilm prevention resulted from lowering the bacterial density by the phage, 79.5% reduction compared to the control, based on SEM imaging.216 To conclude, phages and nanotechnology can be applied as complementary strategic approaches to target bacterial biofilm formation.
Antimicrobial Photodynamic Therapy (aPDT)
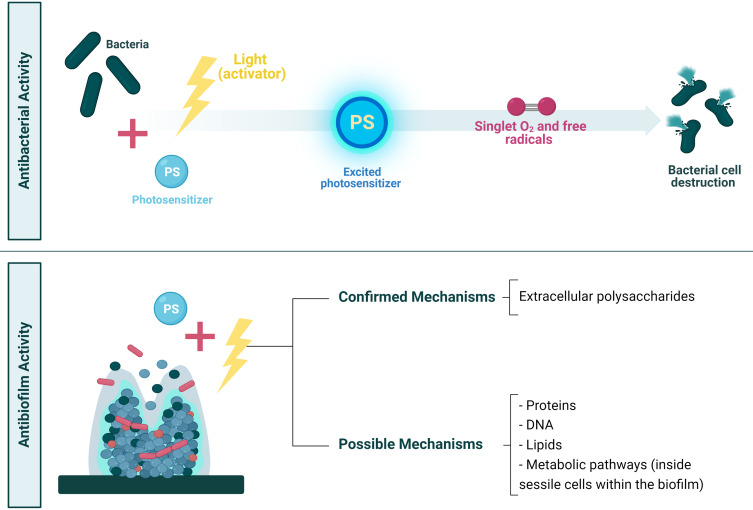
Antimicrobial photodynamic therapy (aPDT) involves a combination of three key components. It requires a visible source of light to activate the photosensitizer appropriately with a specific wavelength of visible light, a non-toxic photosensitizer (PS), and the presence of ambient oxygen that is activated to produce the cytotoxic reactive oxygen species (ROS) and inactivation of the targeted cells (Figure 7). Recently, photodynamic therapy (PDT) studies emerged as a novel non-invasive therapeutic option, which is effective and most efficient for treating localized and superficial infections caused by bacteria present as biofilms, fungi, and viruses.217–219 Also, it is a unique procedure with interesting therapeutic approaches and applications in dentistry to treat biofilm-caused oral infections.217,220
Figure 7.
aPDT mechanism and application as an antibacterial and antibiofilm. The bacteria are absorbed into the photosensitizer and activated to an excited state post-exposure to light at a specific wavelength. Then, from light to molecular oxygen, the photosensitizer (PS) energy can be transferred. When bacteria are formed in a biofilm, aPDT could target the biofilm matrix comprising extracellular polysaccharides or other targets directed towards the bacterial cells within the biofilm.
Microorganism-Photosensitizer (PS) Interactions
The effectiveness of aPDT against different microorganisms depends on the photosensitizer (PS) type, concentration, and class of microorganism. The antibacterial property of aPDT is based on the idea that visible light induces the photosensitizer (PS), which produce ROS. The produced ROS destroys the bacteria unselectively via an oxidative burst, and the confirmed antibiofilm action impacts the integrity of the extracellular matrix (see Figure 7).220–222 The microorganism-photosensitizer interactions depend on the microorganism’s physiochemical features, including relative solubility in water and lipids, constant ionization of factors for light absorption, and efficiently producing oxygen.220 aPDT is more effective against Gram-positive bacteria than Gram-negative bacteria, because of their porous cell wall composed of peptidoglycan and lipoteichoic acid. The PS can reach the cytoplasmic membrane and hinders the structural organization (forming its physical and functional barrier) in Gram-negative bacteria differently. Gram-negative bacteria possess a more complex morphology due to their negatively charged outer cell membrane, which comprises lipopolysaccharide, lipoproteins, proteins (with a porin function), and peptidoglycan. Notably, biofilm reduces the photodynamic activity of PS because of the structural difference in the cell membranes of the microorganisms within the biofilm. Another factor is the presence of other components, such as extracellular polysaccharide matrix and QS factors.218,220 Although numerous planktonic pathogens are killed by aPDT, the sensitivity of biofilm-derived anaerobic bacteria to aPDT is underexplored. The efficiency of aPDT requires microbial cell adsorption to the PS and penetrating the cell wall via subsequent activation through light irradiation.223 aPDT effectively reduces the viability of microbial cells and biofilms in cells and may be an important alternative therapy to traditional dental care techniques to treat many diseases. Photodynamic antimicrobial therapy demonstrates relatively lower toxicity, can enable immediate dental care, has a low cost, multiple PSs are available for each form of the light source, and the treatment is risk-free for the patients.220
The key advantages and benefits of aPDT are their effects on bacteria with substantial differences in the mode of non-selective action, including multiple molecular targets such as proteins, lipids, and nucleic acids. There is a limited adverse effect profile and damage to host tissue. The aPDT exhibits tissue specificity; it allows for curbing many human infectious diseases with no drug resistance and few side effects following multiple therapy sessions.218,224
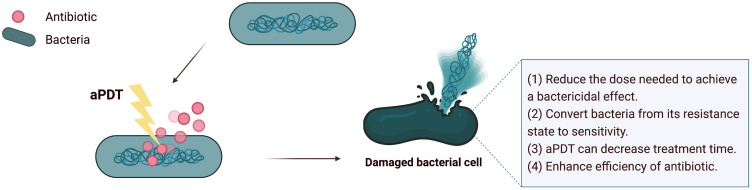
Recent techniques tested the efficacy of combining antimicrobials and aPDT (Figure 8). There is no authenticated protocol for this synergy. However, Vanesa and others suggest that the preferred method is to incorporate aPDT with antimicrobial compounds (synergistically) or perform aPDT periodically associated with long-term antimicrobial drug treatment sessions.225 This synergism aims to enhance antibiotic activity by reducing the dose, toxicity, and treatment time. Also, the likelihood of bacterial resistance to aPDT-antibiotic treatment is low, and studies provided evidence of its ability to revert resistant strains to their susceptible state to specific antibiotics after exposure to sublethal doses of aPDT.225,226
Gene Editing by CRISPR-Cas
Role of CRISPR-Cas in Controlling Bacteria
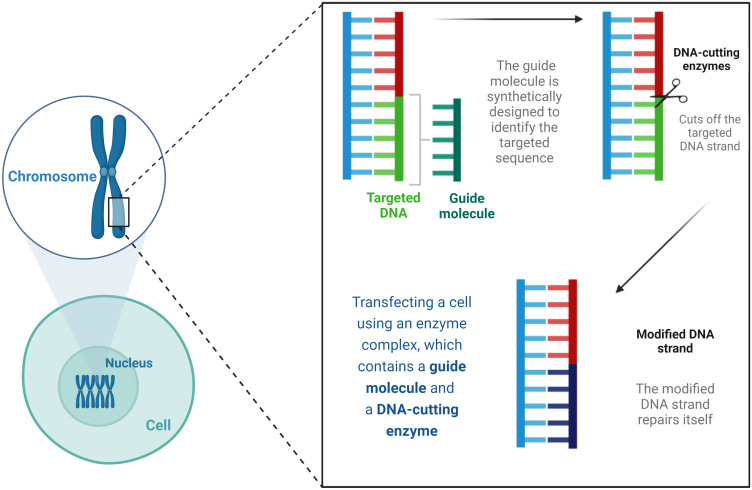
The Clustered Regulatory Interspaced Short Palindromic Repeats (CRISPR) system is emerging as a safe, targeted, and practical approach to treating microbial infections and genetic diseases.227 It requires combining Nanomedicine with CRISPR and specific cleavage of the regulator of bacterial virulence (Cas 9) complex component sensing system, which evolved as bacterial defense mechanisms.227,228 The CRISPR-associated (Cas) genes/proteins expression in human-associated bacteria occurs in diverse Gram-positive and negative bacteria with variable distribution in the human body.228 The CRISPR‐Cas System is present in bacteria and archaea, offering sequence-based adaptive immunity against phages, plasmids, and mobile genetic elements.229 CRISPR technique uses portions of the bacterial DNA. This method for gene editing was first introduced in 2012 in US and Swedish laboratories. As shown in Figure 9, this technique generates directed cuts in a genome when paired with a particular guide protein [ie, Cas9] by using fragments of bacterial DNA.230
Figure 9.
The CRISPR-Cas9 as a concept in gene editing.
Notes: Data from Ho C.230
Abbreviations: DNA, deoxyribonucleic acid.
The CRISPR-Cas structure comprises CRISPR RNAs (crRNAs) and the binding of crRNA, which is unique to the invasive pathogen sequence, resulting in the shredding of the target DNA/RNA sequence by Cas enzymes.231 CRISPR-Cas systems function at a molecular level with adaptive mechanisms known as sequence-specific protection methods that detect invaders and dissect their genetic material.232 This protection mechanism was systematized in three stages, starting with adaptation, as the long precursor of CRISPR locus (Pre-crRNA) is transcribed into crRNA, which is obtained from the invader’s genetic material. The crRNA matures after being incorporated into a CRISPR array. Lastly, an “interference cascade complex” is activated to spot the invader sequence and cleave it by a specific RNA-guided endonuclease.233,234 Recent advances in experimental CRISPR-Cas system research focus on creating animal models and applying functional genomics to screen and develop innovative drugs targeting infectious, immune, and genetic diseases (Figure 10).227
Insights into CRISPR-Based Gene Editing to Control Biofilm Formation
The CRISPR-Cas system protects bacterial cells by identifying and cleaving any invading nucleic acids. It also interferes with bacterial species-specific competitions and the ability to produce several virulence factors during infection, such as regulating gene expression, developing biofilms, repairing the DNA, reacting to stress, and acquiring resistance genes. Scientists can alter the system to provide new insights to understand the role and application of gene editing and modulate CRISPR-Cas using anti-CRISPR agents or antimicrobials against various microorganisms.229
Zuberi et al introduced a novel concept of “CRISPR interference (CRISPRi)” and its role in inhibiting bacterial biofilm formation by targeting a gene essential for QS.235 CRISPRi inhibition produces distinct levels of targeted knockdown, which supports the study of behavioral changes in the bacteria when a gene is expressed at different levels. This study proposed that CRISPRi is a promising technique for inhibiting bacterial biofilm and controlling nosocomial and environmental infections by targeting the luxS gene and intervening with bacterial QS.235 In detail, the luxS gene is an essential gene that encodes for a vital QS molecule, the autoinducer-2 (AI-2) molecule, which has a role in biofilm formation and maturation.236
Research by Zuberi et al used CRISPRi to control urinary tract infections (UTI) and pathogens such as E. coli, specifically in regulating the adhesion property of the bacteria.237 Their idea was to target and knock down the fimH gene expression, which causes the suppression of transcriptional machinery through lodging inactive or “dead” Cas9 at a particular location. These results were assessed using a mannose-sensitive hemagglutination assay and TEM. The authors proposed that this approach be authenticated and applied in treating in vivo infection models to confirm its potential in UTI therapy.237
Noirot-Gros and others used CRISPRi to evaluate its influence on biofilm-associated genes in Pseudomonas fluorescens.238 Cyclic diguanylate (c-di-GMP), a signaling messenger responsible for many bacterial cellular functions, eg, virulence, was monitored with other regulators controlling biofilm formation. They found that CRISPRi inhibition of biofilm formation resulted from the deletion and silencing of genes expressing the biofilm’s thickness and mass.238 CRISPRi also influenced gene expression at the operon level, such as the motility (swarming) and biofilm formation genes (eg, EPS-producing genes).238 A study applied CRISPR/Cas9 gene-editing system to Cedecea neteri (Gram-negative bacteria) isolated from the Aedes mosquitoes.239 The study aimed to mutate the outer membrane protein A (ompA) gene, resulting in an impaired ability to form biofilms and reduced infection prevalence compared to wild-type strains. Using this technique in the site-specific integration of genes will simplify the improvement of para-transgenic control methods to manage arthropod-transmitted infections.239
In short, the CRISPR system used in gene editing alters the gene expression level qualitatively or quantitatively.240 Controlling the gene expression of specific virulence factors, such as forming biofilm and secreting QS molecules, creates a new opportunity to treat different biofilm-associated diseases. Applying this approach to treat and diagnose bacterial infections requires more investigations regarding its pros and cons and how and when it can be applied to achieve its full potential.
