Abstract
Disparities in care, treatment-related toxicity and health-related quality of life (HRQoL) for adolescents and young adults (AYAs, aged 15-39 years) with cancer are under-addressed partly because of limited collection of patient-reported outcomes (PROs) in cancer clinical trials (CCTs). The AYA years include key developmental milestones distinct from younger and older patients, and cancer interrupts attainment of critical life goals. Lack of consensus on a standardized approach to assess HRQoL and treatment-related toxicity in AYA CCTs has limited the ability to improve patient outcomes. The National Cancer Institute’s Clinical Trials Network AYA PRO Task Force was assembled to reach consensus on a core set of PROs and foster its integration into AYA CCTs. Eight key considerations for selecting the core PRO AYA battery components were identified: relevance to AYAs; importance of constructs across the age continuum; prioritization of validated measures; availability of measures without licensing fees; availability in multiple languages; applicability to different cancer types and treatments; ability to measure different HRQoL domains and toxicities; and minimized burden on patients and sites. The Task Force used a modified Delphi approach to identify key components of the PRO battery. The Patient-Reported Outcomes Measurement Information System (PROMIS) and the PRO Common Terminology Criteria for Adverse Events Measurement System met all criteria and were selected to assess HRQoL and treatment toxicity, respectively. Investigators are rapidly incorporating the recommendations of the Task Force into AYA trials. Inclusion of a standardized assessment of HRQoL and treatment toxicities in AYA CCTs is a vital first step to develop interventions to improve health outcomes for AYAs diagnosed with cancer.
Every year approximately 90 000 adolescents and young adults (AYAs, aged 15-39 years) are diagnosed with cancer in the United States, and improvements in treatment have led to a 5-year overall survival of 85% (1). This large and growing population of survivors of AYA cancer warrants an increasing focus on patients’ quality of life during and after treatment. The AYA age range includes critically important developmental stages and milestones that occur during late adolescence, emerging adulthood, and young adulthood that are distinct from children and older adults (2). Some of these milestones include achieving and maintaining independence, establishing social and intimate relationships, starting families, and embarking on career paths (3). A cancer diagnosis in adolescence or young adulthood often presents unique challenges that hinder the achievement of important life goals (4,5). In addition, AYAs experience different types and degrees of treatment toxicity compared with younger and older cancer patients and often have a higher symptom burden during treatment compared with children (6).
Few studies have assessed the impact of a cancer diagnosis and treatment on AYA symptom burden and psychosocial and functional health outcomes. Documented barriers to the assessment of AYA health-related quality of life (HRQoL) have included 1) limited participation of AYAs on clinical trials; 2) few collaborative AYA cancer trials codeveloped by pediatric and medical oncology; 3) historical paucity of validated instruments spanning the AYA age range; 4) burden of collecting patient-reported data; and 5) limited investigator knowledge on how to best gather and analyze this information (2,7-9). However, in recent years, many of these barriers have been addressed. Several collaborative AYA trials are now active or in development within the National Cancer Institute (NCI) Clinical Trials Network (NCTN). Relevant and efficient patient-reported outcome (PRO) measures have been developed, such as the National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS), and electronic data capture is easing the burden of data collection and transfer (10–12). Despite this progress, only 1 in 5 cancer clinical trials focused on the AYA population includes an assessment of patient-reported symptom burden and/or HRQoL (13).
Currently, pediatric and medical oncologists and researchers lack consensus about which core domains of symptom burden and HRQoL should be assessed across AYA age groups, cancer types, and treatment approaches. The absence of a standardized approach to assess HRQoL in cancer clinical trials prevents the identification of AYAs at higher risk for poor HRQoL and hinders the development of interventions to improve short- and long-term health outcomes. Standardization and harmonization of PROs in AYA cancer trials provide opportunities to 1) identify sociodemographic and cancer-related factors associated with high risk for physical and/or psychosocial symptom burden (age, developmental life stage, sex, race and ethnicity, cancer type, treatment approach, treatment phase); 2) assess the timing and duration of distress (during treatment, early posttreatment, long-term survivorship); and 3) identify opportunities for intervention to mitigate patient distress and symptom burden and improve quality of life.
Assembly and charge of the NCTN AYA PRO Task Force
In 2020, the Children’s Oncology Group (COG) was awarded a Childhood Cancer Data Initiative NCI Community Oncology Research Program supplement grant to address the lack of inclusion of PROs in AYA cancer clinical trials. The primary objective was to bring together key stakeholders from across pediatric and medical oncology disciplines on a new NCTN AYA PRO Task Force (herein referred to as “the Task Force”) to come to consensus on a core set of PROs and their measures and guidelines to integrate into future NCTN and NCI Community Oncology Research Program AYA trials (studies that span the AYA age spectrum in cancer types relevant to the AYA population). The development of an AYA PRO core battery provides a key tool to assess HRQoL within and across AYA cancer clinical trials and increases our understanding of the impact of cancer and treatment on AYAs.
