Abstract
Background
Elevated childhood body mass index (BMI), commonly examined as a “once-only” value, increases the risk of cancer and type 2 diabetes (T2D) in adulthood. Continuous exposure to adiposity during childhood may further increase cancer risk. We examined whether longitudinal childhood BMI trajectories were associated with adult obesity-related cancer and the role of adult-onset T2D in these associations.
Methods
Five sex-specific latent class BMI trajectories were generated for 301 927 children (149 325 girls) aged 6-15 years from the Copenhagen School Health Records Register. Information on obesity-related cancers and T2D was obtained from national health registers. Incidence rate ratios (IRR), cumulative incidences, and confidence intervals (CI) were estimated using Poisson regressions.
Results
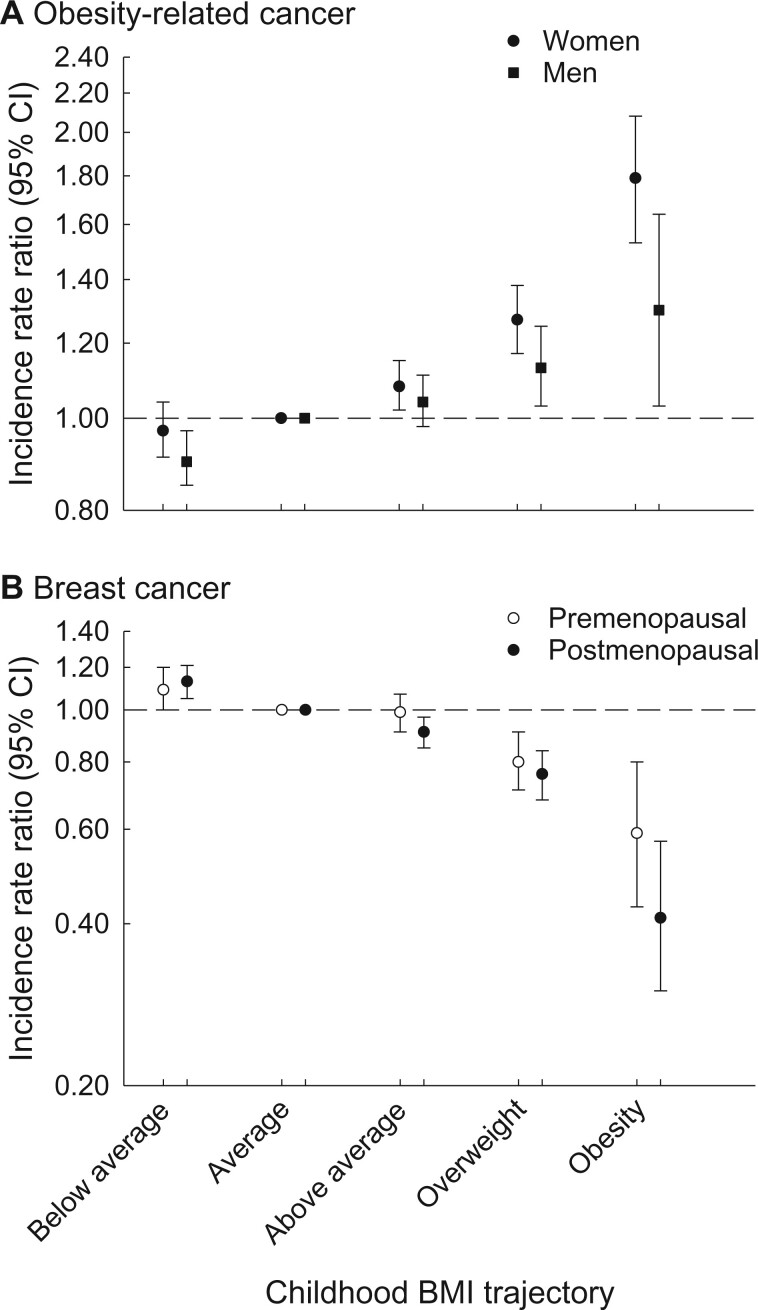
Compared with the average childhood BMI trajectory (containing approximately 40% of individuals), the rate of obesity-related cancer (excluding breast cancer) increased with higher childhood BMI trajectories among women. The highest rates occurred in the overweight (IRR = 1.27, 95% CI = 1.17 to 1.38) and obesity (IRR = 1.79, 95% CI = 1.53 to 2.08) BMI trajectories. Similar patterns were observed among men. In contrast, women with the obesity childhood BMI trajectory had the lowest rate of pre- and postmenopausal breast cancer (IRR = 0.59, 95% CI = 0.43 to 0.80, and IRR = 0.41, 95% CI = 0.30 to 0.57, respectively). For all trajectories, the cumulative risk of obesity-related cancer increased with adult-onset T2D.
Conclusion
Consistent childhood overweight or obesity may increase the rates of adult obesity-related cancer and decrease the rates of breast cancer. Adult-onset T2D conferred additional risk for obesity-related cancer, but the effect did not differ across childhood BMI trajectories.
Excess adult weight, commonly approximated by body mass index (BMI), is associated with increased risks of 13 obesity-related adult cancers (postmenopausal breast, colon and rectum, corpus uteri, esophagus, gallbladder, gastric cardia, kidney, liver, meningioma, multiple myeloma, ovary, pancreas, and thyroid) as identified by the International Agency for Research on Cancer (1). Risks of several of these cancers may be enhanced by type 2 diabetes (T2D) (2-5), potentially independent of adult BMI (6). A limitation of data underpinning the relationship between BMI and cancer, however, has been the use of “once-only” BMI values (7) that are snapshots of BMI and do not capture its development across time.
The cumulative exposure to excess adiposity beginning in childhood is relevant for subsequent cancer risk (8). Mathematical modeling suggests that it takes decades from an initial stem cell mutation to the clinical manifestation of a cancer (9). As shown by us and others, a high BMI at single childhood ages is associated with increased risks of numerous adult cancer forms (10-12). Further supporting the role of child BMI in cancer risk, correlations between child and adult BMI at ages when cancer is often diagnosed are low (13,14). However, relating trajectories of BMI development during childhood to adult cancer may capture BMI patterns that more accurately identify later cancer risk. Apart from 1 study, which examined associations between childhood BMI trajectories and cancer mortality (15), other studies examined noncancer outcomes (16,17) or used recalled child body size only at 2 ages (18-20). Furthermore, although a high BMI in childhood is associated with T2D (21), no studies have investigated the possible influence of T2D on the associations between childhood BMI trajectories and cancer risk.