Nano-Mediated Newly Advancing Approaches
Biofilms contain cells with phenotypic heterogeneity within a self-produced 3D matrix of EPS. The power of nanoparticles (NPs) to infiltrate the entire matrix enables them to engage with the cells within the EPS matrix, susceptible and resistant strains. Also, NPs interfere with the physicochemical interactions in the matrix, which are essential for keeping the stable 3D structure of biofilms.241
Why are Nanoparticles Unique in Treating Bacterial Biofilm?
Primarily, the antibiofilm activity of nanoparticles results from their small size allowing their penetration into the biofilm microenvironment and effectively disrupting its integrity.242 A study used silver-silica dioxide nanoparticles (AgSio2 NPs) as an implant coating to inhibit S. aureus biofilm infections. Results showed size-dependent antibiofilm action; this effect was documented in the smaller NPs (6 nm) due to the amplified release of silver ions compared to the larger particles (11 nm).243 Research also investigated the antibiofilm activity of chitosan-capped gold and silver nanoparticles, biologically fabricated using the tiger milk mushroom (Lignosus rhinocerotis).244 The results showed more antibiofilm activity, 53.21% and 46.24% inhibition against P. aeruginosa and S. aureus biofilms, of smaller-sized metal nanoparticles (< 50 nm).244 Secondly, the larger surface area of nanoparticles enhances surface reactivity and increases the antimicrobial and antibiofilm action.245 Enhanced antibiofilm activity and a larger surface area have been verified in silica nanoparticles,246 metal and metal oxide nanoparticles, such as copper oxide nanoparticles,247 ferrite nanoparticles,248 silver nanoparticles,249 and titanium dioxide nanoparticles.250
Other factors related to the NPs also contribute to the antibiofilm action of NPs, such as hydrophobicity, shape, and surface charge.251 Carefully considering these factors when designing antibiofilm NPs can aid the synthesis of NPs targeting bacterial biofilms. To destroy the bacteria inside the biofilm, NPs may inflict an antimicrobial action directly or deliver therapeutic agents, such as antibiotics or antimicrobials (eg, essential oils, enzymes, or phytochemicals).17
Metal nanoparticles can uniquely control the bacterial signaling system, which has been tested in vitro and in vivo.252 Metal NPs block the synthesis of signaling molecules by interfering with the QS regulatory genes. They also can impede the access of signaling molecules into the neighboring cells by preventing them from binding to other cell’s receptors or degrading these secreted molecules5 (Figure 11).252 Another antibiofilm mechanism of silver NPs is integrating within the bacterial DNA and causing damage.253 In addition, gold NPs trigger ROS-mediated damage,254 while titanium oxide NPs (TiO-NPs) induce EPS lipid-peroxidation.5 Antibiotic-loaded liposomes obstruct the bacterial electron transport system. Some NPs are synthesized to suppress QS, and chitosan NPs can invade the EPS matrix and destroy the biofilm due to their positively charged exterior.5
Recently, a new approach combined the CRISPR-Cas9 gene-editing technique and nanotechnology. Wan and others created a dissolvable patch to treat inflammatory skin disorders.255 They used nanoparticles to deliver the cas9 (gene editing agents) and glucocorticoids into cell nuclei to exploit their corresponding action. The in vivo results and mouse models showed reduced skin inflammation and enhanced glucocorticoid therapy.255 This study motivates research targeting pathogenic bacteria using gene-editing techniques to deliver NPs loaded with appropriate anti-virulence or repurposed antibiotics.
Nanoparticles: Classifications, Synthesis, and Applications
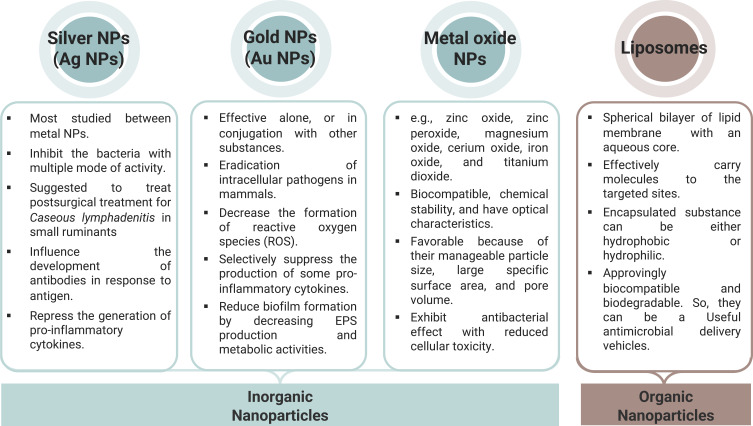
Antimicrobial NPs and nanomaterial coatings with good mechanical and tribological properties (ie, adhesion, friction, and wear resistance) are novel approaches to treating biofilm infections.5,243 NPs are divided in four groups according to their chemical composition: carbon, inorganic, organic, or hybrid NPs.256 This review will focus on the inorganic NPs, metal and metal oxide NPs, and organic NPs (liposomes).
Inorganic nanoparticles (metal and metal oxide NPs) have an inherent antimicrobial activity with a multimode action. They also have an increased antimicrobial effect compared to their bulk form, which allows them to be used either as inhibitors or as carriers for antimicrobials with synergistic effects.257,258
Jang and others synthesized bimetallic (silver and copper) NPs integrated on graphene oxide nanosheets to study their antibiofilm activity.259 They tested the safety and efficacy of this nanocomplex in vitro and in vivo. Biofilm formation was reduced in P. aeruginosa and in vivo assays proved their safety on human dermal fibroblasts. Lastly, an animal model with induced wound infection recovered within seven days when the nanocomposites were applied topically.259
Recently, scientists used inorganic nanoparticles using various capping agents. Khalid and others used functionalized silver and iron oxide NPs (35 and 48 nm) to treat biofilm development and formation of S. aureus and P. aeruginosa.260 Rhamnolipids were used as ligands to increase NPs-bacterial interactions and enhance antimicrobial activity. The results showed high efficiency in inhibiting bacterial adhesion and biofilm formation (>80%). Khalid et al hypothesized that this activity ensued from the synergetic action of ROS production by the metal NPs and the reduced bacterial-adhesion affinity caused by rhamnolipids.260 El-Batal and others chemically synthesized magnesium ferrite nanocomposites loaded with amoxicillin and stabilized them with citric acid to test their antimicrobial and antibiofilm ability.261 The nanocomposites inhibited > 90% of S. aureus and E. coli biofilm. A membrane leakage assay and scanning-electron microscope displayed a perforated bacterial membrane post-treatment. The researchers suggest using this nanosystem as a surface disinfectant as they excelled in the UV-light excitation tests.261 However, Padmavathi et al found that capping copper oxide nanoparticles decreased their antibiofilm activity; the non-coated particles completely eradicated the biofilm of copper-tolerant Staphylococcus lentus, unlike the cetyl trimethyl ammonium bromide-capped NPs.262 They proposed that the capping of the particles restricted the release of the copper ions, thus reducing its efficiency.262
Researchers assessed using biosynthesized or green metal nanoparticles in several applications as biosensors, antimicrobial, antioxidant, and anticancer therapy.263 When metal particles reduce to the nanoscale, it results in intrinsic and unique physiochemical (eg, size, shape), biological, and optical properties.263 The diverse targeting system of NPs lowers the likelihood of bacteria gaining resistance in the future.264,265 Though the green synthesis of NPs is an eco-friendly, non-toxic, and cost-effective alternative to conventional methods, its application has limitations, including stability, toxicity, purity, and the absence of universal synthesis/waste regulations.256,266
In conclusion, inorganic NPs represent a novel approach to treating infections caused by biofilm-forming bacteria. Exploring the aspects of functionalizing the surfaces of these particles or utilizing green practices will boost NP stability, purity, and activity. Risk management of NPs toxicity and setting environmental regulations to handle and dispose of nanomaterials correctly are required to avoid the negative consequences of a thriving technology.
Organic NPs are nanosystems harboring carbon macromolecules (eg, liposomes) used in drug design and delivery, image-channeled treatments, and imaging.267,268 Liposomes are vesicles with a membrane comprising a bilayer of lipids with an aqueous core, which present an innovative technology to carry effective molecules to the targeted sites. The “active” loaded aqueous substance can be hydrophobic or hydrophilic.269,270 Though these particles excel due to their low toxicity and high drug entrapment ability, their use is challenged by their low target specificity, instability, and short half-life in serum.267,271 To overcome these disadvantages, scientists functionalized these particles using ligands (eg, aptamers (Aptamosomes)272,273 or polyethylene glycols (PEGylation)271,274). In detail, aptamosomes are liposomes conjugated with aptamers—a short portion of RNA or DNA designed to improve specificity and affinity.273 These conjugates have been studied recently for cancer therapy or imaging.275,276 More research is vital to explore the ability to formulate unique antimicrobial aptamosomes.