Three key stakeholder roles were identified and targeted for inclusion on the Task Force: 1) AYA oncology clinical trialists; 2) AYA symptom burden and HRQoL experts; and 3) PRO methodologists. A HRQoL framework proposed in AYA survivors of childhood cancer was used and expanded on to identify HRQoL domains relevant and important to AYAs and identify corresponding experts (14). These domains included physical function, fatigue, social function, psychological function, spirituality, body image, fertility, resilience, sexual function, financial toxicity, and treatment toxicity. The NCTN includes 4 adult oncology network groups (Alliance, Eastern Cooperative Oncology Group and American College of Radiology Imaging Network (ECOG-ACRIN), NRG Oncology, and the Southwest Oncology Group (SWOG) and 1 pediatric oncology network group (COG). To maximize the utility and acceptance of the Task Force’s recommendations, it was essential that all network groups were represented.
Seventeen investigators were invited to serve on the Task Force and all accepted. Each NCTN group had a minimum of 2 representatives, and the key stakeholder roles were included as follows: AYA oncology clinical trialists (n = 6), AYA symptom burden and HRQoL experts (n = 13) and PRO methodologists (n = 6). Of note, multiple Task Force members had expertise in more than 1 stakeholder role. In addition, 1 Task Force member was also an AYA cancer survivor and provided perspective from the patient point of view. The Task Force was charged with 1) coming to consensus on a core AYA PRO battery; 2) recommending key time points for assessment; and 3) fostering the core battery’s inclusion in NCTN AYA trials. Between November 2020 and November 2021, the Task Force held 10 virtual meetings, which were audio and video recorded. Their consensus recommendations are described below.
Achieving consensus on an AYA PRO battery
The Task Force first set out to identify key principles for selecting the structure and components of the core PRO AYA battery. The core battery refers to a set of recommended measures to assess HRQoL in AYA cancer patients. Stakeholders reviewed published recommendations on measure selection, and 8 key considerations were selected and agreed on by all members and are shown in Box 1 (11,15). The Task Force noted the battery needed to focus on those areas of health and HRQoL most important to AYAs diagnosed with cancer. Previously validated PRO measures that could be used across the 3 main developmental stages of AYAs (adolescents, emerging adults, and young adults) should be prioritized. The included PRO measures needed to be available and accessible to all AYAs and investigators, available at minimal or no cost, and translated into multiple languages. Measures chosen for the battery needed to be flexible and adaptable, to assess HRQoL domains and toxicities relevant to different studies, different cancers, and different treatment approaches. AYA cancer trials include a diverse group of cancer types and study designs, and the battery, with few modifications, should be able to be included in all relevant AYA trials. Finally, patient and investigator site burden must be minimized without compromising the quality and breadth of the data collected.
Box 1. Adolescent and young adult (AYA) patient-reported outcome core battery selection key considerations.
Health-related quality of life (HRQoL) domains relevant to AYAs
Applicability of the HRQoL construct across the age continuum
Self-report HRQoL measures previously validated, ideally in AYAs across developmental stages
Availability of the HRQoL measure in public domain
Translations and linguistic validation of HRQoL measures in multiple languages
Adaptability of the HRQoL measures to different cancer types and treatment approaches
Flexibility of the HRQoL measures to assess specific HRQoL domains and toxicities relevant to the study
Administration of the HRQoL measures has minimal burden on patients and sites
After the Task Force reached consensus on the 8 guiding principles, a modified Delphi approach was used to identify and select the components of the core AYA PRO battery, including the domains to assess, specific HRQoL measures, and assessment time points. Each meeting Task Force member presented ideas for the battery structure, and revisions were made at subsequent meetings based on stakeholder recommendations. Building from the work of measurement scientists in AYA oncology, the Task Force reviewed measures and measurement frameworks that 1) were embraced by federal health research organizations (eg, NIH, Food and Drug Administration); 2) were embraced by the research community; 3) had strong validation in cancer populations; and 4) met all criteria in the guiding principles. Measures that were available without royalties or other associated fees and had translations available were prioritized to ensure the broadest reach. Task Force members were familiar with the available, relevant measures and measurement frameworks, and thus an additional systematic review of available PRO measures was not conducted (11). Consensus was reached when no further concerns were identified, and all Task Force members agreed on the structure and components of the battery. Selection of the final battery was unanimous.
