Abstract
Left atrial appendage (LAA) closure may prevent atrial fibrillation (AF)-induced thromboembolism. We describe a rare case of right atrial (RA) thrombus after thoracoscopic left atrial appendectomy and pulmonary vein isolation. Careful evaluation for the presence of RA thrombus in patients with persistent AF after LAA occlusion may be necessary. (Level of Difficulty: Intermediate.)
Key Words: atrial fibrillation, echocardiography, left atrial appendectomy, right atrial thrombus
Abbreviations and Acronyms: AF, atrial fibrillation; DOAC, direct oral anticoagulant; LAA, left atrial appendage; LAAO, left atrial appendage occlusion; RA, right atrial; RAA, right atrial appendage; TLAA, thoracoscopic left atrial appendectomy; TPVI, thoracoscopic pulmonary vein isolation
Central Illustration
An 84-year-old man presented for evaluation of sleep apnea and cardiac function. Cardiac examination revealed an irregular pulse (80 beats/min), normal blood pressure (126/80 mm Hg), and the absence of a heart murmur. Electrocardiography revealed atrial fibrillation (AF), and transthoracic echocardiography demonstrated a large right atrial (RA) mass. Written and verbal informed consent was obtained from the patient.
Learning Objectives
-
•
To understand that an RAA thrombus is a rare but important complication in patients with AF.
-
•
To understand the utility of diagnosing RA thrombus using multimodality imaging.
Medical History
The patient’s history included hypertension, persistent nonvalvular AF, and cerebral infarction. The CHA2DS2–VASc score1,2 was 5; therefore, direct oral anticoagulant (DOAC) therapy was initiated. However, the risk of bleeding was high (HAS-BLED score 3), and the patient could not tolerate the anticoagulation therapy because of frequent bleeding events. Two years earlier, he had undergone thoracoscopic left atrial appendectomy (TLAA)3 and thoracoscopic pulmonary vein isolation (TPVI) at another hospital. Anticoagulation therapy was discontinued after TLAA and TPVI. Persistent AF recurred after 6 months; however, DOAC therapy was not resumed.