Utilizing polyethylene glycols (PEGylation) in the liposomal formulation was done to improve the efficacy and delivery and decrease the toxicity of the beta-lactam antibiotic nafcillin.271 PEGylated nafcillin liposomes (PEG-Lipo-NF) were compared to non-functionalized nafcillin liposomes (Lipo-NF), and the free nafcillin against Methicillin-susceptible S. aureus (MSSA). The PEG-Lipo-NF had higher antimicrobial activity than the Lipo-NF or the free antibiotic. The PEG-Lipo-NF had the lowest MIC (0.25 ± 0.01 μg/mL) and improved biofilm inhibition activity (MBIC50 0.5 ± 0.02 μg/mL). Also, in vivo and animal studies showed that PEGylated nafcillin liposomes reduced NF-toxicity and improved cellular viability and liposomal availability.271 In another study, Das and scientists evaluated the dual activity of a PEGylated formula loaded with doxorubicin, epigallocatechin gallate (EGCG), and quercetin against E. coli and a human lymphoblastoid cell line (K-562) as an approach to control chemotherapy-associated secondary microbial infections.274 The PEGylated formula had a loading efficacy of 70.8% ± 5.25 and released the drugs gradually for ten days. The combination of the antioxidant (EGCG) and doxorubicin increased liposomal stability and led to cancer cell death by apoptosis, necrosis, and ROS formation. The antibacterial effect might have resulted from the encapsulated EGCG and quercetin, as the latter have a cell wall damaging ability.274
Liposomes have been studied as vehicles for QS inhibitors, the communication signals between bacteria in biofilms. Liposomes loaded with QS inhibitors showed a dose-dependent activity, higher than unloaded liposomes. This activity persisted and lasted up to 48 hours, unlike the free biofilm inhibitors that lost their effect.277 Studies showed that polyethylene glycol-modified and cationic liposomes could be highly effective antibiofilm agents against P. aeruginosa. The findings demonstrate that the efficacy is linked to the modification level and the liposome’s surface charge.278
Nanotechnology is a promising method. Recent studies suggest combining it with other anti-virulence and anti-QS drugs to achieve ultimate activity while decreasing the probability of future resistance.279–281 Organic and inorganic NPs exhibit superior physical, chemical, mechanical, thermal, and biological properties applied in medicine for their antimicrobial, anti-inflammatory, and antioxidant mechanisms. These NPs are extensively used due to their high stability, multi-functionality, biocompatibility, adhesive and therapeutic properties, and broad applications, as shown in Figure 12.2,5,241,257,264,282,283
Figure 12.
Types of nanoparticles and their application.
Abbreviations: NPs, nanoparticles; Ag NPs, silver nanoparticles; Au NPs, gold nanoparticles; EPS, extracellular polymeric substance.
Conclusion
This review discussed biofilm formation in pathogenic bacteria, its association with virulence factors (such as QS systems), antibiotic resistance, and future therapeutics. Pseudomonas aeruginosa, Acinetobacter baumannii, and Staphylococcus aureus are prevalent biofilm-forming bacteria causing a variety of community and hospital-acquired infections. Targeting bacterial virulence factors, eg, biofilm formation, is a promising approach that aims to destroy the bacterial cell without inducing selective pressure, which is the source of the global resistance issue. The only limitation of such an approach is its dependency on host immunity to fully eradicate the infection. This limitation is especially an issue for immunocompromised individuals. Another possible solution suggests co-administering anti-virulence drugs (eg, antibiofilm) with low doses of antibiotics to attenuate the bacteria by disrupting their biofilm and amplifying the antibiotic’s activity. Therefore, reducing the excessive use of antibiotics, resetting bacterial resistance, and restoring its susceptibility. Although this approach is promising, the results take time. We explored other approaches in this review: inventing novel drugs by repurposing old drugs. Natural compounds, phages, and the CRISPR/Cas technique have proven their role in regulating QS systems and interfering with biofilm formation by regulating gene expression. aPDT is a novel method that damages the integrity of bacterial cell walls and the biofilm matrix. Recent advances in nanomaterials use NPs with multimode activity or combined with other therapeutics, such as phages, phytochemicals, and antibiotics, to manage biofilm-caused infections. Researching innovative methods should achieve maximal efficacy and specificity with minimal toxicity, ensure long-term therapeutic effects, and lower the production cost of valuable drug formulations to control infectious diseases. In addition, advancing these studies to in vivo experimentation and to clinical trials is recommended to prove the capability of these approaches in treating biofilm-associated infections.
Acknowledgment
This work was supported by a grant from King Abdullah International Research Center, National Guard Health Affairs, Riyadh, Saudi Arabia (Grant No. RC20/109/R). The funding agency had no role in the decision to publish or prepare the manuscript. Figures were created with BioRender.com.
Abbreviation
agr, accessory gene regulator genes; AHL, N-acyl homoserine lactones; AI, autoinducers; aPDT, antimicrobial photodynamic therapy; Cas, complex sensing systems; CRISPR, Clustered Regulatory Interspaced Short Palindromic Repeats; ECM, extracellular matrix; eDNA, extracellular DNA; EPS, extracellular polymeric substances; HAI, hospital-acquired infection; MBIC, minimum biofilm inhibitory concentration; MDR, multi-drug resistance; NP/NPs, nanoparticle; PS, photosensitizer; QQ systems, quorum quenching systems; QS systems, quorum sensing systems.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lood R, Frick IM. Protein-Based Strategies to Identify and Isolate Bacterial Virulence Factors. In: Bacterial Pathogenesis. Springer; 2017:3–15. [DOI] [PubMed] [Google Scholar]
- 2.Bassegoda A, Ivanova K, Ramon E, Tzanov T. Strategies to prevent the occurrence of resistance against antibiotics by using advanced materials. Appl Microbiol Biotechnol. 2018;102(5):2075–2089. [DOI] [PubMed] [Google Scholar]
- 3.Diard M, Hardt WD. Evolution of bacterial virulence. FEMS Microbiol Rev. 2017;41(5):679–697. [DOI] [PubMed] [Google Scholar]
- 4.Sharma D, Misba L, Khan AU. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control. 2019;8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee D, Shivapriya PM, Gautam PK, Misra K, Sahoo AK, Samanta SK. A review on basic biology of bacterial biofilm infections and their treatments by nanotechnology-based approaches. Proce National Acad Sci India Section B. 2019;90(2):243–259. [Google Scholar]
- 6.Nordenfelt P, Collin M. Bacterial Pathogenesis: Methods and Protocols. Springer; 2017. [Google Scholar]
- 7.Limoli DH, Jones CJ, Wozniak DJ. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol Spectr. 2015;3(3):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hengge R. Targeting bacterial biofilms by the green tea polyphenol EGCG. Molecules. 2019;24(13):2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karygianni L, Ren Z, Koo H, Thurnheer T. Biofilm Matrixome: extracellular components in structured microbial communities. Trends Microbiol. 2020;28(8):668–681. [DOI] [PubMed] [Google Scholar]
- 10.Solano C, Echeverz M, Lasa I. Biofilm dispersion and quorum sensing. Curr Opin Microbiol. 2014;18:96–104. doi: [DOI] [PubMed] [Google Scholar]
- 11.Sahu PK, Iyer PS, Barage SH, Sonawane KD, Chopade BA. Characterization of the algC Gene Expression Pattern in the Multidrug Resistant Acinetobacter baumannii AIIMS 7 and Correlation with Biofilm Development on Abiotic Surface. Sci World J. 2014;2014:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beloin C, McDougald D. Speciality Grand Challenge for “Biofilms.”. Front Cell Infect Microbiol. 2021;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of antivirulence therapies. Nat Rev Microbiol. 2008;6(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heras B, Scanlon MJ, Martin JL. Targeting virulence not viability in the search for future antibacterials. Br J Clin Pharmacol. 2015;79(2):208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uruén C, Chopo-Escuin G, Tommassen J, Mainar-Jaime RC, Arenas J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics. 2021;10(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Toole G, Kaplan HB, Kolter R. Biofilm Formation as Microbial Development. Annu Rev Microbiol. 2000;54(1):49–79. [DOI] [PubMed] [Google Scholar]
- 17.Lin YK, Yang SC, Hsu CY, Sung JT, Fang JY. The Antibiofilm Nanosystems for Improved Infection Inhibition of Microbes in Skin. Molecules. 2021;26:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh A, Amod A, Pandey P, et al. Bacterial biofilm infections, their resistance to antibiotics therapy and current treatment strategies. Biomed Mater. 2022;17(2):022003. [DOI] [PubMed] [Google Scholar]
- 19.Silva NBS, Marques LA, Röder DDB. Diagnosis of biofilm infections: current methods used, challenges and perspectives for the future. J Appl Microbiol. 2021;131(5):2148–2160. [DOI] [PubMed] [Google Scholar]
- 20.Blair JMA, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJV. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13(1):42–51. [DOI] [PubMed] [Google Scholar]
- 21.Ghai I, Ghai S. Understanding antibiotic resistance via outer membrane permeability. Infect Drug Resist. 2018;11:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang SS, Apisarnthanarak A, Hsu LY. Mechanisms of β-lactam antimicrobial resistance and epidemiology of major community- and healthcare-associated multidrug-resistant bacteria. Adv Drug Deliv Rev. 2014;78:3–13. [DOI] [PubMed] [Google Scholar]
- 23.Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J. Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol. 2010;8(4):251–259. [DOI] [PubMed] [Google Scholar]
- 24.Munita JM, Arias CA. Mechanisms of Antibiotic Resistance. Microbiol Spectr. 2016;23(5):464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varela MF, Stephen J, Lekshmi M, et al. Bacterial resistance to antimicrobial agents. Antibiotics. 2021;10(5):593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ronin D, Boyer J, Alban N, Natoli RM, Johnson A, Kjellerup BV. Current and novel diagnostics for orthopedic implant biofilm infections: a review. APMIS. 2022;130(2):59–81. [DOI] [PubMed] [Google Scholar]