As 1 in 4 obesity-related cancers can be ascribed to high adult BMI levels (22), and an estimated 337 million children and adolescents worldwide have overweight or obesity (23), identifying early indicators of risk is essential to reduce the future cancer burden. We investigated whether longitudinal childhood BMI trajectories are associated with obesity-related cancer in adulthood and the role of adult-onset T2D in these associations.
Methods
Cohort
The study was based on data from the Copenhagen School Health Records Register that include 406 350 children (200 978 girls) born from 1930 to 1996 and who attended public or private schools in Copenhagen (24). From ages 6 to 15 years, children were weighed (without shoes and naked or wearing light clothing, to the nearest 100 g) and height was measured (to the nearest 0.5 cm) by school health doctors or nurses (25). Over time, the frequency of the examinations changed from being performed annually to being performed primarily at school entry and exit (24). A unique government-issued identification number, indicating the date of birth and sex of the individual, was assigned to all individuals alive and living in Denmark on April 2, 1968, or born thereafter. It was recorded in the health record or retrieved for children who finished school before this date (24).
Latent class trajectory modeling
Latent class trajectory models were used to identify sex-specific homogeneous BMI growth patterns during childhood from repeated continuous BMI values (26-28). After examining the proportion of individuals in each trajectory, Bayesian Information Criterion, average posterior probability, relative entropy, odds of correct classification, and graphs of the trajectories (26), a model without random effects and 5 trajectories were identified as optimal (Supplementary Methods, Supplementary Table 1, available online).
Case definitions
Individuals in the Copenhagen School Health Records Register were linked via identification numbers with national health registers to obtain information on vital status, cancer, and T2D. Using the Danish Cancer Registry (29), obesity-related cancers (postmenopausal breast, colon and rectum, corpus uteri, esophagus, gallbladder, gastric cardia, kidney, liver, meningioma, multiple myeloma, ovary, pancreas, thyroid) (7) were identified using the International Classification of Diseases codes version 10 (ICD-10). Analyses were restricted to cancers diagnosed in individuals aged 30 years or older. As it is established that adiposity in girls is inversely associated with breast cancer (10,30), it was examined separately. Breast cancer was defined as premenopausal (younger than 55 years) or postmenopausal (55 years or older) as most women have entered menopause by this age. T2D was identified through several national health registers (Supplementary Table 2, available online). Only diagnoses at age 30 years or older were considered as T2D to avoid misclassification of type 1 diabetes.
Study population
From a recoding project, ICD-10 codes were available in the Danish Cancer Registry from January 1, 1978 onwards. Individuals included in the study were followed from this date or age 30 years until a diagnosis of cancer (except nonmelanoma skin cancer), type 1 diabetes, emigration, death, loss to follow-up, or December 31, 2018 (date of latest available data), whichever came first. Thus, to be eligible for the study, individuals were required to be born between 1930 and 1988 (n = 369 971) and have an identification number (n = 327 130, 88.4%) (Supplementary Figure 1, available online). Exclusions were made for individuals who died (n = 4029, 1.2%), emigrated (n = 8036, 2.5%), or disappeared (n = 22, 0.01%) before the start of follow-up. We further excluded individuals diagnosed with diabetes at ages younger than 30 years (n = 946), cancer before 1978 (n = 758), or aged younger than 30 years (n = 909), or with less than 2 BMI values available (n = 10 503). The analytic study population included 301 927 individuals (149 325 women, 49.5%). The study was approved by the Danish Data Protection Agency (2007-58-0015). According to Danish law, informed consent is not required for purely register-based research of preexisting data.
Statistical analyses
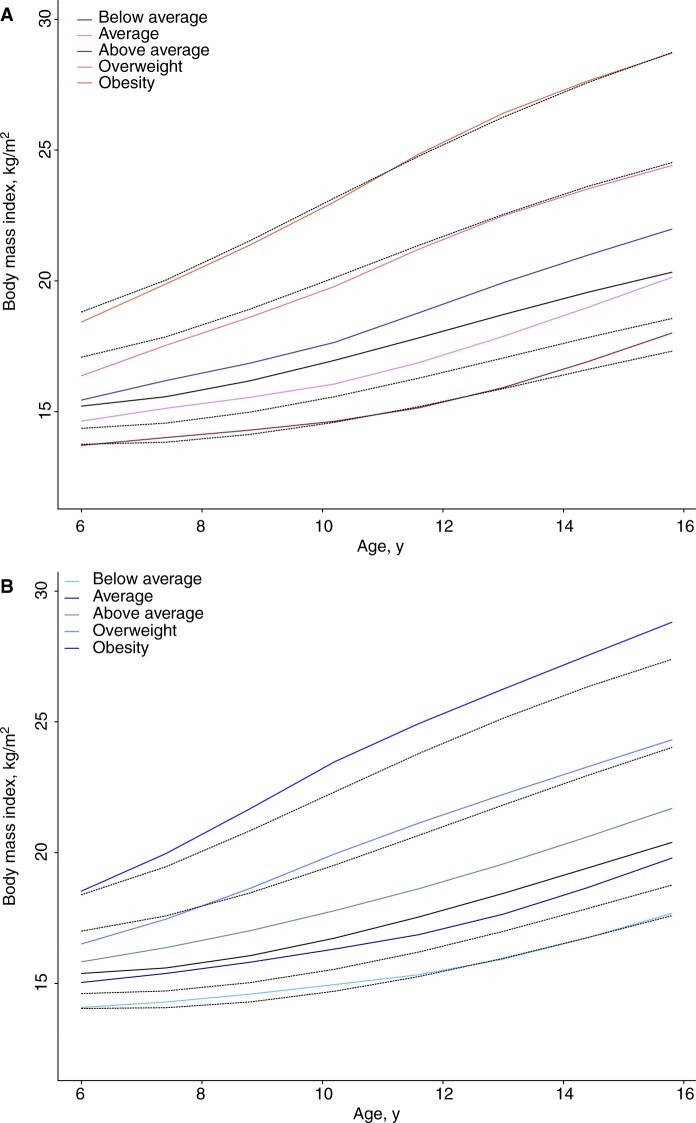
For the descriptive statistics, children were assigned to the trajectory with the highest posterior probability (modal assignment), and the median and interquartile range are presented by childhood BMI trajectory. The trajectories were plotted and compared with the US Centers for Disease Control and Prevention (CDC) 2000 age- and sex-specific BMI reference (see Figure 1) (31).