Differential Diagnosis
Differential diagnoses of the mass included thrombus and tumors (such as myxoma, angiosarcoma, or lymphoma). The site of origin was the right atrial appendage (RAA); Inasmuch as both AF and RA dilation were present, an RA thrombus was suspected. The presence of a stalk and a low-density mass was suspected on contrast-enhanced computed tomography images, and these findings did not rule out a myxoma.
Investigations
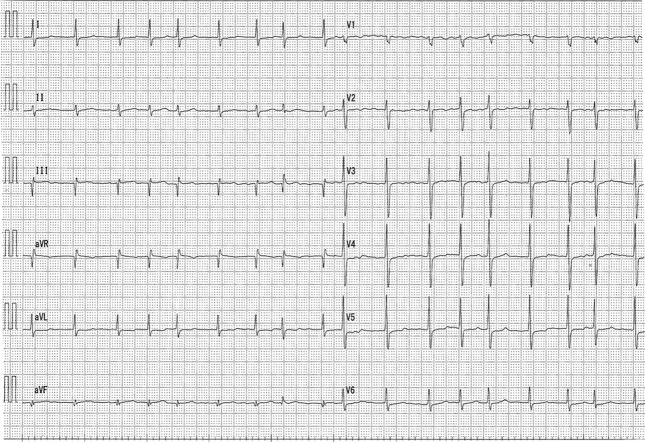
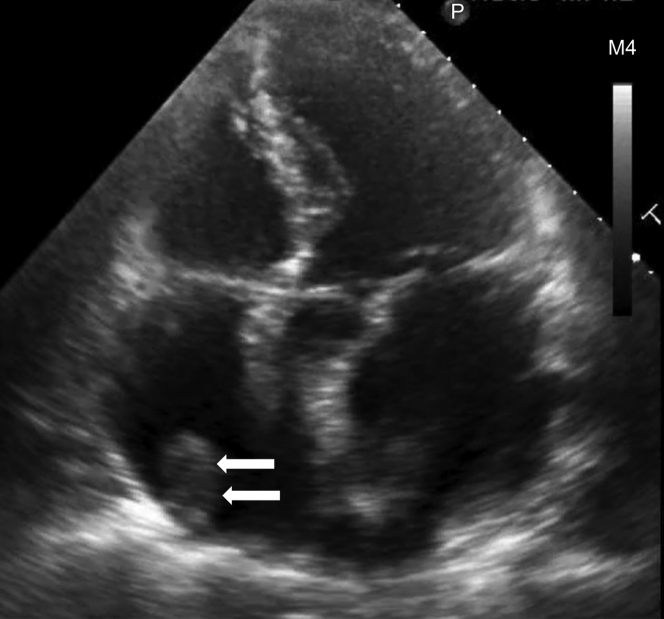
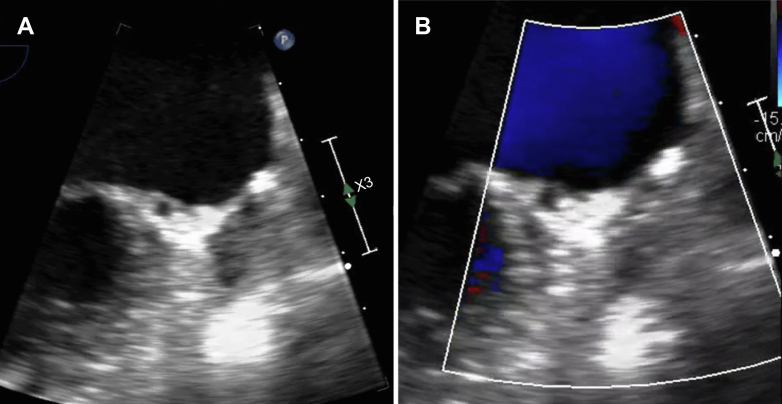
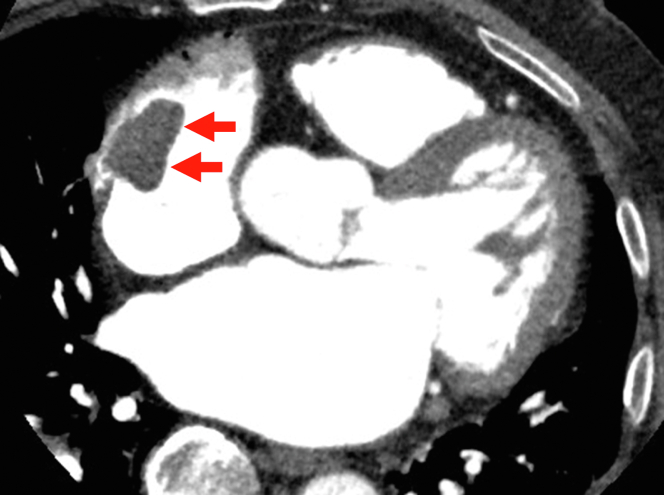
An electrocardiogram showed AF and a ventricular rate of 82 beats/min (Figure 1). The coagulation profile indicated a prothrombin time, an activated partial thromboplastin time, an internal normalized ratio, and a D-dimer level of 14 s, 40.0 s, 1.30, and 0.71 μg/mL, respectively. Transthoracic echocardiography revealed a normal left ventricular ejection fraction (60%) with left atrial and RA dilation (left atrial volume index: 75 mL/m2, RA area: 28 cm2). An echogenic mass was attached to the RA wall (Figure 2). The left atrial appendage (LAA) was occluded (Figure 3). Transesophageal echocardiography demonstrated a large, globular, echogenic mass measuring 3.0 × 2.0 cm and originating from the RAA (Figure 4). Contrast-enhanced computed tomography showed a low-density mass measuring 3.0 cm in the RA (Figure 5).
Figure 1.
Electrocardiogram
Electrocardiogram showing atrial fibrillation.
Figure 2.
Transthoracic Echocardiogram
Transthoracic echocardiogram showing dilated left and right atria (left atrial volume index of 75 mL/m2, right atrium area: 28 cm2) and an echogenic mass attached to the right atrium (arrows).
Figure 3.
Transesophageal Echocardiogram of Left Atrium
(A) Transesophageal echocardiogram after left atrial appendage (LAA) resection. (B) Color Doppler echocardiogram indicates no blood flow into the LAA.
Figure 4.