- 27.Wannigama DL, Hurst C, Pearson L, et al. Simple fluorometric-based assay of antibiotic effectiveness for Acinetobacter baumannii biofilms. Sci Rep. 2019;9(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wannigama DL, Hurst C, Hongsing P, et al. A rapid and simple method for routine determination of antibiotic sensitivity to biofilm populations of Pseudomonas aeruginosa. Ann Clin Microbiol Antimicrob. 2020;19(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sedarat Z, Taylor-Robinson AW. Biofilm formation by pathogenic bacteria: applying a staphylococcus aureus model to appraise potential targets for therapeutic intervention. Pathogens. 2022;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gedefie A, Demsis W, Ashagrie M, et al. Acinetobacter baumannii biofilm formation and its role in disease pathogenesis: a review. Infect Drug Resist. 2021;14:3711–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldman L. Pseudomonas and Related Gram-Negative Bacillary Infections. In: Falagas ME, Rafailidis PI, editors. Goldman-Cecil Medicine. 26th ed. Elsevier, Inc; 2020:1931–1936. [Google Scholar]
- 32.Moradali MF, Ghods S, Rehm BHA. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Y, Liu C, Wang Q, et al. Emergence and expansion of a carbapenem-resistant pseudomonas aeruginosa clone are associated with plasmid-borne bla kpc-2 and virulence-related genes. mSystems. 2021;6(3):e00154–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laverty G, Gorman SP, Gilmore BF. Biomolecular mechanisms of Pseudomonas aeruginosa and Escherichia coli biofilm formation. Pathogens. 2014;3(3):596–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thöming JG, Tomasch J, Preusse M, et al. Parallel evolutionary paths to produce more than one Pseudomonas aeruginosa biofilm phenotype. Npj Biofilms and Microbiomes. 2020;6(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huszczynski SM, Lam JS, Khursigara CM. The role of Pseudomonas aeruginosa lipopolysaccharide in bacterial pathogenesis and physiology. Pathogens. 2020;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Xiang D, Tian F, Ni M. Lipopolysaccharide from biofilm-forming Pseudomonas aeruginosa PAO1 induces macrophage hyperinflammatory responses. J Med Microbiol. 2021;70:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciofu O, Tolker-Nielsen T. Tolerance and resistance of pseudomonas aeruginosa biofilms to antimicrobial agents-how P. aeruginosa Can escape antibiotics. Front Microbiol. 2019;10:913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang Z, Rybtke M, Kragh KN, et al. Transcription of the Alginate Operon in Pseudomonas aeruginosa Is Regulated by c-di-GMP. Microbiol Spectr. 2022;10(4):e00675–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skariyachan S, Sridhar VS, Packirisamy S, Kumargowda ST, Challapilli SB. Recent perspectives on the molecular basis of biofilm formation by Pseudomonas aeruginosa and approaches for treatment and biofilm dispersal. Folia Microbiol (Praha). 2018;63(4):413–432. [DOI] [PubMed] [Google Scholar]
- 41.Yan S, Wu G. Can biofilm be reversed through quorum sensing in pseudomonas aeruginosa? Front Microbiol. 2019;10:1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muhammad MH, Idris AL, Fan X, et al. Beyond risk: bacterial biofilms and their regulating approaches. Front Microbiol. 2020;11:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdelraheem WM, Abdelkader AE, Mohamed ES, Mohammed MS. Detection of biofilm formation and assessment of biofilm genes expression in different Pseudomonas aeruginosa clinical isolates. Meta Gene. 2020;23:100646. [Google Scholar]
- 44.Azam MW, Khan AU. Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov Today. 2019;24(1):350–359. [DOI] [PubMed] [Google Scholar]
- 45.O’Loughlin CT, Miller LC, Siryaporn A, Drescher K, Semmelhack MF, Bassler BL. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc Natl Acad Sci U S A. 2013;110(44):17981–17986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thi MTT, Wibowo D, Rehm BHA. Pseudomonas aeruginosa Biofilms. Int J Mol Sci. 2020;21(22):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chadha J, Harjai K, Chhibber S. Revisiting the virulence hallmarks of Pseudomonas aeruginosa: a chronicle through the perspective of quorum sensing. Environ Microbiol. 2022;24(6):2630–2656. [DOI] [PubMed] [Google Scholar]
- 48.Pye CC, Yu AA, Weese JS. Evaluation of biofilm production by Pseudomonas aeruginosa from canine ears and the impact of biofilm on antimicrobial susceptibility in vitro. Vet Dermatol. 2013;24(4):446–e99. [DOI] [PubMed] [Google Scholar]
- 49.Miryala SK, Anbarasu A, Ramaiah S. Systems biology studies in Pseudomonas aeruginosa PA01 to understand their role in biofilm formation and multidrug efflux pumps. Microb Pathog. 2019;136:103668. [DOI] [PubMed] [Google Scholar]
- 50.Milivojevic D, Šumonja N, Medić S, et al. Biofilm-forming ability and infection potential of Pseudomonas aeruginosa strains isolated from animals and humans. Pathog Dis. 2018;76:4. [DOI] [PubMed] [Google Scholar]
- 51.Orsi GB, Scorzolini L, Franchi C, Mondillo V, Rosa G, Venditti M. Hospital-acquired infection surveillance in a neurosurgical intensive care unit. J Hosp Infect. 2006;64(1):23–29. [DOI] [PubMed] [Google Scholar]
- 52.Azzopardi EA, Azzopardi E, Camilleri L, et al. Gram Negative Wound Infection in Hospitalised Adult Burn Patients-Systematic Review and Metanalysis-. McDowell A. PLoS One. 2014;9(4):e95042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watkins K, Unnikrishnan M. New strategies and targets for antibacterial discovery. In: Drug Discovery Targeting Drug-Resistant Bacteria. Elsevier; 2020:249–272. [Google Scholar]
- 54.Rezzoagli C, Archetti M, Mignot I, Baumgartner M, Kümmerli R. Combining antibiotics with antivirulence compounds can have synergistic effects and reverse selection for antibiotic resistance in Pseudomonas aeruginosa. PLoS Biol. 2020;18(8):e3000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morris FC, Dexter C, Kostoulias X, Uddin MI, Peleg AY. The mechanisms of disease caused by Acinetobacter baumannii. Front Microbiol. 2019;10:1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geisinger E, Isberg RR. Interplay between antibiotic resistance and virulence during disease promoted by multidrug-resistant bacteria. J Infect Dis. 2017;215(suppl_1):S9–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galac MR, Snesrud E, Lebreton F, et al. A diverse panel of clinical Acinetobacter baumannii for research and development. Antimicrob Agents Chemother. 2020;64(10):e00840–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salmani A, Shakerimoghaddam A, Pirouzi A, Delkhosh Y, Eshraghi M. Correlation between biofilm formation and antibiotic susceptibility pattern in Acinetobacter baumannii MDR isolates retrieved from burn patients. Gene Rep. 2020;21:100816. [Google Scholar]
- 59.Lee CR, Lee JH, Park M, et al. Biology of Acinetobacter baumannii: pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front Cell Infect Microbiol. 2017;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. Clinical and Pathophysiological Overview of Acinetobacter Infections: a Century of Challenges. Clin Microbiol Rev. 2017;30(1):409–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi CH, Lee EY, Lee YC, et al. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 2005;7(8):1127–1138. [DOI] [PubMed] [Google Scholar]
- 62.Kim SW, Choi CH, Moon DC, et al. Serum resistance of Acinetobacter baumannii through the binding of factor H to outer membrane proteins. FEMS Microbiol Lett. 2009;301(2):224–231. [DOI] [PubMed] [Google Scholar]
- 63.Lee JS, Choi CH, Kim JW, Lee JC. Acinetobacter baumannii outer membrane protein a induces dendritic cell death through mitochondrial targeting. J Microbiol. 2010;48(3):387–392. [DOI] [PubMed] [Google Scholar]
- 64.Fernández-Cuenca F, Smani Y, Gómez-Sánchez MC, et al. Attenuated virulence of a slow-growing pandrug-resistant Acinetobacter baumannii is associated with decreased expression of genes encoding the porins CarO and OprD-like. Int J Antimicrob Agents. 2011;38(6):548–549. [DOI] [PubMed] [Google Scholar]
- 65.Smani Y, McConnell MJ, Pachón J. Role of Fibronectin in the Adhesion of Acinetobacter baumannii to Host Cells. PLoS One. 2012;7(4):e33073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rumbo C, Tomás M, Moreira EF, et al. The Acinetobacter baumannii Omp33-36 porin is a virulence factor that induces apoptosis and modulates autophagy in human cells. Infect Immun. 2014;82(11):4666–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang N, Ozer EA, Mandel MJ, Hauser AR. Genome-wide identification of Acinetobacter baumannii genes necessary for persistence in the lung. mBio. 2014;5(3):1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang W, Yao Y, Wang S, et al. Immunization with a 22-kDa outer membrane protein elicits protective immunity to multidrug-resistant Acinetobacter baumannii. Sci Rep. 2016;6(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smani Y, Dominguez-Herrera J, Pachon J. Association of the Outer Membrane Protein Omp33 With Fitness and Virulence of Acinetobacter baumannii. J Infect Dis. 2013;208(10):1561–1570. [DOI] [PubMed] [Google Scholar]
- 70.Schweppe DK, Harding C, Chavez JD, et al. Host-Microbe Protein Interactions during Bacterial Infection. Chem Biol. 2015;22(11):1521–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaddy JA, Tomaras AP, Actis LA. The Acinetobacter baumannii 19606 OmpA Protein Plays a Role in Biofilm Formation on Abiotic Surfaces and in the Interaction of This Pathogen with Eukaryotic Cells. Infect Immun. 2009;77(8):3150–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lees-Miller RG, Iwashkiw JA, Scott NE, et al. A common pathway for O -linked protein-glycosylation and synthesis of capsule in Acinetobacter baumannii. Mol Microbiol. 2013;89(5):816–830. [DOI] [PubMed] [Google Scholar]
- 73.Russo TA, Luke NR, Beanan JM, et al. The K1 Capsular Polysaccharide of Acinetobacter baumannii Strain 307-0294 Is a Major Virulence Factor. Infect Immun. 2010;78(9):3993–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iwashkiw JA, Seper A, Weber BS, et al. Identification of a General O-linked Protein Glycosylation System in Acinetobacter baumannii and Its Role in Virulence and Biofilm Formation. PLoS Pathog. 2012;8(6):e1002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geisinger E, Isberg RR. Antibiotic Modulation of Capsular Exopolysaccharide and Virulence in Acinetobacter baumannii. PLoS Pathog. 2015;11(2):e1004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luke NR, Sauberan SL, Russo TA, et al. Identification and characterization of a glycosyltransferase involved in Acinetobacter baumannii lipopolysaccharide core biosynthesis. Infect Immun. 2010;78(5):2017–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin L, Tan B, Pantapalangkoor P, et al. Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. Pirofski L anne, ed. mBio. 2012;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McQueary CN, Kirkup BC, Si Y, et al. Extracellular stress and lipopolysaccharide modulate Acinetobacter baumannii surface-associated motility. J Microbiol. 2012;50(3):434–443. [DOI] [PubMed] [Google Scholar]
