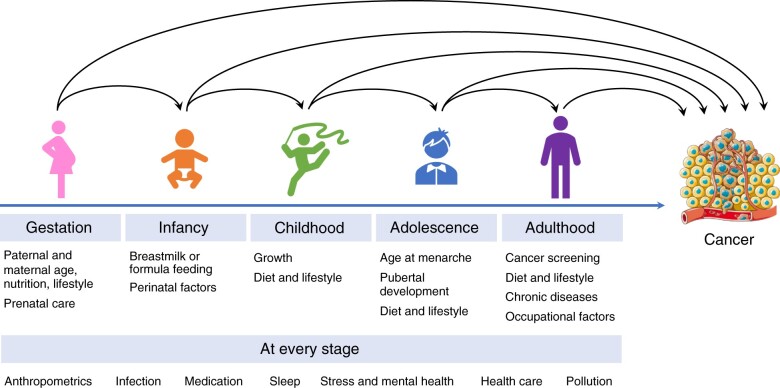
Cancer is a disease of aging, with the vast majority of cancer diagnoses occurring after the age of 50 years (1). However, over the past 3 decades, the incidence of adult cancers has increased in younger adults across the globe, causing a major public health concern (2). Because this surge in early-onset cancer has paralleled worldwide increases in body mass index (BMI), and most of the cancer types increasing in incidence in younger individuals are associated with excess adiposity (referred to as “obesity-related cancer”), the epidemic of early-onset cancer has been attributed, at least in part, to obesity (2). Still, little is known about the risk factors contributing to early-onset cancer. Given the long latency of cancer, it is probable that early-life exposures promote adult cancer development, particularly early-onset cancer (Figure 1). Birthweight and childhood BMI, height, and growth, for example, have been associated with elevated risk of several adulthood cancers (3,4), and prenatal exposures, such as maternal prepregnancy BMI and gestational weight gain, may also contribute to adult cancer risk in offspring (5).
Figure 1.
Proposed research framework to interrogate the role of exposures over the life course in cancer development. Exposures at different ages may influence adulthood cancer risk, both directly and indirectly via relation to exposures in later life. Life course epidemiologic studies are critically needed to identify susceptible windows during which exposures are most influential on cancer risk. Example exposures that may influence cancer risk are listed for each period.
In this issue of the Journal, Jensen and colleagues (6) report findings from their investigation using longitudinal data from the Copenhagen School Health Records Register and the Danish Cancer Registry to evaluate childhood BMI trajectories from ages 6 to 15 years in relation to adult obesity-related cancer incidence among 301 927 individuals. Five sex-specific BMI patterns were selected using latent class trajectory models and named post hoc based on their relation to the US Centers for Disease Control and Prevention Growth Charts (7): below average, average, above average, overweight, and obese. The incidence rate of obesity-related cancer after age 30 years was found to generally increase by heavier BMI trajectory, consistent with previous studies showing a direct association between childhood or adolescent BMI measured at a single time and obesity-related cancer (8-14). Notable exceptions were pre- and postmenopausal breast cancer, both of which were inversely associated with childhood BMI trajectory, confirming previous findings (15). The associations appeared to be stronger for early-onset compared with late-onset cancers. Height at 7 years old was directly associated with obesity-related cancer; adjustment for height did not appreciably change the association of BMI trajectories with cancer. Adult-onset type 2 diabetes was independently associated with obesity-related cancer.
Unlike other studies that were limited to only 1 or 2 measurements of childhood BMI, Jensen et al. (6) analyzed individuals with annual BMI records. The modeling of longitudinal BMI permits a distinction between consistently high BMI and weight gain during a specific period of development, thereby potentiating the identification of a critical period for weight gain as related to future cancer risk. The authors observed that overall, high BMI between the ages of 6 and 15 years was directly associated with obesity-related cancer in adulthood. Surprisingly, all 5 derived BMI patterns were relatively stable across childhood, in contrast with other studies using the same latent class trajectory approach that differentiated high-stable and rapid weight gain patterns (16). One explanation for this discrepancy is that BMI measurements from the Copenhagen School Health Records Register were collected between 1930 and 1996, whereas other studies used data collected between 1990 and 2010 (16). It is possible that the phenotype of childhood obesity characteristic of the modern obesity epidemic was rare at the time when the Copenhagen data were collected, as evidenced by the fact that only 2% of children in the study were characterized with obese BMI trajectory. On one hand, this presents as a limitation in generalizability to the present US population, where as many as 20% of children are considered obese (17). On the other hand, the earlier timing of the study data collection has the advantage of being less confounded by obesogenic diet, lifestyle, and environmental factors that have become more commonplace since the mid-20th century. The observation that the BMI trajectories were already distinct at baseline and did not cross over 9 years of follow-up suggests that the critical period for determining BMI trajectory occurred before age 6 years in this population.