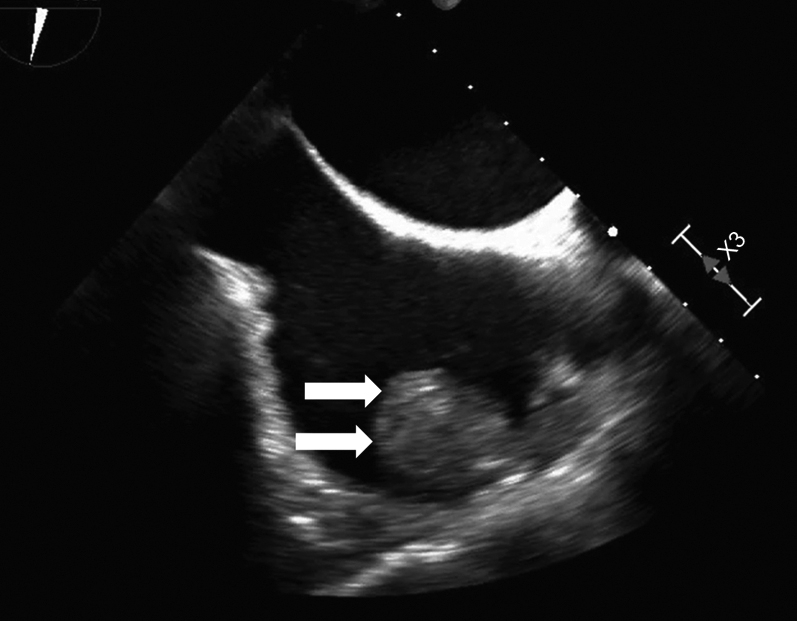
Transesophageal Echocardiogram of Right Atrium
Transesophageal echocardiography showing a large globular echogenic mass measuring 3.0 × 2.0 cm with a stalk originating from the right atrial appendage (arrows).
Figure 5.
Contrast-Enhanced Computed Tomography
Contrast-enhanced computed tomography showing a giant mass (arrows) in the right atrium.
Management (Medical/Interventions)
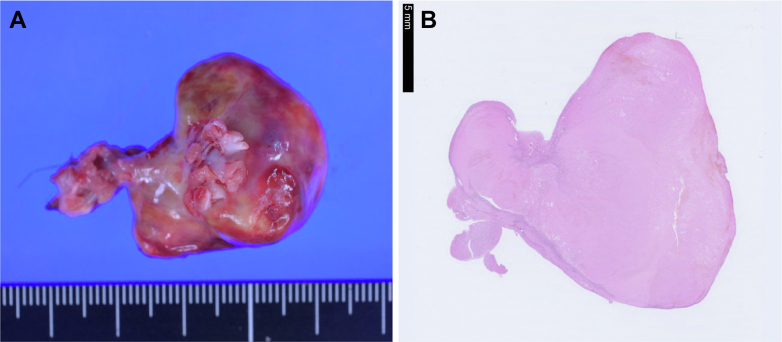
Anticoagulation with a DOAC was initiated. However, a follow-up transthoracic echocardiogram 2 weeks later showed no reduction in the size of the mass, and we were unable to completely rule out a myxoma. To confirm the diagnosis of the RA mass, open-heart surgery and resection were performed; a single nodular mass measuring 40 × 30 × 20 was removed. Histopathologic examination revealed a thrombus with fibrosis at the margins (Figure 6). DOAC was postoperatively prescribed to prevent thrombus recurrence.
Figure 6.
Surgical Specimens
(A) A large thrombus (40 × 30 × 20 mm) is removed from the right atrial appendage. (B) Histopathologic diagnosis reveals a thrombus (blood clot). Tissue staining method is HE staining; magnification is 0.5 ×.
Discussion
The most harmful complications of AF are hemodynamic derangement and systemic emboli.4 LAA is the primary site for thrombus formation in 90% of patients with nonvalvular AF.5 The prevalence of LAA thrombus in patients with AF who do not receive anticoagulant agents is 5% to 27%. Even in patients receiving anticoagulation therapy, the prevalence of LAA thrombus remains as high as 8%.6 Thus, careful evaluation and treatment is necessary in patients with AF.
Left atrial appendage occlusion (LAAO) is recommended for the treatment and prevention of thromboembolisms caused by LAA thrombus in patients with nonvalvular AF, who are at a more than moderate risk of stroke but cannot tolerate anticoagulant therapy because of the high risk of bleeding. TLAA is a relatively simple minimally invasive LAAO procedure to remove the LAA completely.3 The less invasive percutaneous LAAO has recently been recommended as a nonpharmacologic equivalent to anticoagulation in selected patients with nonvalvular AF.1,2,7 Thus, long-term anticoagulation is not required in patients who undergo LAAO and is eventually discontinued.