- 79.McConnell MJ, Actis L, Pachón J. Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev. 2013;37(2):130–155. [DOI] [PubMed] [Google Scholar]
- 80.Beceiro A, Moreno A, Fernández N, et al. Biological Cost of Different Mechanisms of Colistin Resistance and Their Impact on Virulence in Acinetobacter baumannii. Antimicrob Agents Chemother. 2014;58(1):518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Erridge C, Moncayo-Nieto OL, Morgan R, Young M, Poxton IR. Acinetobacter baumannii lipopolysaccharides are potent stimulators of human monocyte activation via Toll-like receptor 4 signalling. J Med Microbiol. 2007;56(2):165–171. [DOI] [PubMed] [Google Scholar]
- 82.Camarena L, Bruno V, Euskirchen G, Poggio S, Snyder M. Molecular Mechanisms of Ethanol-Induced Pathogenesis Revealed by RNA-Sequencing. Roy CR. PLoS Pathog. 2010;6(4):e1000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jacobs AC, Hood I, Boyd KL, et al. Inactivation of phospholipase D diminishes Acinetobacter baumannii pathogenesis. Infect Immun. 2010;78(5):1952–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stahl J, Bergmann H, Göttig S, Ebersberger I, Averhoff B. Acinetobacter baumannii virulence is mediated by the concerted action of three phospholipases D. PLoS One. 2015;10(9):e0138360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fiester SE, Arivett BA, Schmidt RE, et al. Iron-Regulated phospholipase C Activity contributes to the cytolytic activity and virulence of Acinetobacter baumannii. PLoS One. 2016;11(11):e0167068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson TL, Waack U, Smith S, Mobley H, Sandkvist M. Acinetobacter baumannii is dependent on the type II secretion system and its substrate LipA for lipid utilization and in vivo fitness. J Bacteriol. 2016;198(4):711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kwon SO, Gho YS, Lee JC, Kim S. Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. FEMS Microbiol Lett. 2009;297(2):150–156. [DOI] [PubMed] [Google Scholar]
- 88.Jin JS, Kwon SO, Moon DC, et al. Acinetobacter baumannii secretes cytotoxic outer membrane protein a via outer membrane vesicles. PLoS One. 2011;6(2):e17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rumbo C, Fernández-Moreira E, Merino M, et al. Horizontal Transfer of the OXA-24 Carbapenemase Gene via Outer Membrane Vesicles: a New Mechanism of Dissemination of Carbapenem Resistance Genes in Acinetobacter baumannii. Antimicrob Agents Chemother. 2011;55(7):3084–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moon DC, Choi CH, Lee JH, et al. Acinetobacter baumannii outer membrane protein a modulates the biogenesis of outer membrane vesicles. J Microbiol. 2012;50(1):155–160. [DOI] [PubMed] [Google Scholar]
- 91.Jun SH, Lee JH, Kim BR, et al. Acinetobacter baumannii Outer Membrane Vesicles Elicit a Potent Innate Immune Response via Membrane Proteins. PLoS One. 2013;8(8):e71751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li ZT, Zhang RL, Bi XG, et al. Outer membrane vesicles isolated from two clinical Acinetobacter baumannii strains exhibit different toxicity and proteome characteristics. Microb Pathog. 2015;81:46–52. [DOI] [PubMed] [Google Scholar]
- 93.Nho JS, Jun SH, Oh MH, et al. Acinetobacter nosocomialis secretes outer membrane vesicles that induce epithelial cell death and host inflammatory responses. Microb Pathog. 2015;81:39–45. [DOI] [PubMed] [Google Scholar]
- 94.Nairn BL, Lonergan ZR, Wang J, et al. The Response of Acinetobacter baumannii to Zinc Starvation. Cell Host Microbe. 2016;19(6):826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hood MI, Mortensen BL, Moore JL, et al. Identification of an Acinetobacter baumannii Zinc Acquisition System that Facilitates Resistance to Calprotectin-mediated Zinc Sequestration. PLoS Pathog. 2012;8(12):e1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ali HM, Salem MZM, El-Shikh MS, Megeed AA, Alogaibi YA, Talea IA. Investigation of the Virulence Factors and Molecular Characterization of the Clonal Relations of Multidrug-Resistant Acinetobacter baumannii Isolates. J AOAC Int. 2017;100(1):152–158. [DOI] [PubMed] [Google Scholar]
- 97.Zimbler DL, Park TM, Arivett BA, et al. Stress response and virulence functions of the Acinetobacter baumannii NfuA Fe-S scaffold protein. J Bacteriol. 2012;194(11):2884–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Penwell WF, Arivett BA, Actis LA. The Acinetobacter baumannii entA Gene Located Outside the Acinetobactin Cluster Is Critical for Siderophore Production, Iron Acquisition and Virulence. PLoS One. 2012;7(5):e36493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gaddy JA, Actis LA, Arivett BA, Mcconnell MJ, Rafael LR, Pachón J. Role of Acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect Immun. 2012;80(3):1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Juttukonda LJ, Chazin WJ, Skaar EP, Sperandio V. Acinetobacter baumannii coordinates urea metabolism with metal import to resist host-mediated metal limitation. mBio. 2016;7(5):5. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Elhosseiny NM, El-Tayeb OM, Yassin AS, Lory S, Attia AS. The secretome of Acinetobacter baumannii ATCC 17978 type II secretion system reveals a novel plasmid encoded phospholipase that could be implicated in lung colonization. Int J Med Microbiol. 2016;306(8):633–641. [DOI] [PubMed] [Google Scholar]
- 102.Harding CM, Kinsella RL, Palmer LD, Skaar EP, Feldman MF. Medically Relevant Acinetobacter Species Require a Type II Secretion System and Specific Membrane-Associated Chaperones for the Export of Multiple Substrates and Full Virulence. PLoS Pathog. 2016;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carruthers MD, Nicholson PA, Tracy EN, Munson RS. Acinetobacter baumannii Utilizes a Type VI Secretion System for Bacterial Competition. PLoS One. 2013;8(3):e59388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jones CL, Clancy M, Honnold C, et al. Fatal Outbreak of an Emerging Clone of Extensively Drug-Resistant Acinetobacter baumannii with Enhanced Virulence. Clin Infect Dis. 2015;61(2):145–154. [DOI] [PubMed] [Google Scholar]
- 105.Repizo GD, Gagné S, Foucault-Grunenwald ML, et al. Differential Role of the T6SS in Acinetobacter baumannii Virulence. PLoS One. 2015;10(9):e0138265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ruiz FM, Santillana E, Spínola-Amilibia M, Torreira E, Culebras E, Romero A. Crystal Structure of Hcp from Acinetobacter baumannii: a Component of the Type VI Secretion System. PLoS One. 2015;10(6):e0129691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bentancor L, Routray A, Bozkurt-Guzel C, Camacho-Peiro A, Pier GB, Maira-Litrán T. Evaluation of the Trimeric Autotransporter Ata as a Vaccine Candidate against Acinetobacter baumannii Infections. Infect Immun. 2012;80(10):3381–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Russo TA, MacDonald U, Beanan JM, et al. Penicillin‐Binding Protein 7/8 Contributes to the Survival of Acinetobacter baumannii In Vitro and In Vivo. J Infect Dis. 2009;199(4):513–521. [DOI] [PubMed] [Google Scholar]
- 109.Sechi LA, Karadenizli A, Deriu A, et al. PER-1 type beta-lactamase production in Acinetobacter baumannii is related to cell adhesion. Medical Science Monitor. 2004;10(6):180–184. [PubMed] [Google Scholar]
- 110.Koenigs A, Stahl J, Averhoff B, et al. CipA of Acinetobacter baumannii Is a Novel Plasminogen Binding and Complement Inhibitory Protein. J Infect Dis. 2016;213(9):1388–1399. [DOI] [PubMed] [Google Scholar]
- 111.Koenigs A, Zipfel PF, Kraiczy P. Translation elongation factor Tuf of Acinetobacter baumannii is a plasminogen-binding protein. PLoS One. 2015;10(7):e0134418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aranda J, Bardina C, Beceiro A, et al. Acinetobacter baumannii RecA protein in repair of DNA damage, antimicrobial resistance, general stress response, and virulence. J Bacteriol. 2011;193(15):3740–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gebhardt MJ, Gallagher LA, Jacobson RK, et al. Joint Transcriptional Control of Virulence and Resistance to Antibiotic and Environmental Stress in Acinetobacter baumannii. mBio. 2015;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu D, Liu ZS, Hu P, et al. Characterization of surface antigen protein 1 (SurA1) from Acinetobacter baumannii and its role in virulence and fitness. Vet Microbiol. 2016;186:126–138. [DOI] [PubMed] [Google Scholar]
- 115.Elhosseiny NM, Amin MA, Yassin AS, Attia AS. Acinetobacter baumannii universal stress protein A plays a pivotal role in stress response and is essential for pneumonia and sepsis pathogenesis. Int J Med Microbiol. 2015;305(1):114–123. [DOI] [PubMed] [Google Scholar]
- 116.Cerqueira GM, Kostoulias X, Khoo C, et al. A Global Virulence Regulator in Acinetobacter baumannii and Its Control of the Phenylacetic Acid Catabolic Pathway. J Infect Dis. 2014;210(1):46–55. [DOI] [PubMed] [Google Scholar]
- 117.Bhuiyan MS, Ellett F, Murray GL, et al. Acinetobacter baumannii phenylacetic acid metabolism influences infection outcome through a direct effect on neutrophil chemotaxis. Proc Natl Acad Sci U S A. 2016;113(34):9599–9604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tomaras AP, Dorsey CW, Edelmann RE, Actis LA. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology. 2003;149(12):3473–3484. [DOI] [PubMed] [Google Scholar]
- 119.Tomaras AP, Flagler MJ, Dorsey CW, Gaddy JA, Actis LA. Characterization of a two-component regulatory system from Acinetobacter baumannii that controls biofilm formation and cellular morphology. Microbiology. 2008;154(11):3398–3409. [DOI] [PubMed] [Google Scholar]
- 120.Tipton KA, Rather PN. An ompR-envZ Two-Component System Ortholog Regulates Phase Variation, Osmotic Tolerance, Motility, and Virulence in Acinetobacter baumannii Strain AB5075. J Bacteriol. 2017;199:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Subashchandrabose S, Smith S, DeOrnellas V, et al. Acinetobacter baumannii Genes Required for Bacterial Survival during Bloodstream Infection. mSphere. 2016;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Srinivasan VB, Venkataramaiah M, Mondal A, Rajamohan G. Functional Characterization of AbeD, an RND-Type Membrane Transporter in Antimicrobial Resistance in Acinetobacter baumannii. PLoS One. 2015;10(10):e0141314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Álvarez-fraga L, Pérez A, Rumbo-Feal S, et al. Analysis of the role of the LH92_11085 gene of a biofilm hyper-producing Acinetobacter baumannii strain on biofilm formation and attachment to eukaryotic cells. Virulence. 2016;7(4):443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Altınok Ö, Boral B, Ergin A, Eser ÖK. Existence of Biofilm and Biofilm-Associated Virulence Genes in Multi-Drug Resistant Invasive Acinetobacter baumannii Isolates. Mikrobiyol Bul. 2020;54(1):40–49. [DOI] [PubMed] [Google Scholar]