Due to limitations in available data, Jensen and colleagues (6) did not adjust for potential confounders (apart from childhood height and birth year) or distinguish between the direct effect of childhood BMI and indirect effect that functions through adult BMI. A number of confounders may affect both BMI and cancer development and have increased along with obesity since the 1980s: pollution, antibiotic use, physical inactivity, Western diet, height, poor sleep quality, and cesarean delivery (2). Control for confounding by Western-style diet—which is characterized by ultra-processed foods, red and processed meats, and sugar-sweetened beverages—is particularly important, because distinguishing the direct effect of dietary patterns from the effect of diet-induced obesity may inform diet-dependent and independent interventions to reduce the adverse effect of obesity on cancer risk. The association of childhood height, an indication of early-life nutritional status, with cancer risk in the study by Jensen et al. (6) suggests that prenatal, neonatal, and childhood nutrition may directly affect cancer risk. As for adulthood BMI, mediation methods for separating the direct and indirect effects of childhood BMI exist, as do other epidemiologic methods designed to facilitate a life course approach (18,19). Results from a few studies suggest that persistent overweight and obesity in childhood and adulthood are more strongly associated with adult cancer risk than overweight and obesity in childhood only or adulthood only, indicating a potential cumulative effect of adiposity on cancer development (20-23). Future studies applying the life course framework to longitudinal data including both childhood and adulthood BMI can help address whether the effects of childhood BMI on cancer risk can be reduced through weight loss interventions in adulthood (Figure 1).
Jensen et al. (6) provided other notable study design considerations in their study. First, although early-onset cancer incidence is increasing, total incidence remains low relative to late-onset cancer. In stratified analyses, Jensen et al. (6) reported a stronger association of obese BMI trajectory with early-onset compared with late-onset obesity-related cancer among women (incidence rate ratio [IRR] = 2.64, 95% confidence interval [CI] = 1.90 to 3.68 vs IRR = 1.64, 95% CI = 1.38 to 1.95) and men (IRR = 1.88, 95% CI = 1.12 to 3.15 vs IRR = 1.20, 95% CI = 0.92 to 1.56). Owing to the uniquely large sample size of the Danish registry, the confidence intervals had almost no overlap, providing strong support for the particular importance of childhood BMI for early-onset cancer. Second, use of retrospective data on early-life exposures from cohorts established in adulthood engender possible biases due to differential recall by adult BMI or health as well as bias introduced because individuals who are most susceptible to effects of exposure may not survive outcome-free to the age of recruitment (left truncation). For instance, a cohort study that recruits individuals at age 50 years and excludes prevalent cancer cases will overlook individuals who experienced cancer diagnoses or mortality before age 50 years, a period when the effects of early-life risk factors on cancer may be most consequential. Jensen and colleagues (6) largely avoided left truncation by following individuals continuously from childhood, although they did exclude 946 diabetes cases and 1667 cancer cases that occurred either before age 30 years or before 1978 (the year when International Classification of Diseases (ICD)-10 codes became available in the Danish Cancer Registry). Finally, the cohort used by Jensen et al. (6), like most existing longitudinal cohorts, was predominantly non-Hispanic White. In the United States, racial and ethnic minorities are more likely to experience preventable diseases, including some cancers, because of the joint contributions of disproportionate exposure to environmental risk factors and possible genetic differences (24,25). The inclusion of racial and ethnic minorities in future cohort studies ought to be a priority in the study of early-life risk factors for cancer.