Figure 1.
Mean childhood body mass index (BMI) trajectories by sex. Shown are the estimated mean BMI trajectories for each of the identified trajectories among girls (A) and boys (B), in relation to the BMI percentiles (10th, 25th, 50th, 85th and 95th) from the US Centers for Disease Control and Prevention growth charts illustrated with the black lines. BMI = body mass index.
Primary outcomes were obesity-related cancer and pre- and postmenopausal breast cancer. Secondary outcomes were specific obesity-related cancer forms. Sex-specific incidence rate ratios (IRR) were estimated using multivariable Poisson regressions. In these analyses, the posterior probabilities assigned to each trajectory (rather than modal assignment) were used as the exposures to account for potential misclassification. The interpretation of the posterior probability estimates is similar to belonging to a class with complete certainty (posterior probability = 100%). Log-risk time was the offset, and the timescale (age in 1-year intervals) and year of birth were included as covariates. Because of nonlinearity, age and year of birth were modeled as cubic splines with 3 knots. Using the likelihood ratio test, interactions were tested between child BMI trajectories and birth cohort and T2D (included as a time-varying covariate), respectively. Post hoc sensitivity analyses were conducted adjusting child BMI trajectories for height at 7 years (early childhood height is strongly correlated with adult height) (32). For comparison purpose, associations only with height are presented in supplementary material (Supplementary Table 3, available online). Post hoc sensitivity analyses were conducted for early and late-onset obesity-related cancers (younger than 50 years or 50 years and older at diagnosis) and for breast cancer by defining menopausal status at ages younger than 50 years as premenopausal and 50 years and older as postmenopausal.
Sex-specific 10-year cumulative risks of obesity-related cancer were estimated at ages 55 and 65 years as this is when most of these cancers occur. They were calculated for individuals with and without T2D using the conditional survival function (33), which accounts for the competing risk of death (34). The 95% confidence intervals (CI) for the estimated cumulative risks were simulated using the bootstrap method with 1000 repetitions.
Statistical analyses were performed using STATA version 16 (35) and R version 3.6.1 (36). Two-sided confidence intervals and P values were calculated, and a priori the level of significance was set at 5%.
Results
Of the 5 childhood BMI trajectories, most children had the average trajectory and the fewest had the obesity trajectory (Figure 1, Table 1). The trajectories approximated the curves from the CDC BMI reference. During 8.1 million person-years of observation, 7285 women and 7261 men developed obesity-related cancer of which 846 and 634 cases were diagnosed at ages younger than 50 years, respectively (Table 2). Among women, 3812 premenopausal and 6406 postmenopausal breast cancers were diagnosed.
Table 1.
Characteristics of individuals in the Copenhagen School Health Record Register by sex and childhood BMI trajectorya
| Characteristics | Total | Childhood BMI trajectory |
||||
|---|---|---|---|---|---|---|
| Below average | Average | Above average | Overweight | Obesity | ||
| Women | ||||||
| No. of women (%) | 149 325 | 31 232 (20.9) | 59 357 (39.8) | 39 974 (26.8) | 15 215 (10.2) | 3547 (2.4) |
| Year of birth, median (IQR) | 149 325 | 1951 (1942-1964) | 1949 (1940-1962) | 1949 (1941-1963) | 1952 (1943-1967) | 1961 (1947-1978) |
| BMI at age 7 y, median (IQR), kg/m2 | 140 284 | 14.0 (13.6-14.5) | 15.0 (14.6-15.5) | 16.0 (15.5-16.6) | 17.2 (16.5-18.0) | 19.2 (18.1-20.4) |
| Height at age 7 y, median (IQR), m | 140 284 | 1.20 (1.17-1.24) | 1.21 (1.18-1.25) | 1.22 (1.19-1.26) | 1.24 (1.20-1.27) | 1.25 (1.22-1.29) |
| Obesity-related cancer, No. (%) | 7285 (4.9) | 1360 (4.4) | 2917 (4.9) | 2059 (5.2) | 771 (5.1) | 178 (5.0) |
| Premenopausal breast cancer, No. (%)b | 3812 (2.6) | 892 (2.9) | 1547 (2.6) | 1030 (2.6) | 301 (2.0) | 42 (1.2) |
| Postmenopausal breast cancer, No. (%)b | 6406 (4.3) | 1477 (4.7) | 2807 (4.7) | 1638 (4.1) | 445 (2.9) | 39 (1.1) |
| Type 2 diabetes, No. (%) | 11 696 (7.8) | 1887 (6.0) | 3969 (6.7) | 3412 (8.5) | 1809 (11.9) | 619 (17.5) |
| Men | ||||||
| No. of men (%) | 152 602 | 35 208 (23.1) | 64 817 (42.5) | 37 990 (24.9) | 12 046 (7.9) | 2541 (1.7) |
| Year of birth, median (IQR) | 152 602 | 1951 (1942-1964) | 1949 (1941-1963) | 1949 (1940-1962) | 1952 (1943-1967) | 1961 (1947-1978) |
| BMI at age 7 y, median (IQR), kg/m2 | 143 481 | 14.3 (13.9-14.7) | 15.3 (14.9-15.7) | 16.2 (15.7-16.7) | 17.2 (16.5-17.9) | 19.1 (18.0-20.3) |
| Height at age 7 y, median (IQR), m | 143 481 | 1.21 (1.18-1.25) | 1.22 (1.19-1.25) | 1.23 (1.20-1.27) | 1.25 (1.22-1.29) | 1.27 (1.24-1.31) |
| Obesity-related cancer, No. (%) | 7261 (4.8) | 1530 (4.3) | 3283 (5.1) | 1865 (4.9) | 510 (4.2) | 73 (2.9) |
| Type 2 diabetes, No. (%) | 17 546 (11.5) | 3490 (9.9) | 7002 (10.8) | 4670 (12.3) | 1929 (16.0) | 455 (17.9) |
For the characteristics of individuals in each of the childhood BMI trajectories, individuals were assigned to the trajectory with the highest posterior probability (modal assignment). BMI = body mass index; IQR = interquartile range.