This patient had a high risk for stroke (CHA2DS2-VASc score of 5) but also had a high risk for bleeding (HAS-BLED score of 3); therefore, long-term anticoagulation might have been difficult. Because percutaneous LAAO was not approved in Japan at the time of treatment, TLAA and TPVI were performed. DOAC therapy was discontinued after TLAA. AF recurred 6 months postoperatively; however, DOAC therapy was not resumed, and evaluation of thrombus was not conducted. It may be necessary to evaluate for the presence of heart thrombus using imaging methods in patients in whom anticoagulation after LAAO has been discontinued.
The presence of LAA thrombus has drawn considerable attention as a cause of thromboembolism; however, few studies have reported an RAA thrombus in patients with AF. Guidelines for the testing or treatment of RAA thrombosis have not yet been established. Cresti et al8 stated that anatomical features explain the lower prevalence of thrombus formation in the RAA. Previous studies have shown that in patients with AF, the incidence of thrombosis detected by transesophageal echocardiography is lower in the RAA (0.4%-6%) than in the LAA (6%-15%). Furthermore, few studies have reported the occurrence of RAA thrombus after LAAO. The incidence of RAA thrombus in patients with AF is low; however, an RAA thrombus can cause life-threatening complications, including pulmonary embolism and paradoxical thrombus migration through a patent foramen ovale, with risk of systemic embolization.9 Previous authors have suggested that the management of RAA thrombus should be the same as that of LAA thrombus in patients with AF.10 Therefore, the detection of RAA thrombus is essential and needs to be carefully managed.
Treatment options for RAA thrombus include anticoagulation, thrombolysis, and surgery. Medical or surgical management is case dependent. Optimal management guidelines of RAA thrombus in patients with AF after LAAO have not yet been established. Further studies are required to better elucidate the optimal management for detection and treatment of RAA thrombus after LAAO in patients with AF.
Follow-Up
After RA thrombectomy, DOAC treatment was continued, and follow-up imaging examination (after 18 months) indicated no thrombus recurrence. The patient remained well, experiencing no episodes of major bleeding, cardiogenic stroke, or pulmonary embolism.
Conclusions
RAA thrombus occurred in a patient with persistent AF in whom anticoagulant therapy was discontinued after TLAA. Determining the appearance of RAA thrombus may be useful, because although uncommon, this condition may cause life-threatening complications, including pulmonary embolism or paradoxical thrombosis.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Kirchhof P., Benussi S., Kotecha D., et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 2.January C.T., Wann L.S., Calkins H., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64(21):e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Ohtsuka T., Ninomiya M., Nonaka T., et al. Thoracoscopic stand-alone left atrial appendectomy for thromboembolism prevention in nonvalvular atrial fibrillation. J Am Coll Cardiol. 2013;62:103–107. doi: 10.1016/j.jacc.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Faletra F.F., Saric M., Saw J., Lempereur M., et al. Imaging for patient's selection and guidance of LAA and ASD percutaneous and surgical closure. J Am Coll Cardiol Img. 2021;14:3–21. doi: 10.1016/j.jcmg.2019.06.032. [DOI] [PubMed] [Google Scholar]
- 5.Osmancik P., Herman D., Neuzil P., et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol. 2020;75:3122–3135. doi: 10.1016/j.jacc.2020.04.067. [DOI] [PubMed] [Google Scholar]
- 6.Zhan Y., Joza J., Al Rawahi M., et al. Assessment and management of the left atrial appendage thrombus in patients with nonvalvular atrial fibrillation. Can J Cardiol. 2018;34:252–261. doi: 10.1016/j.cjca.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Nogami A., Kurita T., Kusano K., et al. JCS/JHRS 2021 guideline focused update on non-pharmacotherapy of cardiac arrhythmias. Circ J. 2022;86:337–363. doi: 10.1253/circj.CJ-21-0162. [DOI] [PubMed] [Google Scholar]
- 8.Cresti A., García-Fernández M.A., Miracapillo G., et al. Frequency and significance of right atrial appendage thrombi in patients with persistent atrial fibrillation or atrial flutter. J Am Soc Echocardiogr. 2014;27:1200–1207. doi: 10.1016/j.echo.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Kukla P., McIntyre W.F., Koracevic G., et al. Relation of atrial fibrillation and right-sided cardiac thrombus to outcomes in patients with acute pulmonary embolism. Am J Cardiol. 2015;115:825–830. doi: 10.1016/j.amjcard.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 10.Richardson A.C., Omar M., Velarde G., et al. Right atrial appendage thrombus in atrial fibrillation: A case report and review of the literature. J Investig Med High Impact Case Rep. 2021;9 doi: 10.1177/23247096211010048. [DOI] [PMC free article] [PubMed] [Google Scholar]