In addition to biological and interventional implications, a better understanding of the effects of childhood obesity and other early-life risk factors on cancer, particularly early-onset cancer, may have the potential to inform tailored screening. For example, both the US Preventive Services Task Force and the American Cancer Society lowered the recommended starting age for colorectal cancer screenings from 50 years to 45 years because of the increasing incidence of early-onset colorectal cancer (26,27). To improve the cost-effectiveness of early screening, epidemiologic risk factors can be leveraged to identify individuals at high risk of cancer (28). However, the discriminatory ability of known risk factors remains limited. Given the findings from Jensen et al. (6) on the possible strong effect of early-life risk factors on cancer, incorporating exposures throughout the life course could lead to better risk assessment and more effective risk stratification tools for tailored screening.
Although the need for long-term investment in large, diverse, longitudinal cohorts beginning at birth or early childhood is not unique to cancer, the rising incidence of early-onset cancer cases raises the urgency of this call to action. In the meantime, studies leveraging existing cohorts and registry data can be helpful to generate new hypotheses. The associations between childhood BMI trajectories and adult cancer reported by Jensen et al. (6) are preliminary findings in the broader investigation of early-life exposures and cancer risk and highlight the need for further well-designed life course epidemiologic studies (Figure 1).
Funding
None.
Notes
Role of the funder: Not applicable.
Disclosures: The authors have no disclosures. MS, a JNCI Associate Editor and coauthor of this editorial, was not involved in the review process or decision to publish this editorial.
Author contributions: Conceptualization, all authors; writing—original draft, all authors; writing—review and editing, all authors.
Contributor Information
Alaina M Bever, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Harvard-MIT Division of Health Sciences and Technology, Harvard Medical School, Boston, MA, USA.
Mingyang Song, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Clinical and Translational Epidemiology Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Data availability
No data were generated or analyzed for this editorial.
References
- 1. National Cancer Institute. Risk factors: age - NCI. https://www.cancer.gov/about-cancer/causes-prevention/risk/age. Published April 29, 2015. Accessed September 11, 2022.
- 2. Ugai T, Sasamoto N, Lee HY, et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat Rev Clin Oncol. 2022;19(10):656-673. doi: 10.1038/s41571-022-00672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aarestrup J, Bjerregaard LG, Meyle KD, et al. Birthweight, childhood overweight, height and growth and adult cancer risks: a review of studies using the Copenhagen School Health Records Register. Int J Obes. 2020;44(7):1546-1560. doi: 10.1038/s41366-020-0523-9. [DOI] [PubMed] [Google Scholar]
- 4. Clinton SK, Giovannucci EL, Hursting SD.. The World Cancer Research Fund/American Institute for Cancer research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr. 2020;150(4):663-671. doi: 10.1093/jn/nxz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fu BC, Chowdhury-Paulino IM, Giovannucci EL, Mucci LA.. Prenatal and perinatal factors and risk of cancer in middle and older adulthood among men. Cancer Epidemiol Biomarkers Prev. 2021;30(10):1841-1845. doi: 10.1158/1055-9965.EPI-21-0316. [DOI] [PubMed] [Google Scholar]
- 6. Jensen BW, Aarestrup J, Blond K, et al. Childhood body mass index trajectories, adult-onset type 2 diabetes and obesity-related cancers. J Natl Cancer Inst. 2023;115(1):43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;314:1-27. [PubMed] [Google Scholar]
- 8. Jeffreys M, Davey Smith G, Martin RM, Frankel S, Gunnell D.. Childhood body mass index and later cancer risk: a 50-year follow-up of the Boyd Orr study. Int J Cancer. 2004;112(2):348-351. doi: 10.1002/ijc.20423. [DOI] [PubMed] [Google Scholar]
- 9. Levi Z, Kark JD, Barchana M, et al. Measured body mass index in adolescence and the incidence of colorectal cancer in a cohort of 1.1 million males. Cancer Epidemiol Biomarkers Prev. 2011;20(12):2524-2531. doi: 10.1158/1055-9965.EPI-11-0531. [DOI] [PubMed] [Google Scholar]