Pre- and postmenopausal breast cancers were defined based on age at diagnosis as younger than age 55 years or age 55 years and older, respectively.
Table 2.
Number of women and men diagnosed with specific obesity-related cancer formsa
| Cancer form | ICD-10 codea | Women, No. | Men, No. |
|---|---|---|---|
| Colon | C18 | 1890 | 2138 |
| Corpus uteri | C54 and C55 | 1355 | |
| Esophagus | C15 | 205 | 699 |
| Gallbladder | C23 | 51 | 22 |
| Gastric cardia | C16.0 | 71 | 320 |
| Kidney | C64 | 418 | 812 |
| Liver | C22 | 210 | 590 |
| Meningioma | C70 | 8 | 7 |
| Multiple myeloma | C90.0 | 227 | 268 |
| Ovary | C56 | 1088 | |
| Pancreas | C25 | 698 | 865 |
| Rectum | C19.9 and C20.9 | 850 | 1443 |
| Thyroid | C73 | 214 | 97 |
| Breast | C50 and D05 | 10 218 | |
| Total | 17 503 | 7261 |
From 1978 onward, ICD-10 codes are available in the Danish Cancer Registry, because of a recoding project in 2004 where all cancers diagnosed from 1978 to 2004 were converted into ICD-10 codes (29). ICD = International Disease Classification.
Compared with women with the average childhood BMI trajectory (referent), women with the above average (IRR = 1.08, 95% CI = 1.02 to 1.15), overweight (IRR = 1.27, 95% CI = 1.17 to 1.38), or obesity (IRR = 1.79, 95% CI = 1.53 to 2.08) childhood BMI trajectories had higher rates of obesity-related cancer, whereas no statistically significant difference in the rate was observed for women with the below average childhood BMI trajectory (IRR = 0.97, 95% CI = 0.91 to 1.04) (Figure 2, A; Supplementary Table 4, available online). Adjusting the analyses for childhood height did not change the results, and the associations remained statistically significant for the overweight and obesity trajectories (Supplementary Table 5, available online). The IRR for early onset obesity-related cancer was higher for the obesity trajectory than for late-onset obesity-related cancer, however, the confidence intervals were wide and overlapping (Supplementary Table 4, available online).
Figure 2.
Rates of cancer among women and men by childhood BMI trajectory. Shown are the incidence rate ratios between childhood BMI trajectories and the rates of obesity-related cancer (A) and breast cancer (B). Individuals with the average childhood BMI trajectory were used as the referent. The analyses were adjusted for birth year. The error bars represent the 95% confidence intervals (CIs). BMI = body mass index.
Compared with men in the reference group, men with the overweight (IRR = 1.13, 95% CI = 1.03 to 1.25) or obesity (IRR = 1.30, 95% CI = 1.03 to 1.64) childhood BMI trajectories had higher rates of obesity-related cancer (Figure 2, A; Supplementary Table 4, available online). In contrast, men with the below average (IRR = 0.90, 95% CI = 0.85 to 0.97) childhood BMI trajectory had a statistically significantly lower rate of obesity-related cancer compared with the referent, whereas no association was observed for the above average (IRR = 1.04, 95% CI = 0.98 to 1.11) childhood BMI trajectory. Adjusting the analyses for childhood height did essentially not change the results (Supplementary Table 5, available online). The IRR was higher for early onset than late-onset obesity-related cancer for the obesity trajectory, but the confidence intervals were wide and overlapping (Supplementary Table 4, available online).
For breast cancer, the associations were reversed. Women with the below average childhood BMI trajectory had higher rates of premenopausal (IRR = 1.09, 95% CI = 1.00 to 1.20) and postmenopausal (IRR = 1.13, 95% CI = 1.05 to 1.21) breast cancer compared with the referent. The lowest rates of breast cancer were observed for women with the overweight (premenopausal: IRR = 0.80, 95% CI = 0.71 to 0.91; postmenopausal: IRR = 0.76, 95% CI = 0.68 to 0.84) and obesity (premenopausal: IRR = 0.59, 95% CI = 0.43 to 0.80; postmenopausal: IRR = 0.41, 95% CI = 0.30 to 0.57) childhood BMI trajectories (Figure 2, B; Supplementary Table 6, available online). Women with the above average childhood BMI trajectory had similar or slightly lower rates of premenopausal (IRR = 0.99, 95% CI = 0.91 to 1.07) and postmenopausal (IRR = 0.91, 95% CI = 0.85 to 0.97) breast cancer compared with the referent. Changing the definition of age at menopause minimally affected the results (Supplementary Table 6, available online) as did adjusting the childhood BMI trajectories for childhood height, and the associations remained statistically significant (Supplementary Table 5, available online).
In the exploratory analyses of associations between childhood BMI trajectories and specific cancer forms among women, the 3 most common cancers (colon, corpus uteri, and ovary) showed a similar pattern to the overall results (Table 3). In men, 2 of the 3 most prevalent cancer forms (colon and pancreatic) showed associations in accord with the overall results, whereas no association was observed for rectal cancer (Table 3). Adjusting the analyses of these specific cancer forms for childhood height attenuated the associations observed among women, whereas the associations were essentially unchanged for men (Supplementary Table 5, available online).
Table 3.