- 125.Colquhoun JM, Rather PN. Insights Into Mechanisms of Biofilm Formation in Acinetobacter baumannii and Implications for Uropathogenesis. Front Cell Infect Microbiol. 2020;10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Srisakul S, Wannigama DL, Higgins PG, et al. Overcoming addition of phosphoethanolamine to lipid A mediated colistin resistance in Acinetobacter baumannii clinical isolates with colistin-sulbactam combination therapy. Sci Rep. 2022;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Toltzis P. Staphylococcus epidermidis and Other Coagulase-Negative Staphylococci. In: Principles and Practice of Pediatric Infectious Diseases. Elsevier; 2018:706–712.e4. [Google Scholar]
- 128.Taylor TA, Unakal CG. Staphylococcus Aureus. StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 129.Silva V, Capelo JL, Igrejas G, Poeta P. Molecular Epidemiology of Staphylococcus aureus Lineages in Wild Animals in Europe: a Review. Antibiotics. 2020;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Oliveira D, Borges A, Simões M. Staphylococcus aureus toxins and their molecular activity in infectious diseases. Toxins. 2018;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Quave CL, Horswill AR. Flipping the switch: tools for detecting small molecule inhibitors of staphylococcal virulence. Front Microbiol. 2014;5:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Craft KM, Nguyen JM, Berg LJ, Townsend SD. Methicillin-resistant Staphylococcus aureus (MRSA): antibiotic-resistance and the biofilm phenotype. Medchemcomm. 2019;10(8):1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Luther MK, Parente DM, Caffrey AR, et al. Clinical and Genetic Risk Factors for Biofilm-Forming Staphylococcus aureus. Antimicrob Agents Chemother. 2018;62:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zapotoczna M, O’Neill E, O’Gara JP. Untangling the diverse and redundant mechanisms of Staphylococcus aureus biofilm formation. PLoS Pathog. 2016;12(7):e1005671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nguyen HTT, Nguyen TH, Otto M. The staphylococcal exopolysaccharide PIA – biosynthesis and role in biofilm formation, colonization, and infection. Comput Struct Biotechnol J. 2020;18:3324–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Foster TJ, Geoghegan JA, Ganesh VK, Höök M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12(1):49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Foster TJ. Surface Proteins of Staphylococcus aureus. Microbiol Spectr. 2019;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Otto M. Staphylococcal Biofilms. Microbiol Spectr. 2018;6(4):658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Enany S, Alexander LC. The Rise of Virulence and Antibiotic Resistance in Staphylococcus Aureus. InTech; 2017. [Google Scholar]
- 140.Reffuveille F, Josse J, Vallé Q, Mongaret C, Gangloff SC. Staphylococcus aureus Biofilms and their Impact on the Medical Field. In: The Rise of Virulence and Antibiotic Resistance in Staphylococcus Aureus. InTech; 2017:187–214. [Google Scholar]
- 141.Ruhal R, Kataria R. Biofilm patterns in gram-positive and gram-negative bacteria. Microbiol Res. 2021;251:126829. [DOI] [PubMed] [Google Scholar]
- 142.Jamal M, Ahmad W, Andleeb S, et al. Bacterial biofilm and associated infections. J Chin Med Assoc. 2018;81(1):7–11. [DOI] [PubMed] [Google Scholar]
- 143.Krzyściak P, Chmielarczyk A, Pobiega M, Romaniszyn D, Wójkowska‐Mach J. Acinetobacter baumannii isolated from hospital‐acquired infection: biofilm production and drug susceptibility. APMIS. 2017;125(11):1017–1026. [DOI] [PubMed] [Google Scholar]
- 144.Olsen I. Biofilm-specific antibiotic tolerance and resistance. Eur J Clin Microbiol Infect Dis. 2015;34(5):877–886. [DOI] [PubMed] [Google Scholar]
- 145.Sharma G, Sharma S, Sharma P, et al. Escherichia coli biofilm: development and therapeutic strategies. J Appl Microbiol. 2016;121(2):309–319. [DOI] [PubMed] [Google Scholar]
- 146.Gupta S, Kumar P, Rathi B, et al. Targeting of Uropathogenic Escherichia coli papG gene using CRISPR-dot nanocomplex reduced virulence of UPEC. Sci Rep. 2021;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Law SKK, Tan HS. The role of quorum sensing, biofilm formation, and iron acquisition as key virulence mechanisms in Acinetobacter baumannii and the corresponding anti-virulence strategies. Microbiol Res. 2022;260:127032. [DOI] [PubMed] [Google Scholar]
- 148.Kaushik V, Tiwari M, Joshi R, Tiwari V. Therapeutic strategies against potential antibiofilm targets of multidrug‐resistant Acinetobacter baumannii. J Cell Physiol. 2022;237(4):2045–2063. [DOI] [PubMed] [Google Scholar]
- 149.Trifan A, Luca SV, Greige-Gerges H, Miron A, Gille E, Aprotosoaie AC. Recent advances in tackling microbial multidrug resistance with essential oils: combinatorial and nano-based strategies. Crit Rev Microbiol. 2020;46(3):338–357. [DOI] [PubMed] [Google Scholar]
- 150.Gentile V, Frangipani E, Bonchi C, Minandri F, Runci F, Visca P. Iron and Acinetobacter baumannii Biofilm formation. Pathogens. 2014;3(3):704–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Upmanyu K, Singh R. Factors mediating Acinetobacter baumannii biofilm formation: opportunities for developing therapeutics. Curr Res Microb Sci. 2022;3:100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vestby LK, Grønseth T, Simm R, Nesse LL. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics. 2020;9(2):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dieltjens L, Appermans K, Lissens M, et al. Inhibiting bacterial cooperation is an evolutionarily robust anti-biofilm strategy. Nat Commun. 2020;11(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shao L, Dong Y, Chen X, Xu X, Wang H. Modeling the elimination of mature biofilms formed by Staphylococcus aureus and Salmonella spp. In: Using Combined Ultrasound and Disinfectants. Ultrason Sonochem; 2020:69. [DOI] [PubMed] [Google Scholar]
- 155.Nadar S, Khan T, Patching SG, Omri A. Development of Antibiofilm Therapeutics Strategies to Overcome Antimicrobial Drug Resistance. Microorganisms. 2022;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tursi SA, Puligedda RD, Szabo P, et al. Salmonella Typhimurium biofilm disruption by a human antibody that binds a pan-amyloid epitope on curli. Nat Commun. 2020;11(1):1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Steenackers H, Hermans K, Vanderleyden J, de Keersmaecker SCJ. Salmonella biofilms: an overview on occurrence, structure, regulation and eradication. Food Res Int. 2012;45(2):502–531. [Google Scholar]
- 158.Cadavid E, Echeverri F. The search for natural inhibitors of biofilm formation and the activity of the autoinductor C6-AHL in Klebsiella pneumoniae ATCC 13884. Biomolecules. 2019;9(2):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Santiago AJ, Burgos-Garay L. Bacteriophage treatment of carbapenemase-producing Klebsiella pneumoniae in a multispecies biofilm: a potential biocontrol strategy for healthcare facilities. AIMS Microbiol. 2020;6(1):43–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chhibber S, Gondil VS, Sharma S, et al. Approach for Combating Klebsiella pneumoniae Biofilm Using Histidine Functionalized Silver Nanoparticles. Front Microbiol. 2017;8:1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kaplan JB. Therapeutic potential of biofilm-dispersing enzymes. Int J Artif Organs. 2009;32(9):545–554. [DOI] [PubMed] [Google Scholar]
- 162.Tosato MG, Schilardi P. Synergistic effect of carboxypterin and methylene blue applied to antimicrobial photodynamic therapy against mature biofilm of Klebsiella pneumoniae. Heliyon. 2020;6(3):e03522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bilici K, Atac N, Muti A, et al. Broad spectrum antibacterial photodynamic and photothermal therapy achieved with indocyanine green loaded SPIONs under near infrared irradiation. Biomater Sci. 2020;8(16):4616–4625. [DOI] [PubMed] [Google Scholar]
- 164.Vuotto C, Longo F, Balice MP, Donelli G, Varaldo PE. Antibiotic resistance related to biofilm formation in Klebsiella pneumoniae. Pathogens. 2014;3(3):743–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Muhammad MH, Idris AL, Fan X, et al. Beyond Risk: bacterial Biofilms and Their Regulating Approaches. Front Microbiol. 2020;11(928):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Suresh MK, Biswas R, Biswas L. An update on recent developments in the prevention and treatment of Staphylococcus aureus biofilms. Int J Med Microbiol. 2019;309(1):1–12. [DOI] [PubMed] [Google Scholar]
- 167.Le KY, Park MD, Otto M. Immune Evasion Mechanisms of Staphylococcus epidermidis Biofilm Infection. Front Microbiol. 2018;9:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen W, Xie TT, Zeng H. Formation, Antibiotic Resistance, and Control Strategies of Staphylococcus Epidermidis Biofilm. IntechOpen; 2020. [Google Scholar]
- 169.Bhattacharyya P, Agarwal B, Goswami M, Maiti D, Baruah S, Tribedi P. Zinc oxide nanoparticle inhibits the biofilm formation of Streptococcus pneumoniae. Antonie Van Leeuwenhoek. 2018;111(1):89–99. [DOI] [PubMed] [Google Scholar]
- 170.Wang J, Song M, Pan J, et al. Quercetin impairs Streptococcus pneumoniae biofilm formation by inhibiting sortase A activity. J Cell Mol Med. 2018;22(12):6228–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yadav MK, Go YY, Chae SW, Song JJ. The Small Molecule DAM Inhibitor, Pyrimidinedione, Disrupts Streptococcus pneumoniae Biofilm Growth In Vitro. PLoS One. 2015;10(10):e0139238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lu L, Hu W, Tian Z, et al. Developing natural products as potential anti-biofilm agents. Chin Med. 2019;14(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Martínez OF, Cardoso MH, Ribeiro SM, Franco OL. Recent advances in anti-virulence therapeutic strategies with a focus on dismantling bacterial membrane microdomains, toxin neutralization, quorum-sensing interference and biofilm inhibition. Front Cell Infect Microbiol. 2019;9:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bazaid AS, Aldarhami A, Patel M, et al. The Antimicrobial Effects of Saudi Sumra Honey against Drug Resistant Pathogens: phytochemical Analysis, Antibiofilm, Anti-Quorum Sensing, and Antioxidant Activities. Pharmaceuticals. 2022;15(10):1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ghosh S, Lahiri D, Nag M, et al. Phytocompound Mediated Blockage of Quorum Sensing Cascade in ESKAPE Pathogens. Antibiotics. 2022;11(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Singkham-In U, Higgins PG, Wannigama DL, Hongsing P, Chatsuwan T. Rescued chlorhexidine activity by resveratrol against carbapenem-resistant Acinetobacter baumannii via down-regulation of AdeB efflux pump. PLoS One. 2020;15(12):e0243082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bandeira junior G, Sutili FJ, Gressler LT, et al. Antibacterial potential of phytochemicals alone or in combination with antimicrobials against fish pathogenic bacteria. J Appl Microbiol. 2018;125(3):655–665. [DOI] [PubMed] [Google Scholar]