- 10. Zhang X, Wu K, Giovannucci EL, et al. Early life body fatness and risk of colorectal cancer in U.S. women and men--results from two large cohort studies. Cancer Epidemiol Biomarkers Preve. 2015;24(4):690-697. doi: 10.1158/1055-9965.EPI-14-0909-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Möller E, Adami HO, Mucci LA, et al. Lifetime body size and prostate cancer risk in a population-based case-control study in Sweden. Cancer Causes Control. 2013;24(12):2143-2155. doi: 10.1007/s10552-013-0291-0. [DOI] [PubMed] [Google Scholar]
- 12. Levi Z, Kark JD, Afek A, et al. Measured body mass index in adolescence and the incidence of pancreatic cancer in a cohort of 720,000 Jewish men. Cancer Causes Control. 2012;23(2):371-378. doi: 10.1007/s10552-011-9886-5 [DOI] [PubMed] [Google Scholar]
- 13. Levi Z, Kark JD, Shamiss A, et al. Body mass index and socioeconomic status measured in adolescence, country of origin, and the incidence of gastroesophageal adenocarcinoma in a cohort of 1 million men. Cancer. 2013;119(23):4086-4093. doi: 10.1002/cncr.28241. [DOI] [PubMed] [Google Scholar]
- 14. Leiba A, Kark JD, Afek A, et al. Adolescent obesity and paternal country of origin predict renal cell carcinoma: a cohort study of 1.1 million 16 to 19-year-old males. J Urol. 2013;189(1):25-29. doi: 10.1016/j.juro.2012.08.184. [DOI] [PubMed] [Google Scholar]
- 15. Rosner B, Eliassen AH, Toriola AT, et al. Weight and weight changes in early adulthood and later breast cancer risk. Int J Cancer. 2017;140(9):2003-2014. doi: 10.1002/ijc.30627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mattsson M, Maher GM, Boland F, Fitzgerald AP, Murray DM, Biesma R.. Group-based trajectory modelling for BMI trajectories in childhood: a systematic review. Obes Rev. 2019;20(7):998-1015. doi: 10.1111/obr.12842. [DOI] [PubMed] [Google Scholar]
- 17. Petersen R. Racial and ethnic disparities in adult obesity in the United States: CDC’s tracking to inform state and local action. Prev Chronic Dis. 2019;16:E46. doi: 10.5888/pcd16.180579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37:17-32. doi: 10.1146/annurev-publhealth-032315-021402. [DOI] [PubMed] [Google Scholar]
- 19. Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C.. Life course epidemiology. J Epidemiol Community Health. 2003;57(10):778-783. doi: 10.1136/jech.57.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mariosa D, Smith-Byrne K, Richardson TG, et al. Body size at different ages and risk of 6 cancers: a Mendelian randomization and prospective cohort study. J Natl Cancer Inst. 2022;114(9):1296-1300. doi: 10.1093/jnci/djac061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petrick JL, Jensen BW, Sørensen TIA, Cook MB, Baker JL.. Overweight patterns between childhood and early adulthood and esophageal and gastric cardia adenocarcinoma risk: linkage of Copenhagen schools health records register and Danish conscription database. Obesity (Silver Spring). 2019;27(9):1520-1526. doi: 10.1002/oby.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jensen BW, Bjerregaard LG, Ängquist L, et al. Change in weight status from childhood to early adulthood and late adulthood risk of colon cancer in men: a population-based cohort study. Int J Obes (Lond). 2018;42(10):1797-1803. doi: 10.1038/s41366-018-0109-y. [DOI] [PubMed] [Google Scholar]
- 23. Song M, Willett WC, Hu FB, et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138(10):2383-2395. doi: 10.1002/ijc.29981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention - CDC. CDC Health disparities and inequalities report—United States, 2011. MMWR Suppl. 2011;60(1):1-2. https://www.cdc.gov/mmwr/preview/ind2011_su.html#HealthDisparities2011. Accessed January 14, 2011. [PubMed] [Google Scholar]
- 25. Zavala VA, Bracci PM, Carethers JM, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021;124(2):315-332. doi: 10.1038/s41416-020-01038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davidson KW, Barry MJ, Mangione CM, et al. ; US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi: 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
- 27. Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250-281. doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- 28. Wang K, Ma W, Wu K, et al. Long-term colorectal cancer incidence and mortality after colonoscopy screening according to individuals’ risk profiles. J Natl Cancer Inst. 2021;113(9):1177-1185. doi: 10.1093/jnci/djab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were generated or analyzed for this editorial.