Associations between childhood BMI trajectory and specific obesity-related cancer forms by sexa
| Cancer form | Sex | Childhood BMI trajectory |
||||
|---|---|---|---|---|---|---|
| Below average | Average | Above average | Overweight | Obesityb | ||
| IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | ||
| Colon | Women | 0.88 (0.77 to 1.01) | 1.00 (Referent) | 1.09 (0.97 to 1.23) | 1.12 (0.95 to 1.33) | 1.52 (1.10 to 2.10) |
| Men | 0.86 (0.76 to 0.97) | 1.00 (Referent) | 1.04 (0.93 to 1.16) | 1.13 (0.95 to 1.35) | 1.38 (0.90 to 2.12) | |
| Corpus uteri | Women | 0.98 (0.83 to 1.15) | 1.00 (Referent) | 1.23 (1.07 to 1.42) | 1.49 (1.24 to 1.79) | 3.22 (2.43 to 4.26) |
| Esophagus | Women | 0.90 (0.59 to 1.39) | 1.00 (Referent) | 1.25 (0.87 to 1.78) | 1.51 (0.95 to 2.41) | — |
| Men | 1.15 (0.94 to 1.41) | 1.00 (Referent) | 1.13 (0.93 to 1.39) | 1.46 (1.09 to 1.95) | 1.60 (0.79 to 3.27) | |
| Gastric cardia | Women | 1.18 (0.56 to 2.47) | 1.00 (Referent) | 1.36 (0.71 to 2.60) | 2.28 (1.08 to 4.81) | — |
| Men | 0.85 (0.62 to 1.17) | 1.00 (Referent) | 1.03 (0.77 to 1.37) | 1.02 (0.63 to 1.63) | — | |
| Kidney | Women | 0.91 (0.68 to 1.23) | 1.00 (Referent) | 1.04 (0.81 to 1.35) | 1.33 (0.96 to 1.85) | 1.68 (0.89 to 3.16) |
| Men | 0.90 (0.73 to 1.10) | 1.00 (Referent) | 1.22 (1.02 to 1.46) | 1.17 (0.88 to 1.55) | 1.82 (1.01 to 3.26) | |
| Liver | Women | 1.00 (0.67 to 1.49) | 1.00 (Referent) | 0.92 (0.63 to 1.33) | 1.71 (1.11 to 2.63) | — |
| Men | 0.73 (0.57 to 0.93) | 1.00 (Referent) | 1.03 (0.84 to 1.27) | 1.11 (0.80 to 1.54) | 1.31 (0.60 to 2.88) | |
| Multiple myeloma | Women | 0.88 (0.59 to 1.31) | 1.00 (Referent) | 1.13 (0.81 to 1.58) | 1.03 (0.63 to 1.69) | — |
| Men | 0.84 (0.59 to 1.18) | 1.00 (Referent) | 1.07 (0.79 to 1.46) | 0.85 (0.49 to 1.48) | — | |
| Ovary | Women | 1.06 (0.89 to 1.26) | 1.00 (Referent) | 0.95 (0.80 to 1.11) | 1.17 (0.95 to 1.46) | 2.01 (1.40 to 2.89) |
| Pancreas | Women | 0.93 (0.74 to 1.16) | 1.00 (Referent) | 1.12 (0.92 to 1.36) | 1.21 (0.92 to 1.58) | 1.60 (0.94 to 2.70) |
| Men | 0.78 (0.64 to 0.96) | 1.00 (Referent) | 1.07 (0.90 to 1.27) | 1.33 (1.03 to 1.72) | 1.77 (0.99 to 3.16) | |
| Rectum | Women | 1.07 (0.88 to 1.30) | 1.00 (Referent) | 0.98 (0.82 to 1.18) | 1.18 (0.92 to 1.51) | 0.61 (0.30 to 1.28) |
| Men | 1.06 (0.93 to 1.22) | 1.00 (Referent) | 0.92 (0.80 to 1.06) | 0.97 (0.77 to 1.23) | 0.70 (0.34 to 1.45) | |
| Thyroid | Women | 1.12 (0.76 to 1.67) | 1.00 (Referent) | 1.10 (0.76 to 1.59) | 1.35 (0.85 to 2.14) | 1.87 (0.85 to 4.11) |
| Men | 0.60 (0.33 to 1.11) | 1.00 (Referent) | 0.92 (0.55 to 1.54) | 0.91 (0.41 to 2.06) | — | |
Because of the low case number, analyses were not performed for gallbladder and meningioma. BMI = body mass index; CI = confidence interval; IRR = incidence rate ratio.
“—” signifies results not presented if <5 cases per regulations on data reporting set by the Danish National Statistics Center.
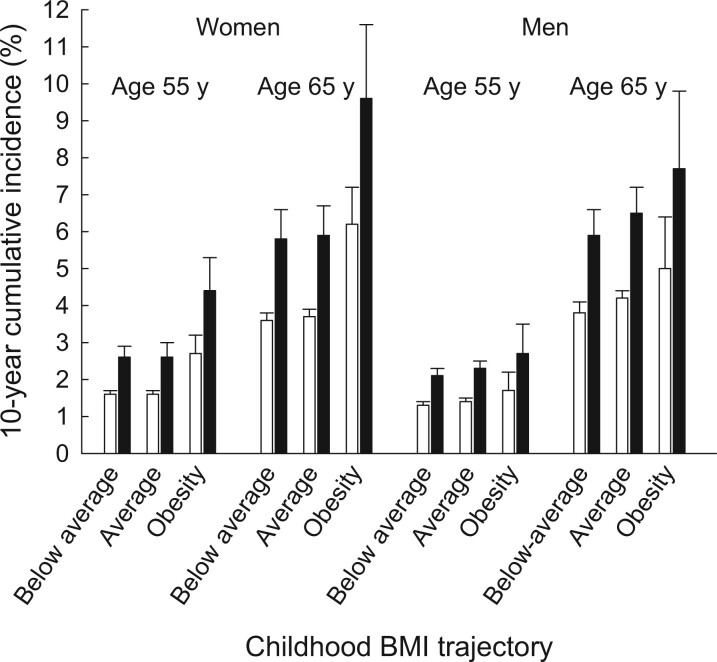
T2D was statistically significantly associated with the rate of obesity-related cancer in models adjusted for childhood BMI trajectories (women: IRR = 1.67, 95% CI = 1.48 to 1.88; men: IRR = 1.65, 95% CI = 1.49 to 1.82). Nonetheless, adult-onset T2D did not statistically significantly modify the associations between childhood BMI trajectories and obesity-related cancer among men or women (P ≥ .55). A comparison between 2 women aged 65 years with the obesity childhood BMI trajectory, where one had T2D and the other did not, showed that the 10-year risk difference for being diagnosed with an obesity-related cancer was 3.4 percentage points higher for the woman with T2D (Figure 3;Supplementary Table 7, available online). A similar comparison in men showed that the risk difference was 2.7 percentage points higher in the man with T2D than in the man without T2D.