Recommended AYA PRO core battery
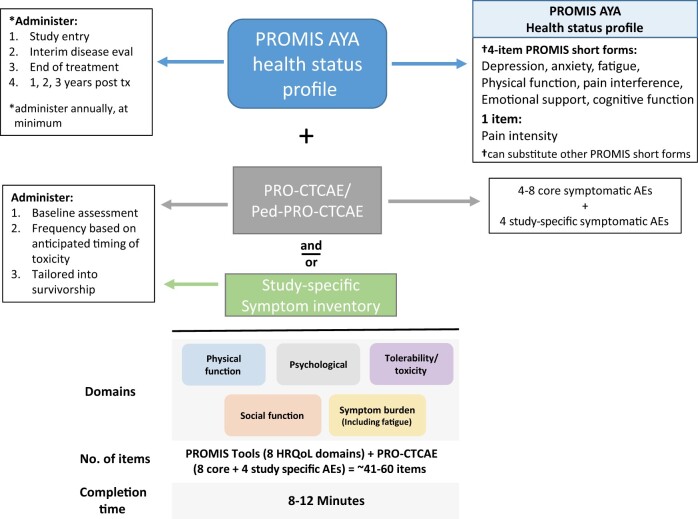
Early in the process, the Task Force identified the 2 main components of the battery: 1) assessment of HRQoL domains and 2) assessment of treatment tolerability and toxicity (Figure 1). The following describes the final recommendations for the AYA PRO Core Battery, including the rationale for the inventories selected and the assessment time points suggested.
Figure 1.
AYA PRO core battery. The AYA PRO core battery includes assessment of HRQoL via the PROMIS Health Status Profile and treatment tolerability and toxicity via the PRO-CTCAE and Ped-PRO-CTCAE and/or another study-specific symptom inventory. The core battery should be adapted to meet the needs of each study question. *Administer annually, at minimum. †Can substitute other PROMIS short forms. AE = adverse events; AYA = adolescent and young adult; eval = evaluation; HRQoL = health-related quality of life; Ped-PRO-CTCAE = Pediatric Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events; PRO = patient-reported outcome; PRO-CTCAE = Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events; PROMIS = Patient-Reported Outcomes Measurement Information System; tx = treatment.
Assessment of HRQoL
HRQoL domains most relevant and important to the AYA cancer population were prioritized for inclusion in the core battery (16). The Task Force reviewed prior studies that identified patient-reported high-priority HRQoL domains. In an observational study of developmentally diverse AYA patients and survivors, the most important HRQOL domains identified by participants were physical function, pain, cognitive function, social support, and finances (17). In a recent study by Siembida et al. (18), AYAs ranked HRQOL domains in order of importance. The authors noted the importance of individual HRQOL domains varied based on AYA treatment status (on vs off) and developmental stage. For example, pain was more frequently identified as a priority domain for AYAs undergoing treatment compared with those who had completed treatment, and depression was more commonly ranked as a priority domain for young adults who completed treatment compared with emerging adults and adolescents who completed treatment. The Task Force reached consensus on the domains to be included in the core battery, and they were captured in a recent systematic review by Sodergren et al. (19) and include 1) physical well-being, including pain and fatigue; 2) cognitive functioning; 3) relationships and social functioning; and 4) emotional well-being, including fear, worry, and depression. It was essential that the core battery had the capacity to efficiently measure each of these key domains.
The NIH’s PROMIS was developed to provide researchers with access to publicly available PRO measures that have strong evidence for their validity and reliability across a broad range of diseases and conditions (20-22). PROMIS instruments assess a wide range of HRQoL domains, including depression, anxiety, fatigue, cognition, pain, and social and physical functioning, and have been validated in multiple cancer populations (11,23-27). Importantly, PROMIS instruments assess the majority of the HRQoL domains identified as most important by AYAs diagnosed with and treated for cancer and have both pediatric (aged 8-17 years) and adult versions of the measures (11). Although the relative importance of these domains differs for late adolescents, emerging adults, and young adults, PROMIS has been validated across the AYA age and developmental stage spectrum and a diverse population of patients (2,28-31). Linking metrics between pediatric and adult forms have been estimated for many of the PROMIS HRQoL domains, including physical functioning, pain interference, fatigue, depression, anxiety, and anger (32,33). PROMIS content is copyrighted, and permission is required to use the measures to ensure appropriate application, however, the content is widely available and easily accessible without royalties. The English versions of all PROMIS measures are free, and distribution fees for translated versions of PROMIS measures are waived in nonindustry-sponsored academic studies (34,35). PROMIS measures should be accessed directly from the PROMIS website (https://www.healthmeasures.net/search-view-measures) as they are occasionally updated. All PROMIS instruments have short-form versions that have been validated as standalone measures, and many offer computer adaptive testing–based assessment that tailors the PRO measure to each individual to allow for more efficient measurement (36–38). Electronic delivery of PROMIS tools has been used in prior cancer trials (39). PROMIS met all 8 guiding principles for assessment of HRQoL in the core AYA PRO battery.
The AYA PRO core battery assesses relevant HRQoL domains via the newly designated PROMIS AYA Health Status Profile. Figure 1 provides one example of this profile, which consists of multiple standalone PROMIS short-form measures that are selected based on the HRQoL domains important to AYAs and that are likely to be impacted during and after cancer treatment. The profile is flexible and adaptable, as investigators can swap in or out specific lengths of short-form measures (for example, using an 8-item PROMIS fatigue measure instead of the 4-item version because fatigue is a secondary outcome in a study) to meet their study objectives. For HRQoL domains that are being used as primary or secondary PRO endpoints, the Task Force recommends using longer short forms of PROMIS measures to enhance the reliability and content validity of the measure. To minimize patient burden, the Task Force recommends the total time for completion of all patient-reported measures not exceed 15-30 minutes in 1 assessment.