- 178.Mishra R, Panda AK, de Mandal S, Shakeel M, Bisht SS, Khan J. Natural Anti-biofilm Agents: strategies to Control Biofilm-Forming Pathogens. Front Microbiol. 2020;11:566325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Singh VK, Mishra A, Jha B. Anti-quorum sensing and anti-biofilm activity of Delftia tsuruhatensis extract by attenuating the quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa. Front Cell Infect Microbiol. 2017;7:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Reen FJ, Gutiérrez-Barranquero JA, Parages ML, Gara F. Coumarin: a novel player in microbial quorum sensing and biofilm formation inhibition. Appl Microbiol Biotechnol. 2018;102(5):2063–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang J, Rui X, Wang L, Guan Y, Sun X, Dong M. Polyphenolic extract from Rosa rugosa tea inhibits bacterial quorum sensing and biofilm formation. Food Control. 2014;42:125–131. [Google Scholar]
- 182.Batohi N, Lone SA, Marimani M, Wani MY, Al-Bogami AS, Ahmad A. Citral and its derivatives inhibit quorum sensing and biofilm formation in Chromobacterium violaceum. Arch Microbiol. 2021;203(4):1451–1459. [DOI] [PubMed] [Google Scholar]
- 183.Azeredo J, García P, Drulis-Kawa Z. Targeting biofilms using phages and their enzymes. Curr Opin Biotechnol. 2021;68:251–261. [DOI] [PubMed] [Google Scholar]
- 184.Ingle AP, Wagh S, Biswas J, Mondal M, Feitosa CM, Rai M. Phyto-Fabrication of Different Nanoparticles and Evaluation of their Antibacterial and Anti-Biofilm Efficacy. Curr Nanosci. 2020;16(6):1002–1015. [Google Scholar]
- 185.Mogha K. Biofilm - A threat to dairy industry. Ind J Dairy Sci. 2014;67(6):459–466. [Google Scholar]
- 186.Girennavar B, Cepeda ML, Soni KA, et al. Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. Int J Food Microbiol. 2008;125(2):204–208. [DOI] [PubMed] [Google Scholar]
- 187.Bruni R, Barreca D, Protti M, et al. Botanical Sources, Chemistry, Analysis, and Biological Activity of Furanocoumarins of Pharmaceutical Interest. Molecules. 2019;24(11):2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.D’Almeida RE, Molina RRDI, Viola CM, et al. Comparison of seven structurally related coumarins on the inhibition of Quorum sensing of Pseudomonas aeruginosa and Chromobacterium violaceum. Bioorg Chem. 2017;73:37–42. [DOI] [PubMed] [Google Scholar]
- 189.Snoussi M, Noumi E, Hajlaoui H, et al. Phytochemical Profiling of Allium subhirsutum L. Aqueous Extract with Antioxidant, Antimicrobial, Antibiofilm, and Anti-Quorum Sensing Properties: in Vitro and In Silico Studies. Plants. 2022;11(4):495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Awadelkareem AM, Al-Shammari E, Elkhalifa AO, et al. Anti-Adhesion and Antibiofilm Activity of Eruca sativa Miller Extract Targeting Cell Adhesion Proteins of Food-Borne Bacteria as a Potential Mechanism: combined In Vitro-In Silico Approach. Plants. 2022;11(5):610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.de Moura DF, Rocha TA, De melo Barros D, et al. Evaluation of the antioxidant, antibacterial, and antibiofilm activity of the sesquiterpene nerolidol. Arch Microbiol. 2021;203(7):4303–4311. [DOI] [PubMed] [Google Scholar]
- 192.Emam M, Abdel-Haleem DR, Salem MM, et al. Phytochemical Profiling of Lavandula coronopifolia Poir. Aerial Parts Extract and Its Larvicidal, Antibacterial, and Antibiofilm Activity Against Pseudomonas aeruginosa. Molecules. 2021;26(6):1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang M, Meng F, Gu W, et al. Effects of Natural Products on Bacterial Communication and Network-Quorum Sensing. Biomed Res Int. 2020;2020:8638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ackermann HW. Bacteriophage observations and evolution. Res Microbiol. 2003;154(4):245–251. [DOI] [PubMed] [Google Scholar]
- 195.Breitbart M, Rohwer F. Here a virus, there a virus, everywhere the same virus? Trends Microbiol. 2005;13(6):278–284. [DOI] [PubMed] [Google Scholar]
- 196.Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vandamme EJ. Phage therapy and phage control: to be revisited urgently!! J Chem Technol Biotechnol. 2014;89(3):329–333. [Google Scholar]
- 198.Kortright KE, Chan BK, Koff JL, Turner PE. Phage Therapy: a Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe. 2019;25(2):219–232. [DOI] [PubMed] [Google Scholar]
- 199.Fernández L, Gutiérrez D, García P, Rodríguez A. The perfect bacteriophage for therapeutic applications—A quick guide. Antibiotics. 2019;8(3):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hermoso JA, García JL, García P. Taking Aim on bacterial pathogens: from phage therapy to enzybiotics. Curr Opin Microbiol. 2007;10(5):461–472. [DOI] [PubMed] [Google Scholar]
- 201.Ferriol-González C, Domingo-Calap P. Phages for Biofilm Removal. Antibiotics. 2020;9(5):268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vázquez R, García P. Synergy Between Two Chimeric Lysins to Kill Streptococcus pneumoniae. Front Microbiol. 2019;10:1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Łubowska N, Piechowicz L. Staphylococcus aureus biofilm and the role of bacteriophages in its eradication. Postepy Hig Med Dosw. 2018;72:101–107. [Google Scholar]
- 204.Topka-Bielecka G, Dydecka A, Necel A, et al. Bacteriophage-derived depolymerases against bacterial biofilm. Antibiotics. 2021;10(2):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pires DP, Oliveira H, Melo LDR, Sillankorva S, Azeredo J. Bacteriophage-encoded depolymerases: their diversity and biotechnological applications. Appl Microbiol Biotechnol. 2016;100(5):2141–2151. [DOI] [PubMed] [Google Scholar]
- 206.Loessner MJ. Bacteriophage endolysins—current state of research and applications. In: Current Opinion in Microbiology. Vol. 8. Elsevier; 2005:480–487. [DOI] [PubMed] [Google Scholar]
- 207.Young R, Wang IN, Roof WD. Phages will out: strategies of host cell lysis. Trends Microbiol. 2000;8(3):120–128. [DOI] [PubMed] [Google Scholar]
- 208.Hasan M, Ahn J. Evolutionary Dynamics between Phages and Bacteria as a Possible Approach for Designing Effective Phage Therapies against Antibiotic-Resistant Bacteria. Antibiotics. 2022;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pei R, Lamas-Samanamud GR. Inhibition of Biofilm Formation by T7 Bacteriophages Producing Quorum-Quenching Enzymes. Appl Environ Microbiol. 2014;80(17):5340–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Silpe JE, Bassler BL. Phage-encoded LuxR-type receptors responsive to host-produced bacterial quorum-sensing autoinducers. mBio. 2019;10(2):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu S, Lu H, Zhang S, Shi Y, Chen Q. Phages against Pathogenic Bacterial Biofilms and Biofilm-Based Infections: a Review. Pharmaceutics. 2022;14(2):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Luong T, Salabarria AC, Roach DR. Phage Therapy in the Resistance Era: where Do We Stand and Where Are We Going? Clin Ther. 2020;42(9):1659–1680. [DOI] [PubMed] [Google Scholar]
- 213.Esteban PP, Alves DR, Enright MC, et al. Enhancement of the antimicrobial properties of bacteriophage-K via stabilization using oil-in-water nano-emulsions. Biotechnol Prog. 2014;30(4):932–944. [DOI] [PubMed] [Google Scholar]
- 214.Esteban PP, Jenkins ATA, Arnot TC. Elucidation of the mechanisms of action of Bacteriophage K/nano-emulsion formulations against S. aureus via measurement of particle size and zeta potential. Colloids Surf B Biointerfaces. 2016;139:87–94. [DOI] [PubMed] [Google Scholar]
- 215.Yu P, Wang Z, Marcos-Hernandez M, et al. Bottom-up biofilm eradication using bacteriophage-loaded magnetic nanocomposites: a computational and experimental study. Environ Sci Nano. 2019;6(12):3539–3550. [Google Scholar]
- 216.Liu ZH, Chiang MT, Lin HY. Lytic Bacteriophage as a Biomaterial to Prevent Biofilm Formation and Promote Neural Growth. Tissue Eng Regen Med. 2022;1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Koshi E, Mohan A, Rajesh S, Philip K. Antimicrobial photodynamic therapy: an overview. J Indian Soc Periodontol. 2011;15(4):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Polat E, Kang K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines. 2021;9(6):584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Dascalu LM, Moldovan M, Prodan D, et al. Assessment and Characterization of Some New Photosensitizers for Antimicrobial Photodynamic Therapy (aPDT). Materials. 2020;13(13):3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Carrera ET, Dias HB, Corbi SCT, et al. The application of antimicrobial photodynamic therapy (aPDT) in dentistry: a critical review. Laser Phys. 2016;26(12):123001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hu X, Huang YY, Wang Y, Wang X, Hamblin MR. Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Front Microbiol. 2018;9:1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cieplik F, Deng D, Crielaard W, et al. Antimicrobial photodynamic therapy–what we know and what we don’t. Crit Rev Microbiol. 2018;44(5):571–589. [DOI] [PubMed] [Google Scholar]
- 223.Tavares LJ, de Avila ED, Klein MI, Panariello BHDD, Spolidório DMPP, Pavarina AC. Antimicrobial photodynamic therapy alone or in combination with antibiotic local administration against biofilms of Fusobacterium nucleatum and Porphyromonas gingivalis. J Photochem Photobiol B. 2018;188:135–145. [DOI] [PubMed] [Google Scholar]
- 224.Ghorbani J, Rahban D, Aghamiri S, Teymouri A, Bahador A. Photosensitizers in antibacterial photodynamic therapy: an overview. Laser Ther. 2018;27(4):293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Perez-Laguna V, Gilaberte Y, Millán-Lou MI, et al. A combination of photodynamic therapy and antimicrobial compounds to treat skin and mucosal infections: a systematic review. Photochem Photobiol Sci. 2019;18(5):1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chibebe junior J, Fuchs BB, Sabino CP, et al. Photodynamic and Antibiotic Therapy Impair the Pathogenesis of Enterococcus faecium in a Whole Animal Insect Model. PLoS One. 2013;8(2):e55926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dubey AK, Kumar Gupta V, Kujawska M, et al. Exploring nano-enabled CRISPR-Cas-powered strategies for efficient diagnostics and treatment of infectious diseases. J Nanostructure Chem. 2022;1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Louwen R, Staals RHJ, Endtz HP, van Baarlen P, van der Oost J. The Role of CRISPR-Cas Systems in Virulence of Pathogenic Bacteria. Microbiol Mol Biol Rev. 2014;78(1):74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gong T, Zeng J, Tang B, Zhou X. CRISPR-Cas systems in oral microbiome: from immune defense to physiological regulation. Mol Oral Microbiol. 2020;35(2):41–48. [DOI] [PubMed] [Google Scholar]
- 230.Ho C CRISPR gene-editing controversy shows old ideas about East and West still prevail. The Conversation; 2016. Available from: https://theconversation.com/crispr-gene-editing-controversy-shows-old-ideas-about-east-and-west-still-prevail-66918. Accessed December 15, 2022.