Figure 3.
The 10-year cumulative incidences (in percentages) of obesity-related cancers among women and men. The 10-year cumulative risks of obesity-related cancer were estimated for women and men at age 55 and 65 years for individuals in each childhood BMI trajectory with (black) and without (white) adult-onset T2D and accounting for the competing risk of death. The error bars represent the 95% confidence intervals. BMI = body mass index; T2D = type 2 diabetes.
Discussion
Among the 5 sex-specific childhood BMI trajectories identified, higher rates of obesity-related cancer were observed for women and men with trajectories that were above average compared with the average childhood BMI trajectory. Among men, a lower rate of obesity-related cancer was observed for those with the below average childhood BMI trajectory. In contrast, the overweight and obesity childhood BMI trajectories were associated with lower rates of breast cancer in women, and the highest rates of pre- and postmenopausal breast cancer were observed for women with the below average childhood BMI trajectory. Adult-onset T2D conferred an additional risk of being diagnosed with an obesity-related cancer across all childhood BMI trajectories.
The rates of obesity-related cancer generally increased across all childhood BMI trajectories. We found that consistent overweight or obesity in childhood was associated with a decreased rate of breast cancer, a paradoxical observation that is explained in other studies by a disproportionally greater effect on risk of early adulthood weight gain in women with normal childhood weight (37). Notably, we found higher rates of obesity-related cancer associated with trajectories even below the CDC threshold for overweight. Thus, it is of great concern as 18% of contemporary Danish children (38) and 42% of American children (39) are classified as having overweight or obesity, meaning that far too many children may be at increased risk of obesity-related cancer in adulthood. Together with the long latency period of some cancer forms (9), our results suggest that childhood is a critical period that should be considered in cancer prevention planning.
As the definition of obesity-related cancer is based on associations with adult adiposity (1), it may not necessarily apply to childhood adiposity. However, we previously reported positive associations between childhood BMI at individual ages and risks of many of these cancer forms (10); a mendelian randomization study links genetically predicted childhood BMI with some cancer forms as well (40).
We found that associations between childhood BMI trajectories and specific cancer forms generally reflected the overall results, especially in women. However, among men, rectal cancer notably was not associated with childhood BMI trajectories despite high case numbers. Including childhood height in the analyses had little effect, suggesting that height is not driving the associations observed. Although the IRRs were higher for early onset obesity-related cancer, they should be interpreted cautiously as the confidence intervals were wide.
No previous studies examined childhood BMI or body size trajectories specifically in relation to incident cancer (16,17). A Finnish study found differential associations between sex-specific BMI trajectories from birth to age 11 years with cancer mortality (15). A French study found that women with trajectories including a larger body shape around the age at menarche had lower risks of postmenopausal breast cancer than those with a smaller body shape at this age (19). This finding was supported by results from an American study (18) but not by results from a Mexican study (20). Thus, our results are generally in accord with the previous literature on breast cancer.
Our novel investigation of whether T2D impacted the associations showed a higher cumulative incidence of obesity-related cancer in women and men with T2D for all childhood BMI trajectories. Nonetheless, the effect of adult-onset T2D on the rate of obesity-related cancer did not depend on the childhood BMI trajectory. Given that obesity in children is linked with T2D and that T2D is emerging at younger ages in contemporary populations (41,42), our results suggest that this association warrants further investigation.
Suggested mechanisms linking adiposity and obesity-related cancer include dysregulation of insulin, insulin-like growth factor 1, adiponectin and alterations in sex-hormone levels as well as chronic exposure to low-grade inflammation, which increase local inflammation and cell division and inhibit cell apoptosis (43). T2D leads to similar metabolic changes thus supporting the possibility of an increased risk of obesity-related cancer among individuals with T2D (2). However, shared risk factors including obesity, physical inactivity (44), and genetics (5) complicate the understanding of the association between T2D and cancer. Moreover, antidiabetes medication may influence cancer risk, but its role is unclear (45). Exposure to the metabolic alterations caused by excess adiposity already from childhood is a plausible mechanism potentially explaining the increased risk of obesity-related cancer. For breast cancer, mechanisms underlying the links with childhood adiposity remain speculative but include effects of altering sex-hormone levels and effects on the developing breast (37,46).
Our study has several strengths. The longitudinal data with repeated measurements of weight and height enabled modeling of BMI development through childhood rather than using BMI at single time points. As a result, the clinical relevance of our results is enhanced as it supports comparisons with BMI curves across several ages rather than requiring measurements at exact ages, which are often unavailable. Possible misclassification among the trajectories was accounted for by using posterior probabilities for trajectory membership in the analyses. Cancer diagnoses were obtained from a validated national register where 86% of cancers are morphologically verified (29). T2D was included in the analyses as a time-varying covariable thus accounting for the time exposed to T2D before cancer was diagnosed. The results are likely generalizable to contemporary children as the associations did not change across the study period, which included pre-World War II, World War II occupation, and the subsequent introduction of the social welfare state. Further, the combination of the population-based cohort, the universal and free health care available in Denmark, and the low loss to follow-up because of the national registers minimizes potential effects of selection bias and enhances the generalizability of our results.
It is a limitation that our cohort includes mainly White individuals, which may limit the generalization of the results to other ethnicities. The definition of T2D changed across time; however, because of the structure of our data in relation to age, time period, and the general increase in T2D over time, most T2D cases were identified after 1995, thus changes in its definition likely did not affect our results. Information on socioeconomic status of the child, smoking, and physical activity and adult BMI was unavailable. As these factors may have changed across the time span of the study, their effect on the results is uncertain. To examine effects of child BMI on adult disease, child and adult BMI values are often included in the same model. Depending on the approach used, including BMIs at both time points may estimate the effects of change in BMI from child to adult ages and not necessarily their independent effects. Furthermore, as child and adult BMI lie on the same pathway, the inclusion of adult BMI may represent an inappropriate overadjustment (47). Finally, the identified trajectories are based on a subjective judgement of the optimal model. However, the high average posterior probabilities indicate a good model fit, and the similarities with the CDC BMI reference support that the trajectories reflect patterns of childhood growth.