The trajectory of HRQoL in AYAs with cancer has not been well reported and prior research suggests that many HRQoL domains can remain impaired or worsen long after initial diagnosis (40-42). The Task Force recommends administration of the PROMIS AYA Health Status Profile at study entry, intermittently throughout treatment, at the end of treatment, and annually, when possible, through at least the first 3-5 years of posttreatment survivorship.
Assessment of treatment tolerability and toxicity
The NCI’s PRO version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) was developed to standardize the self-report assessment of treatment-related tolerability and toxicity-associated symptom burden in patients with cancer (43,44). The PRO-CTCAE’s item library was designed to mirror the provider-reported CTCAE and includes items to assess 78 symptomatic adverse events (AEs) in adults and 62 symptomatic AEs in children and adolescents with cancer. The pediatric version of the PRO-CTCAE (Ped-PRO-CTCAE) is available for use in patients aged between 7 and 17 years, thus the PRO-CTCAE system can be used across the full AYA age spectrum (45-47). A caregiver version is also available for patients who are unable to self-report. The PRO-CTCAE measurement system is freely available in the public domain (https://healthcaredelivery.cancer.gov/pro-ctcae/) and has been translated into multiple languages (48,49). The flexible PRO-CTCAE measurement system allows investigators to select only the subset of symptomatic AEs to assess for a study based on anticipated toxicities, allowing for brief, efficient assessment of toxicity-related symptom burden. Electronic symptom monitoring utilizing the adult and/or the pediatric versions of the PRO-CTCAE has been effectively used in clinical trials (50,51).
The AYA PRO core battery assesses toxicity-related symptom burden via use of targeted items from the PRO-CTCAE and/or use of a study-specific symptom inventory as shown in Figure 1. For patients aged younger than 18 years, corresponding items from the Ped-PRO-CTCAE are recommended in place of the adult version of PRO-CTCAE. In addition to including approximately 4 study-specific symptomatic AEs, the Task Force recommends utilizing 4-8 additional symptomatic AEs that are commonly experienced by AYAs across disease groups and trials, such as nausea, diarrhea, pain, and fatigue. This will allow for direct comparison of symptom toxicities across the AYA cancer population. When targeted toxicities are expected, study-specific treatment toxicity measures are recommended alone or in combination with the PRO-CTCAE, such as peripheral neuropathy. In addition to the item libraries offered by the PRO-CTCAE and the Ped-PRO-CTCAE, the European Organization for Research and Treatment of Cancer and the Functional Assessment of Cancer Therapy “family of instruments” offer validated symptom scales (52,53). These scales are of varying length and have been used across cancer types and treatments. The approach to symptom toxicity assessment with the PRO-CTCAE is flexible and adaptable as investigators can substitute specific items based on anticipated toxicities and those that are most burdensome to AYAs.
Whereas many toxicities, such as nausea and vomiting, are self-limited during therapy, other toxicities, such as neuropathy, fatigue, and problems with concentration, can persist long after treatment has concluded. To date, however, few studies have reported the duration and intensity of toxicities in AYAs (54,55). The Task Force recommends assessing symptomatic AEs at study baseline, throughout treatment especially when toxicity is anticipated, and routinely through posttreatment survivorship and to strongly consider aligning assessment of PRO-CTCAE AEs with clinician-reported AEs, as prior studies report limited concordance between patient- and provider-reported toxicity burden (56,57).
Inventory delivery and data capture
The NCTN AYA PRO Task Force reviewed the current state of PRO measure delivery within the NCTN and provided recommendations for delivery of the AYA PRO core battery. Currently, most NCTN trials are utilizing paper-and-pencil administration of PRO measures, which is inefficient and burdensome for sites and patients, requires multiple steps for data transfer, and can lead to human error in data entry and transfer (58). AYAs are a tech-savvy population, comfortable with handheld devices, text messaging, and email (59). Electronic delivery, capture, and transfer of PRO data has been shown to minimize missing data and be efficient, effective, and accessible and is preferred by many patients and researchers. The Task Force agreed that although barriers exist to the widespread adoption of electronic PRO (ePRO) assessment within the NCTN, when possible, ePROs should be used for AYA trials, with a backup option for paper-and-pencil surveys. Investigators should also consider using computer adaptive tests (available from PROMIS), which reduce patient burden without sacrificing measurement precision. Network groups will need to develop mechanisms to use and scale electronic capture in their trials. The ECOG-ACRIN System for Easy Entry of PROs has been developed to meet this need, and the ePRO capture and transfer system has been used across multiple trials with high success. Other groups, including NRG, have tested the use of VisionTree Optimal Care (VisionTree, San Diego, CA, USA) in their trials, which provides a similar approach to electronic data capture.