- 231.Yadav N, Narang J, Chhillar AK, Rana JS. CRISPR: a new paradigm of theranostics. Nanomedicine. 2021;33:102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hille F, Charpentier E. CRISPR-Cas: biology, mechanisms and relevance. Philos Trans R Soc B Biol Sci. 2016;371(1707):20150496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wiedenheft B, van Duijn E, Bultema J, et al. RNA-guided complex from a bacterial immune system enhances target recognition through seed sequence interactions. Proc Natl Acad Sci U S A. 2011;108(25):10092–10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Paul B, Montoya G. CRISPR-Cas12a: functional overview and applications. Biomed J. 2020;43(1):8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zuberi A, Misba L, Khan AU. CRISPR interference (CRISPRi) inhibition of luxS gene expression in E. coli: an approach to inhibit biofilm. Front Cell Infect Microbiol. 2017;7:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Huang R, Li M, Gregory RL. Bacterial interactions in dental biofilm. Virulence. 2011;2(5):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zuberi A, Ahmad N, Khan AU. CRISPRi induced suppression of fimbriae gene (fimH) of a Uropathogenic Escherichia coli: an approach to inhibit microbial biofilms. Front Immunol. 2017;8:1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Noirot-Gros MF, Forrester S, Malato G, Larsen PE, Noirot P. CRISPR interference to interrogate genes that control biofilm formation in Pseudomonas fluorescens. Sci Rep. 2019;9(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hegde S, Nilyanimit P, Kozlova E, et al. CRISPR/Cas9-mediated gene deletion of the ompA gene in symbiotic Cedecea neteri impairs biofilm formation and reduces gut colonization of Aedes aegypti mosquitoes. PLoS Negl Trop Dis. 2019;13(12):e0007883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Moon S, Kim DY, Ko JH, Kim YS. Recent advances in the CRISPR genome editing tool set. Exp Mol Med. 2019;51(11):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Makabenta JM, Nabawy A, Li CH, Schmidt-Malan S, Patel R, Rotello VM. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol. 2021;19(1):23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Abdalla SSI, Katas H, Azmi F, Busra MFM. Antibacterial and Anti-Biofilm Biosynthesised Silver and Gold Nanoparticles for Medical Applications: mechanism of Action, Toxicity and Current Status. Curr Drug Deliv. 2020;17(2):88–100. [DOI] [PubMed] [Google Scholar]
- 243.Geissel FJ, Platania V, Gogos A, et al. Antibiofilm activity of nanosilver coatings against Staphylococcus aureus. J Colloid Interface Sci. 2022;608:3141–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mohammad MA, Faris Taufeq FY. Roles of chitosan in synthesis, antibacterial and anti-biofilm properties of bionano silver and gold. RSC Adv. 2022;12(30):19297–19312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Luzala MM, Muanga CK, Kyana J, et al. A Critical Review of the Antimicrobial and Antibiofilm Activities of Green-Synthesized Plant-Based Metallic Nanoparticles. Nanomaterials. 2022;12(11):1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Al-Azawi M, Hadi SM, Mohammed C. Synthesis of silica nanoparticles via green approach by using hot aqueous extract of Thuja orientalis leaf and their effect on biofilm formation. Iraqi J Agric Sci. 2019;50:245–255. [Google Scholar]
- 247.Shehabeldine AM, Amin BH, Hagras FA, et al. Potential Antimicrobial and Antibiofilm Properties of Copper Oxide Nanoparticles: time-Kill Kinetic Essay and Ultrastructure of Pathogenic Bacterial Cells. Appl Biochem Biotechnol. 2022;1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Maksoud MIAA, El-Sayyad GS, Ashour AH, et al. Antibacterial, antibiofilm, and photocatalytic activities of metals-substituted spinel cobalt ferrite nanoparticles. Microb Pathog. 2019;127:144–158. [DOI] [PubMed] [Google Scholar]
- 249.Takamiya AS, Monteiro DR, Gorup LF, et al. Biocompatible silver nanoparticles incorporated in acrylic resin for dental application inhibit Candida albicans biofilm. Mater Sci Eng C Mater Biol Appl. 2021;118. [DOI] [PubMed] [Google Scholar]
- 250.Dias HB, Bernardi MIB, Bauab TM, Hernandes AC. Titanium dioxide and modified titanium dioxide by silver nanoparticles as an anti biofilm filler content for composite resins. Dent Mater. 2019;35(2):e36–e46. [DOI] [PubMed] [Google Scholar]
- 251.Al-Wrafy FA, Al-Gheethi AA, Ponnusamy SK, Noman EA, Fattah SA. Nanoparticles approach to eradicate bacterial biofilm-related infections: a critical review. Chemosphere. 2022;288:132603. [DOI] [PubMed] [Google Scholar]
- 252.Qais FA, Khan MS, Ahmad I. Nanoparticles as Quorum Sensing Inhibitor: prospects and Limitations. In: Biotechnological Applications of Quorum Sensing Inhibitors. Springer; 2018:227–244. [Google Scholar]
- 253.Mao BH, Chen ZY, Wang YJ, Yan SJ. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Sci Rep. 2018;8(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Darabpour E, Kashef N, Amini SM, Kharrazi S, Djavid GE. Fast and effective photodynamic inactivation of 4-day-old biofilm of methicillin-resistant Staphylococcus aureus using methylene blue-conjugated gold nanoparticles. J Drug Deliv Sci Technol. 2017;37:134–140. [Google Scholar]
- 255.Wan T, Pan Q, Ping Y. Microneedle-assisted genome editing: a transdermal strategy of targeting NLRP3 by CRISPR-Cas9 for synergistic therapy of inflammatory skin disorders. Sci Adv. 2021;7(11):eabe2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Barhoum A, García-Betancourt ML, Jeevanandam J, et al. Review on Natural, Incidental, Bioinspired, and Engineered Nanomaterials: history, Definitions, Classifications, Synthesis, Properties, Market, Toxicities, Risks, and Regulations. Nanomaterials. 2022;12(2):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kiani MH, Imran M, Raza A, Shahnaz G. Multi-functionalized nanocarriers targeting bacterial reservoirs to overcome challenges of multi drug-resistance. DARU J Pharm Sci. 2020;28(1):319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Franco D, Calabrese G, Guglielmino SPP, Conoci S. Metal-Based Nanoparticles: antibacterial Mechanisms and Biomedical Application. Microorganisms. 2022;10(9):1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Jang J, Lee JM. Development of Antibiofilm Nanocomposites: ag/Cu Bimetallic Nanoparticles Synthesized on the Surface of Graphene Oxide Nanosheets. ACS Appl Mater Interfaces. 2020;12(32):35826–35834. [DOI] [PubMed] [Google Scholar]
- 260.Khalid HF, Tehseen B, Sarwar Y, et al. Biosurfactant coated silver and iron oxide nanoparticles with enhanced anti-biofilm and anti-adhesive properties. J Hazard Mater. 2019;364:441–448. [DOI] [PubMed] [Google Scholar]
- 261.El-Batal AI, Al-Hazmi NE, Farrag AA, et al. Antimicrobial synergism and antibiofilm activity of amoxicillin loaded citric acid-magnesium ferrite nanocomposite: effect of UV-illumination, and membrane leakage reaction mechanism. Microb Pathog. 2022;164:105440. [DOI] [PubMed] [Google Scholar]
- 262.Padmavathi AR, Sriyutha Murthy P, Das A, Nishad PA, Pandian R, Rao TS. Copper oxide nanoparticles as an effective anti-biofilm agent against a copper tolerant marine bacterium, Staphylococcus lentus. Biofouling. 2019;35(9):1007–1025. [DOI] [PubMed] [Google Scholar]
- 263.Rónavári A, Igaz N, Adamecz DI, et al. Green Silver and Gold Nanoparticles: biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules. 2021;26(4):844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Vallet-Regí M, González B, Izquierdo-Barba I. Nanomaterials as Promising Alternative in the Infection Treatment. Int J Mol Sci. 2019;20(15):3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Nisar P, Ali N, Rahman L, Ali M, Shinwari ZK. Antimicrobial activities of biologically synthesized metal nanoparticles: an insight into the mechanism of action. J Biol Inorg Chem. 2019;24(7):929–941. [DOI] [PubMed] [Google Scholar]
- 266.Zhang D, Ma XL, Gu Y, Huang H, Zhang GW. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front Chem. 2020;8:3256. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 267.Xu M, Yim W, Zhou J, et al. The Application of Organic Nanomaterials for Bioimaging, Drug Delivery, and Therapy: spanning Various Domains. IEEE Nanotechnol Mag. 2021;15(4):8–28. [Google Scholar]
- 268.Ng KK, Zheng G. Molecular Interactions in Organic Nanoparticles for Phototheranostic Applications. Chem Rev. 2015;115(19):11012–11042. [DOI] [PubMed] [Google Scholar]
- 269.Gonzalez Gomez A, Hosseinidoust Z. Liposomes for Antibiotic Encapsulation and Delivery. ACS Infect Dis. 2020;6(5):896–908. [DOI] [PubMed] [Google Scholar]
- 270.Chauhan SB, Gupta V. Recent Advances in Liposome. Res J Pharm Technol. 2020;13(4):2051–2056. [Google Scholar]
- 271.Alavi SE, Koohi Moftakhari Esfahani, M, Raza, A, Adelnia, H, Ebrahimi Shahmabad, H. PEG-grafted liposomes for enhanced antibacterial and antibiotic activities: an in vivo study. NanoImpact. 2022;25:100384. [DOI] [PubMed] [Google Scholar]
- 272.Chen XF, Zhao X, Yang Z. Aptamer-Based Antibacterial and Antiviral Therapy against Infectious Diseases. J Med Chem. 2021;64(24):17601–17626. [DOI] [PubMed] [Google Scholar]
- 273.Allemailem KS, Almatroudi A, Alsahli MA, et al. Recent advances in understanding oligonucleotide aptamers and their applications as therapeutic agents. 3 Biotech. 2020;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Das A, Konyak PM, Das A, Dey SK, Saha C. Physicochemical characterization of dual action liposomal formulations: anticancer and antimicrobial. Heliyon. 2019;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ghandhariyoun N, Jaafari MR, Nikoofal-Sahlabadi S, Taghdisi SM, Moosavian SA. Reducing Doxorubicin resistance in breast cancer by liposomal FOXM1 aptamer: in vitro and in vivo. Life Sci. 2020;262. [DOI] [PubMed] [Google Scholar]
- 276.Zhou C, You T, Jang H, et al. Aptamer-Conjugated Polydiacetylene Colorimetric Paper Chip for the Detection of Bacillus thuringiensis Spores. Sensors. 2020;20:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hallan SS, Marchetti P, Bortolotti D, et al. Design of Nanosystems for the Delivery of Quorum Sensing Inhibitors: a Preliminary Study. Molecules. 2020;25(23):5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ibaraki H, Kanazawa T, Chien WYY, et al. The effects of surface properties of liposomes on their activity against Pseudomonas aeruginosa PAO-1 biofilm. J Drug Deliv Sci Technol. 2020;57:101754. [Google Scholar]
- 279.Ayllon M, Abatchev G, Bogard A, Whiting R, Hobdey SE, Fologea D. Liposomes prevent in vitro hemolysis induced by streptolysin o and lysenin. Membranes. 2021;11(5):364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kumar S, Paliya BS, Singh BN. Superior inhibition of virulence and biofilm formation of Pseudomonas aeruginosa PAO1 by phyto-synthesized silver nanoparticles through anti-quorum sensing activity. Microb Pathog. 2022;170:105678. [DOI] [PubMed] [Google Scholar]
- 281.da Silveira SA, Perez A. Liposomes as novel anti-infectives targeting bacterial virulence factors? Expert Rev Anti Infect Ther. 2015;13(5):531–533. [DOI] [PubMed] [Google Scholar]
- 282.Agarwal H, Nakara A, Shanmugam VK. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: a review. Biomed Pharmacother. 2019;109:2561–2572. [DOI] [PubMed] [Google Scholar]
- 283.Mauricio MD, Guerra-Ojeda S, Marchio P, et al. Nanoparticles in medicine: a focus on vascular oxidative stress. Oxid Med Cell Longev. 2018;2018:879. [DOI] [PMC free article] [PubMed] [Google Scholar]