Our study showed that individuals with overweight or obesity childhood BMI trajectories have higher rates of obesity-related cancer as adults but lower rates of breast cancer in women. Adult-onset T2D conferred additional risk for obesity-related cancer, but its effects did not differ across childhood BMI trajectories.
Funding
This work was supported by Novo Nordisk Foundation (grant number NNF17OC0028338) to JLB; the World Cancer Research Fund (grant number 2018/1760) to JLB; and the Dr Sofus Carl Emil Friis and wife Olga Doris’ Fund to JLB.
Notes
Role of the funder: The funders of this study had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: BWJ, JA, KB, AGR, JLB have no conflicts of interest to declare. DV and MEJ have received research grants from Sanofi Aventis, Novo Nordisk A/S, and Boehringer Ingelheim and MEJ also from AMGEN and Astra Zeneca. DV received a research grant from Bayer A/S and holds shares in Novo Nordisk A/S.
Author contributions: Conceptualization: BWJ, JLB; data curation: JLB; formal analyses: BWJ, DV; investigation: all authors; methodology: BWJ, JLB, DV; project administration: JLB; resources: JLB; software: JLB, DV; supervision: JLB, DV; visualisation: all authors; writing- original draft: BWJ; writing- review & editing: all authors.
Acknowledgements: The Copenhagen School Health Records Register was initiated and planned by Dr Thorkild I.A. Sørensen and built by the Institute of Preventive Medicine, The Capital Region of Denmark. We appreciate the statistical assistance from Dr Bendix Carstensen, Steno Diabetes Center Copenhagen.
Prior presentation: Preliminary results were presented orally at the European and International Congress on Obesity September 2, 2020, online conference.
Supplementary Material
Contributor Information
Britt W Jensen, Center for Clinical Research and Prevention, Copenhagen University Hospital—Bispebjerg and Frederiksberg, Copenhagen, Denmark.
Julie Aarestrup, Center for Clinical Research and Prevention, Copenhagen University Hospital—Bispebjerg and Frederiksberg, Copenhagen, Denmark.
Kim Blond, Center for Clinical Research and Prevention, Copenhagen University Hospital—Bispebjerg and Frederiksberg, Copenhagen, Denmark.
Marit E Jørgensen, Clinical Epidemiological Research, Steno Diabetes Center Copenhagen, Herlev, Denmark; Steno Diabetes Center Greenland, Nuuk, Greenland; National Institute of Public Health, University of Southern Denmark, Copenhagen, Denmark.
Andrew G Renehan, Division of Cancer Sciences, School of Medical Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK.
Dorte Vistisen, Clinical Epidemiological Research, Steno Diabetes Center Copenhagen, Herlev, Denmark; Department of Public Health, University of Copenhagen, Copenhagen, Denmark.
Jennifer L Baker, Center for Clinical Research and Prevention, Copenhagen University Hospital—Bispebjerg and Frederiksberg, Copenhagen, Denmark.
Data availability
Data supporting the analyses in this work cannot be directly shared due to data protection regulations as they contain personally identifiable information. Access to these data can be provided to researchers under certain circumstances, pending approval by the steering committee for the Copenhagen School Health Records Register and agreements with the Center for Clinical Research and Prevention, Copenhagen University Hospital—Bispebjerg and Frederiksberg, Copenhagen, Denmark.
References
- 1. International Agency for Research on Cancer. Absence of Excess Body Fatness. Lyon: IARC Handbook of Cancer Prevention; 2018. http://publications.iarc.fr/570. Accessed August 15, 2022. [Google Scholar]
- 2. Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60(4):207-221. [DOI] [PubMed] [Google Scholar]
- 3. Goto A, Yamaji T, Sawada N, et al. Diabetes and cancer risk: a Mendelian randomization study. Int J Cancer. 2020;146(3):712-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JPA.. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350(1):g7607. [DOI] [PubMed] [Google Scholar]
- 5. Yuan S, Kar S, Carter P, et al. Is type 2 diabetes causally associated with cancer risk? Evidence from a two-sample Mendelian randomization study. Diabetes. 2020;69(7):1588-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M.. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. 2018;6(2):95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K; for the International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer–viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arnold M, Jiang L, Stefanick ML, et al. Duration of adulthood overweight, obesity, and cancer risk in the Women’s Health Initiative: a longitudinal study from the United States. PLoS Med. 2016;13(8):e1002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meza R, Jeon J, Renehan AG, Luebeck EG.. Colorectal cancer incidence trends in the United States and United Kingdom: evidence of right- to left-sided biological gradients with implications for screening. Cancer Res. 2010;70(13):5419-5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aarestrup J, Bjerregaard LG, Meyle KD, et al. Birthweight, childhood overweight, height and growth and adult cancer risks: a review of studies using the Copenhagen School Health Records Register. Int J Obes. 2020;44(7):1546-1560. [DOI] [PubMed] [Google Scholar]
- 11. Zhang X, Wu K, Giovannucci EL, et al. Early life body fatness and risk of colorectal cancer in U.S. women and men-results from two large cohort studies. Cancer Epidemiol Biomarkers Prev. 2015;24(4):690-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Engeland A, Tretli S, Bjorge T.. Height, body mass index, and ovarian cancer: a follow-up of 1.1 million Norwegian women. J Natl Cancer Inst. 2003;95(16):1244-1248. [DOI] [PubMed] [Google Scholar]
- 13. Aarestrup J, Bjerregaard LG, Gamborg M, et al. Tracking of body mass index from 7 to 69 years of age. Int J Obes (Lond). 2016;40(9):1376-1383. [DOI] [PubMed] [Google Scholar]
- 14. Rosner B, Eliassen AH, Toriola AT, et al. Weight and weight changes in early adulthood and later breast cancer risk. Int J Cancer. 2017;140(9):2003-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. von Bonsdorff MB, Tormakangas T, Rantanen T, et al. Early life body mass trajectories and mortality in older age: findings from the Helsinki Birth Cohort Study. Ann Med. 2015;47(1):34-39. [DOI] [PubMed] [Google Scholar]