NCTN AYA trial inclusion and adaptation of the core PRO battery
The Task Force developed a multipronged approach to disseminate the battery and foster its integration into AYA cross-network trials. To date, members have presented the AYA PRO battery in more than 20 meetings and fora, including both pediatric and adult oncology NCTN group meetings, on COG Responsible Investigator webinars, in a NCI blog post, and in meetings with NCI Division of Cancer Prevention leadership and patient advocates (60). Task Force members have also been charged with additional targeted dissemination of the AYA PRO battery within their respective network groups such as within the Alliance, NRG, SWOG, AYA, and outcomes research committees and working groups. In addition, the Task Force developed a frequently asked questions (FAQs) document to address common questions about the AYA PRO battery (see the Supplementary Material, available online). The FAQs address topics including the rationale for the battery, information on how it was developed, and recommendations about how to integrate it into future AYA trials. Additional input and guidance from AYA cancer patients will be important to help identify key HRQoL study questions, as well as help optimize PRO delivery and response rates. AYA study committees and disease-specific committees include patient advocates, and they will serve an important role in adapting recommendations from the Task Force for individual studies.
There are currently 5 planned NCTN cross-network AYA trials that are utilizing the battery for key HRQoL and/or toxicity endpoints. These include osteosarcoma studies, as well as studies in acute lymphoblastic leukemia, Hodgkin lymphoma, and non-Hodgkin lymphoma. AOST2031 is a phase 3 trial (https://clinicaltrials.gov/ct2/show/NCT05235165) comparing open vs thoracoscopic management of pulmonary metastasis in children, adolescents, and adults with osteosarcoma and will be the first trial to adapt and include the battery. The following is an example of how the battery has been easily adapted to meet the needs of that trial. As a first step, the AYA PRO core battery was adapted to include 2 slightly longer PROMIS short forms, assessing pain interference (8 items) and upper extremity functioning (8 items for pediatric patients and 7 items for adult patients), given the hypothesized effect of the randomized surgical approaches on these domains. These were followed by other PROMIS 4-item short forms, designed to characterize the participant’s HRQoL over time. Certified translations in Spanish and French of each of the short forms were obtained from HealthMeasures.net of the PROMIS Health Organization as part of the written use agreement. Next, the key symptom to be monitored serially was pain intensity, using the PROMIS pain intensity single item, contained in the core battery. Finally, study time points and assessment windows were designed to reflect the intended treatment and anticipated recovery over the 4- to 6-week study period.
Evaluation and optimization of the core battery
Achieving consensus on a core PRO battery for AYA cancer trials is the first step in assessing and improving HRQoL for AYAs diagnosed with cancer, however, there are limitations in our process, and the utility of the battery needs to be systematically evaluated. The Task Force would have benefited from the inclusion of more AYA patient advocates with differing cancer diagnoses and treatments and diverse sociodemographic backgrounds. In addition, increased racial and ethnic diversity of other stakeholders on the Task Force would have added valuable perspectives to address disparities faced by underserved AYA cancer patients and survivors. A variety of metrics will be assessed to evaluate the uptake and utility of the core battery and improve it. The number and diversity of AYA trials including the battery is being assessed, as well as how the battery is being adopted within each trial. Given the inherent flexibility in the measures included in the battery, it will be important to determine whether studies use the recommended core set of measures or make additional changes to meet individual study needs. If domains are consistently included or excluded from trials, updates will be made to the AYA Health Status Profile. Additional metrics relevant to the use of electronic data capture will be assessed, including the mode of survey delivery (email, text message, paper), response rates, number of reminders, missing item responses, and patient and site burden.
Future directions
The AYA PRO core battery provides investigators with a new tool to efficiently assess the impact of a cancer diagnosis and treatment on AYAs’ HRQoL and symptom toxicities across cancer subtypes and clinical trial designs. The battery directly addresses the historical challenges that have limited the assessment of AYA PROs by offering a rational, standardized measurement approach that minimizes the burden of data collection for patients and research sites. The rapid incorporation of the battery into multiple AYA cross-network trials highlights its flexibility and generalizability across disease groups and its acceptability by AYA clinical trialists.
The NCTN AYA PRO Task Force continues to work with investigators across the NCTN to help adapt the battery to their specific study needs. We will study the implementation and utilization of the core battery in AYA trials to refine and optimize its use in future trials. In addition, the Task Force is developing an online AYA PRO resource guide that will house the AYA PRO core battery and FAQs, provide additional relevant AYA PRO measurement tools, list NCTN AYA trials that utilize the battery, share AYA PRO study tools and templates, and enumerate key unanswered questions on AYA HRQoL and symptom burden. The inclusion of a standardized assessment of HRQoL and symptom toxicities is the first step toward the development of interventions aimed at improving short- and long-term outcomes for AYAs with cancer.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health [grant number UG1 CA189955] (MR and SP).
Notes
Role of the funder: The funders had no role in the design of the study, conduct of the study, analysis, interpretation of data, or decision to submit the manuscript for publication.
Disclosures: The authors have no conflicts of interest. PAG, the JNCI Editor-in-Chief and a co-author on this commentary, was not involved in the editorial review or decision to publish this manuscript.
Author contributions: Conceptualization, methodology, writing—original draft, writing—review & editing: all authors.
Supplementary Material
Contributor Information
Michael E Roth, Division of Pediatrics and Patient Care, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Susan K Parsons, Department of Medicine, Division of Hematology/Oncology, Tufts Medical Center, and the Tufts University School of Medicine, Boston, MA, USA.
Patricia A Ganz, Department of Medicine, Division of Hematology Oncology, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
Lynne I Wagner, Department of Social Sciences and Health Policy, Wake Forest School of Medicine and the Wake Forest Baptist Comprehensive Cancer Center, Winston Salem, NC, USA.
Pamela S Hinds, Department of Nursing Science, Children’s National Hospital, George Washington University School of Medicine, Washington, DC, USA.
Sarah Alexander, Division of Haematology/Oncology, The Hospital for Sick Children, Department of Pediatrics, University of Toronto, Toronto, ON, Canada.
Kristin Bingen, Division of Pediatric Psychology and Developmental Medicine, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, USA.
Sharon L Bober, Department of Psychosocial Oncology and Palliative Care, Dana-Farber Cancer Institute and Harvard Medical School, Boston, MA, USA.
Julienne Brackett, Pediatric Hematology-Oncology, Department of Pediatrics, Texas Children’s Hospital, Houston, TX, USA.
David Cella, Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL, USA.
N Lynn Henry, Division of Hematology/Oncology, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA.
Daniel J Indelicato, Department of Radiation Oncology, University of Florida, Jacksonville, FL, USA.
Rebecca H Johnson, Division of Pediatric Hematology/Oncology, Mary Bridge Children’s Hospital, MultiCare Health System, Tacoma, WA, USA.
Tamara P Miller, Aflac Cancer and Blood Disorders Center, Department of Pediatrics, Emory University, Atlanta, GA, USA.
Shoshana M Rosenberg, Department of Population Health Sciences, Weill Cornell Medicine, New York, NY, USA.
Kathryn H Schmitz, Public Health Sciences, Penn State College of Medicine, Hershey, PA, USA.
Gita Thanarajasingam, Division of Haematology, Mayo Clinic, Rochester, MN, USA.
Bryce B Reeve, Department of Population Health Sciences, Department of Pediatrics, Duke University School of Medicine, Durham, NC, USA.
John M Salsman, Department of Social Sciences and Health Policy, Wake Forest School of Medicine and the Wake Forest Baptist Comprehensive Cancer Center, Winston Salem, NC, USA.
Data availability
All data generated in this initiative will be made freely available in the public domain.
References
- 1. Miller KD, Fidler‐Benaoudia M, Keegan TH, et al. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70(6):443-459. [DOI] [PubMed] [Google Scholar]
- 2. Siembida EJ, Reeve BB, Zebrack BJ, et al. Measuring health‐related quality of life in adolescent and young adult cancer survivors with the National Institutes of Health Patient‐Reported Outcomes Measurement Information System®: comparing adolescent, emerging adult, and young adult survivor perspectives. Psychooncology. 2021;30(3):303-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tanner JL, Arnett JJ.. The emergence of emerging adulthood: the new life stage between adolescence and young adulthood. In: Furlong A, ed. Routledge Handbook of Youth and Young Adulthood. London: Routledge; 2016;50-56. [Google Scholar]
- 4. Smith AW, Keegan T, Hamilton A, et al. ; for the AYA HOPE Study Collaborative Group. Understanding care and outcomes in adolescents and young adults with cancer: a review of the AYA HOPE study. Pediatr Blood Cancer. 2019;66(1):e27486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salsman JM, Bingen K, Barr RD, et al. Understanding, measuring, and addressing the financial impact of cancer on adolescents and young adults. Pediatr Blood Cancer. 2019;66(7):e27660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bukowinski AJ, Burns KC, Parsons K, et al. Toxicity of cancer therapy in adolescents and young adults (AYAs). Semin Oncol Nurs. 2015;31(3):216-226. [DOI] [PubMed] [Google Scholar]
- 7. Siembida EJ, Loomans‐Kropp HA, Trivedi N, et al. Systematic review of barriers and facilitators to clinical trial enrollment among adolescents and young adults with cancer: identifying opportunities for intervention. Cancer. 2020;126(5):949-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albers LF, Bergsma FB, Mekelenkamp H, et al. Discussing sexual health with adolescent and young adults with cancer: a qualitative study among healthcare providers. J Canc Educ. 2022;37(1):133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith AW, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: an update on progress and recommendations for the future. Cancer. 2016;122(7):988-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weiss AR, Nichols CR, Freyer DR.. Enhancing adolescent and young adult oncology research within the National Clinical Trials Network: rationale, progress, and emerging strategies. Semin Oncol. 2015;42(5):740-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salsman JM, Danhauer SC, Moore JB, et al. Optimizing the measurement of health‐related quality of life in adolescents and young adults with cancer. Cancer. 2020;126(22):4818-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bennett AV, Jensen RE, Basch E.. Electronic patient‐reported outcome systems in oncology clinical practice. CA Cancer J Clin. 2012;62(5):336-347. [DOI] [PubMed] [Google Scholar]