- 16. Mattsson M, Maher GM, Boland F, Fitzgerald AP, Murray DM, Biesma R.. Group-based trajectory modelling for BMI trajectories in childhood: a systematic review. Obes Rev. 2019;20(7):998-1015. [DOI] [PubMed] [Google Scholar]
- 17. Blond K, Aarestrup J, Vistisen D, et al. Associations between body mass index trajectories in childhood and cardiovascular risk factors in adulthood. Atherosclerosis. 2020;314:10-17. [DOI] [PubMed] [Google Scholar]
- 18. Song M, Willett WC, Hu FB, et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138(10):2383-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fagherazzi G, Guillas G, Boutron-Ruault MC, Clavel-Chapelon F, Mesrine S.. Body shape throughout life and the risk for breast cancer at adulthood in the French E3N cohort. Eur J Cancer Prev. 2013;22(1):29-37. [DOI] [PubMed] [Google Scholar]
- 20. Amadou A, Torres Mejia G, Fagherazzi G, et al. Anthropometry, silhouette trajectory, and risk of breast cancer in Mexican women. Am J Prev Med. 2014;46(3):S52-S64. [DOI] [PubMed] [Google Scholar]
- 21. Zimmermann E, Bjerregaard LG, Gamborg M, Vaag AA, Sørensen TIA, Baker JL.. Childhood body mass index and development of type 2 diabetes throughout adult life–a large-scale Danish cohort study. Obesity (Silver Spring). 2017;25(5):965-971. [DOI] [PubMed] [Google Scholar]
- 22. Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16(1):36-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baker JL, Olsen LW, Andersen I, Pearson S, Hansen B, Sørensen TI.. Cohort profile: the Copenhagen School Health Records Register. Int J Epidemiol. 2009;38(3):656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baker JL, Sorensen TI.. The Copenhagen School Health Records Register. Scand J Public Health. 2011;39(suppl 7):87-90. [DOI] [PubMed] [Google Scholar]
- 26. Lennon H, Kelly S, Sperrin M, et al. Framework to construct and interpret latent class trajectory modelling. BMJ Open. 2018;8(7):e020683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagin DS, Odgers CL.. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109-138. [DOI] [PubMed] [Google Scholar]
- 28. Proust-Lima C, Philipps V, Liquet B.. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J Stat Soft. 2017;78(2):1-56. [Google Scholar]
- 29. Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(suppl 7):42-45. [DOI] [PubMed] [Google Scholar]
- 30. World Cancer Research Fund International/American Institute for Cancer Research. Continuous update project expert report 2018: diet, nutrition, physical activity and breast cancer. https://www.wcrf.org/wp-content/uploads/2021/02/Breast-cancer-report.pdf. Accessed August 15, 2022.
- 31. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1-190. [PubMed] [Google Scholar]
- 32. Pedersen DC, Meyle KD, Angquist L, et al. Changes and correlations in height from 7 to 69 years of age across the birth years of 1930 to 1989. Am J Hum Biol. 2020;32(4):e23378. [DOI] [PubMed] [Google Scholar]
- 33. Andersen PK, Geskus RB, de Witte T, Putter H.. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41(3):861-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vistisen D, Andersen GS, Hulman A, et al. A validated prediction model for end-stage kidney disease in type 1 diabetes. Diabetes Care. 2021;44(4):901-907. [DOI] [PubMed] [Google Scholar]
- 35. StataCorp. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC; 2019. https://www.stata.com/. Accessed August 15, 2022. [Google Scholar]
- 36. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. https://www.R-project.org/. Accessed August 15, 2022. [Google Scholar]
- 37. Renehan AG, Pegington M, Harvie MN, et al. Young adulthood body mass index, adult weight gain and breast cancer risk: the PROCAS Study (United Kingdom). Br J Cancer. 2020;122(10):1552-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bruun JM, Bjerregaard LG, Due P, et al. Prevention of Overweight Among Children and Adolescents. [In Danish: Forebyggelse af overvægt blandt børn og unge] København: Vidensråd for Forebyggelse; 2021. [Google Scholar]
- 39. Fryar CD, Carroll MD, Afful J.. Prevalence of Overweight, Obesity, and Severe Obesity Among Children and Adolescents Aged 2-19 Years: United States, 1963-1965 Through 2017-2018. Center for Disease Control and Prevention; 2020.
- 40. Fang X, Wang X, Song Z, et al. Causal association of childhood obesity with cancer risk in adulthood: a Mendelian randomization study. Int J Cancer. 2021;149(7):1421-1425. [DOI] [PubMed] [Google Scholar]
- 41. Alberti G, Zimmet P, Shaw J, et al. ; for the Consensus Workshop Group. Type 2 diabetes in the young: the evolving epidemic: the international diabetes federation consensus workshop. Diabetes Care. 2004;27(7):1798-1811. [DOI] [PubMed] [Google Scholar]
- 42. Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in incidence of type 1 and type 2 diabetes among youths—selected counties and Indian reservations, United States. MMWR Morb Mortal Wkly Rep. 2020;69(6):161-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Renehan AG, Zwahlen M, Egger M.. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15(8):484-498. [DOI] [PubMed] [Google Scholar]
- 44. Johnson JA, Carstensen B, Witte D, et al. ; for the Diabetes and Cancer Research Consortium. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia. 2012;55(6):1607-1618. [DOI] [PubMed] [Google Scholar]
- 45. Badrick E, Renehan AG.. Diabetes and cancer: 5 years into the recent controversy. Eur J Cancer. 2014;50(12):2119-2125. [DOI] [PubMed] [Google Scholar]
- 46. Macias H, Hinck L.. Mammary gland development. Wiley Interdiscip Rev Dev Biol. 2012;1(4):533-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. VanderWeele TJ. On the relative nature of overadjustment and unnecessary adjustment. Epidemiology. 2009;20(4):496-499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the analyses in this work cannot be directly shared due to data protection regulations as they contain personally identifiable information. Access to these data can be provided to researchers under certain circumstances, pending approval by the steering committee for the Copenhagen School Health Records Register and agreements with the Center for Clinical Research and Prevention, Copenhagen University Hospital—Bispebjerg and Frederiksberg, Copenhagen, Denmark.