- 13. Berkman AM, Murphy KM, Siembida EJ, et al. Inclusion of patient-reported outcomes in adolescent and young adult phase III therapeutic trials: an analysis of cancer clinical trials registered on ClinicalTrials.gov. Value Health. 2021;24(12):1820-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nightingale CL, Quinn GP, Shenkman EA, et al. Health-related quality of life of young adult survivors of childhood cancer: a review of qualitative studies. J Adolesc Young Adult Oncol. 2011;1(3):124-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aaronson N, Alonso J, Burnam A, et al. Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res. 2002;11(3):193-205. [DOI] [PubMed] [Google Scholar]
- 16. Husson O, Reeve BB, Darlington A-S, et al. Next step for global adolescent and young adult oncology: a core patient-centered outcome set. J Natl Cancer Inst. 2022;114(4):496-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salsman JM, Snyder MA, Zebrack B, et al. Measuring quality of life in adolescents and young adults (AYAS) with cancer: a promising solution? Ann Behav Med. 2016;50:S67. [Google Scholar]
- 18. Siembida ER, Zebrack B, Roth M, Murphy K, Snyder MA, Salsman JM.. Health-related quality of life in adolescent and young adult cancer survivors: measuring what matters most? In: Annual Meeting of the International Society for Quality of Life Research; Prague, Czech Republic; October 2022. [Google Scholar]
- 19. Sodergren SC, Husson O, Robinson J, et al. ; for the EORTC Quality of Life Group. Systematic review of the health-related quality of life issues facing adolescents and young adults with cancer. Qual Life Res. 2017;26(7):1659-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeWalt DA, Rothrock N, Yount S, et al. ; for the PROMIS Cooperative Group. Evaluation of item candidates: the PROMIS qualitative item review. Med Care. 2007;45(5 suppl 1):S12-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reeve BB, Hays RD, Bjorner JB, et al. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care. 2007;45(5):S22-S31. [DOI] [PubMed] [Google Scholar]
- 22. Garcia SF, Cella D, Clauser SB, et al. Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. J Clin Oncol. 2007;25(32):5106-5112. [DOI] [PubMed] [Google Scholar]
- 23. Pilkonis PA, Choi SW, Reise SP, et al. ; for the PROMIS Cooperative Group. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18(3):263-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hahn EA, DeVellis RF, Bode RK, et al. ; for the PROMIS Cooperative Group. Measuring social health in the patient-reported outcomes measurement information system (PROMIS): item bank development and testing. Qual Life Res. 2010;19(7):1035-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150(1):173-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS™ sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2011;10(1):6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weinfurt KP, Lin L, Bruner DW, et al. Development and initial validation of the PROMIS® sexual function and satisfaction measures version 2.0. J Sex Med. 2015;12(9):1961-1974. [DOI] [PubMed] [Google Scholar]
- 28. Hinds PS, Nuss SL, Ruccione KS, et al. PROMIS pediatric measures in pediatric oncology: valid and clinically feasible indicators of patient‐reported outcomes. Pediatr Blood Cancer. 2013;60(3):402-408. [DOI] [PubMed] [Google Scholar]
- 29. Reeve BB, McFatrich M, Mack JW, et al. Expanding construct validity of established and new PROMIS pediatric measures for children and adolescents receiving cancer treatment. Pediatr Blood Cancer. 2020;67(4):e28160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hinds PS, Wang J, Cheng YI, et al. PROMIS pediatric measures validated in a longitudinal study design in pediatric oncology. Pediatr Blood Cancer. 2019;66(5):e27606. [DOI] [PubMed] [Google Scholar]
- 31. Jensen RE, Potosky AL, Reeve BB, et al. Validation of the PROMIS physical function measures in a diverse US population-based cohort of cancer patients. Qual Life Res. 2015;24(10):2333-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reeve BB, Thissen D, DeWalt DA, et al. Linkage between the PROMIS® pediatric and adult emotional distress measures. Qual Life Res. 2016;25(4):823-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tulsky DS, Kisala PA, Boulton AJ, et al. Determining a transitional scoring link between PROMIS® pediatric and adult physical health measures. Qual Life Res. 2019;28(5):1217-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Terwee C, Roorda L, De Vet H, et al. Dutch-Flemish translation of 17 item banks from the patient-reported outcomes measurement information system (PROMIS). Qual Life Res. 2014;23(6):1733-1741. [DOI] [PubMed] [Google Scholar]
- 35. Devine J, Klasen F, Moon J, et al. Translation and cross-cultural adaptation of eight pediatric PROMIS® item banks into Spanish and German. Qual Life Res. 2018;27(9):2415-2430. [DOI] [PubMed] [Google Scholar]
- 36. Cella D, Choi SW, Condon DM, et al. PROMIS® adult health profiles: efficient short-form measures of seven health domains. Value Health. 2019;22(5):537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Papuga MO, Dasilva C, McIntyre A, et al. Large-scale clinical implementation of PROMIS computer adaptive testing with direct incorporation into the electronic medical record. Health Syst. 2018;7(1):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salsman JM, Schalet BD, Park CL, et al. Assessing meaning & purpose in life: development and validation of an item bank and short forms for the NIH PROMIS®. Qual Life Res. 2020;29(8):2299-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tran TXM, Park J, Lee J, et al. Utility of the Patient-Reported Outcomes Measurement Information System (PROMIS) to measure primary health outcomes in cancer patients: a systematic review. Support Care Cancer. 2021;29(4):1723-1739. [DOI] [PubMed] [Google Scholar]
- 40. Quinn GP, Gonçalves V, Sehovic I, et al. Quality of life in adolescent and young adult cancer patients: a systematic review of the literature. Patient Relat Outcome Meas. 2015;6:19-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith AW, Bellizzi KM, Keegan TH, et al. Health-related quality of life of adolescent and young adult patients with cancer in the United States: the Adolescent and Young Adult Health Outcomes and Patient Experience study. J Clin Oncol. 2013;31(17):2136-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kaul S, Avila JC, Mutambudzi M, et al. Mental distress and health care use among survivors of adolescent and young adult cancer: a cross‐sectional analysis of the National Health Interview Survey. Cancer. 2017;123(5):869-878. [DOI] [PubMed] [Google Scholar]
- 43. Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106(9):dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reeve BB, McFatrich M, Mack JW, et al. Validity and reliability of the pediatric patient-reported outcomes version of the common terminology criteria for adverse events. J Natl Cancer Inst. 2020;112(11):1143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reeve BB, McFatrich M, Lin L, et al. Validation of the caregiver pediatric patient‐reported outcomes version of the common terminology criteria for adverse events measure. Cancer. 2021;127(9):1483-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hinds PS, Pinheiro LC, McFatrich M, et al. Recommended scoring approach for the pediatric patient‐reported outcomes version of the Common Terminology Criteria for Adverse Events. Pediatr Blood Cancer. 2021;69(6):e29452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arnold B, Mitchell SA, Lent L, et al. ; for the PRO-CTCAE Spanish Translation and Linguistic Validation Study Group. Linguistic validation of the Spanish version of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Support Care Cancer. 2016;24(7):2843-2851. [DOI] [PubMed] [Google Scholar]
- 49. Miyaji T, Iioka Y, Kuroda Y, et al. Japanese translation and linguistic validation of the US National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). J Patient Rep Outcomes. 2017;1(1):8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Basch E, Stover AM, Schrag D, et al. Clinical utility and user perceptions of a digital system for electronic patient-reported symptom monitoring during routine cancer care: findings from the PRO-TECT trial. J Clin Oncol Clin Cancer Inform. 2020;4:947-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leahy AB, Schwartz LA, Li Y, et al. Electronic symptom monitoring in pediatric patients hospitalized for chemotherapy. Cancer. 2021;127(16):2980-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Webster K, Cella D, Yost K.. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fayers P, Bottomley A, EORTC Quality of Life Group.. Quality of life research within the EORTC—the EORTC QLQ-C30. Eur J Cancer. 2002;38(suppl 4):125-133. [DOI] [PubMed] [Google Scholar]
- 54. Baker KS, Syrjala KL.. Long-term complications in adolescent and young adult leukemia survivors. Hematol Am Soc Hematol Educ Program. 2018;2018(1):146-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Childhood Cancers and Disability, Aiuppa L, Cartaxo T, Spicer CM, Volberding PA, eds. Childhood Cancer and Functional Impacts Across the Care Continuum. Washington, DC: National Academies Press; 2020. [PubMed]
- 56. Atkinson TM, Ryan SJ, Bennett AV, et al. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24(8):3669-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Freyer DR, Lin L, Mack JW, et al. Lack of concordance in symptomatic adverse event reporting by children, clinicians, and caregivers: implications for cancer clinical trials. J Clin Oncol. 2022;40(15):1623-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Judson TJ, Bennett AV, Rogak LJ, et al. Feasibility of long-term patient self-reporting of toxicities from home via the Internet during routine chemotherapy. J Clin Oncol. 2013;31(20):2580-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Silver L. Smartphone ownership is growing rapidly around the world, but not always equally. Pew Research Center; 2019. https://www.pewresearch.org/global/2019/02/05/smartphone-ownership-is-growing-rapidly-around-the-world-but-not-always-equally/. Accessed March 15, 2022.
- 60. National Cancer Institute Staff. New task force focuses on quality of life for AYAs with cancer. current cancer blog. August 4, 2021. https://www.cancer.gov/news-events/cancer-currents-blog/2021/aya-cancer-patient-reported-quality-of-life. Accessed March 15, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated in this initiative will be made freely available in the public domain.