Abstract
Aim
The aim of this study was to examine the relationship between ghrelin levels and the subjective effects of alcohol in heavy drinkers, and to compare them to healthy controls.
Methods
Ghrelin levels were collected as part of two laboratory studies. Both groups received either IV infusion of saline or high dose of alcohol (100 mg%). In the study of heavy drinkers, ghrelin was gathered on all subjects, but data was analyzed only for participants who received placebo (N=12). Healthy controls (N=20) came from another study that collected data on family history. Ghrelin levels and measures of alcohol effects (BAES, VAS, NDS, YCS [see manuscript for details]) were collected at 4 timepoints: baseline, before infusion, during infusion and after infusion.
Results
IV alcohol significantly reduced ghrelin levels and higher fasting ghrelin levels were associated with more intense subjective alcohol effects. There were no differences in fasting ghrelin levels or subjective effects between heavy drinkers and controls. However, while both groups showed similar decline in ghrelin levels following alcohol infusion, on the placebo day, ghrelin levels in the healthy subjects increased significantly and exponentially over time while for the heavy drinkers ghrelin levels remained flat.
Conclusions
Our findings support the role of ghrelin in reward mechanisms for alcohol. Contrary to others, we found no differences in fasting ghrelin levels or subjective experiences of alcohol between heavy drinkers and healthy controls. However, the group differences on the IV placebo day may be a possible indication of ghrelin abnormalities in heavy drinkers.
INTRODUCTION
Research over the past two decades suggests that ghrelin, a 28-amino acid peptide, has a role in alcohol reward, craving and risk for relapse in heavy drinkers and those with alcohol use disorder (AUD) (Zallar et al., 2017; Morris et al., 2018; Farokhnia et al., 2019; Jerlhag, 2019). Although originally identified as important in regulating appetite and food intake, subsequent research has shown that ghrelin has a role in a wide range of other physiological functions like energy balance and stress response (Mani and Zigman, 2017; Stone et al., 2020). Exact mechanisms linking AUD to the ghrelin system are not fully understood. It is believed that ghrelin acts on brain regions and neural circuits responsible for reward processing and stress regulation (Meyer et al., 2014; Koob and Volkow, 2016). Therefore, in AUD (Al Massadi et al., 2019; Deschaine and Leggio, 2020), ghrelin suppression, which occurs following alcohol consumption, may eventually lead to upregulation of ghrelin secretion with repeated alcohol consumption. This compensatory change is thought to lead to increases in mesolimbic dopamine release in response to food and alcohol (Al Massadi et al., 2019) and is supported by evidence of increased ghrelin levels in abstinent individuals with AUD (Kim et al., 2005; Kraus et al., 2005; Leggio et al., 2012; Kim et al., 2013).
This bidirectional interplay between alcohol and ghrelin has been well documented in various human studies. Oral alcohol intake and intravenous (IV) alcohol administration reduces ghrelin levels in those with AUD and healthy controls (Kraus et al., 2005; Addolorato et al., 2006; Badaoui et al., 2008; de Timary et al., 2012; Koopmann et al., 2012; Leggio et al., 2012; Kim et al., 2013; Leggio et al., 2013; Deschaine and Leggio, 2022). Results comparing ghrelin levels between those with AUD and controls are inconsistent. Some studies show higher ghrelin levels in those with AUD (Kim et al., 2005; Kraus et al., 2005; Wurst et al., 2007), whereas others show lower ghrelin levels in those with AUD (Addolorato et al., 2006; Badaoui et al., 2008; de Timary et al., 2012). The inconsistencies have been attributed to differences in the form of ghrelin (total vs acyl + des-acyl), length of AUD, timing of blood collected and other methodological differences.
Findings from laboratory studies that manipulate the ghrelin system provide further support for the role of ghrelin in AUD. Ghrelin administration in two double-blind, placebo-controlled studies showed increase in alcohol craving (Leggio et al., 2014) and alcohol self-administration (Farokhnia et al., 2018). Forced water intake resulted in decreases in ghrelin and alcohol craving (Koopmann et al., 2017).
Finally, a number of laboratory studies, including studies from our group, show a positive relationship between ghrelin and alcohol craving, reward sensitivity, cue-induced brain activity as well as impulsivity and subjective effects of alcohol (Leggio et al., 2012, Wurst et al., 2007, Akkişi Kumsar and Dilbaz, 2015, Ralevski et al., 2017, Ralevski et al., 2018, Sha et al., 2021). Our group was the first to show a positive relationship between fasting ghrelin levels and stimulant/sedative subjective effects of alcohol in healthy subjects. This study was designed to investigate the effects of IV alcohol on ghrelin levels and to examine the relationship between ghrelin levels and the subjective effects of alcohol in heavy drinkers.
METHODS
Participants
The participants for this study came from two sources. The group of heavy drinkers were a part of a larger double-blind, laboratory study designed to test the effects of a medication (2 different doses of minocycline) and placebo on subjective effects of alcohol (Petrakis et al., 2019) [NCT 02187211]. The group of control subjects came from a study that examined the effects of family history on the subjective effects of alcohol (Kerfoot et al., 2013). Both studies were approved by the Yale University Human Subjects Committee (HIC) and VA Healthcare Human Subjects Subcommittee. All participants signed consent before any procedures were initiated. Heavy drinkers: The data collection for the heavy drinkers was initiated about halfway through the original study and ghrelin samples were collected on all subjects (N = 35) but were analyzed only on those participants that received placebo so there would be no medication effect. Male and female heavy drinkers (N = 12) between the ages of 21 and 55, with no current medical problems were eligible if they consumed ≥10 standard alcoholic drinks per week and had 1–5 weekly ‘binge’ drinking episodes (5 plus drinks per occasion for men; 4 plus drinks for women). Current mood, anxiety, psychotic and substance use disorders (excluding alcohol, marijuana and tobacco), history of major medical illnesses, pregnant females, or those in alcohol withdrawal (having a Clinical Institute Withdrawal Assessment Scale, CIWA, score of 4 or greater) were not eligible to participate. Healthy controls: Data for the sample of healthy controls consisted of N = 20 participants who were male and female social drinkers, between the ages of 21 and 30 years old, who had no Axis I disorders (except alcohol abuse), were not alcohol naïve, and were medically and neurologically healthy (Ralevski et al., 2017).
Procedures
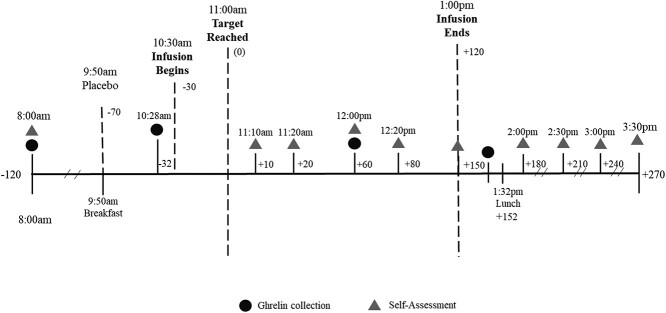
Heavy drinkers: As part of the original study, all participants were carefully screened using the Structured Clinical Interview for DSM-IV (SCID) to determine psychiatric diagnoses. Data on daily alcohol use were collected using the Time-Line Follow-Back Assessment Method. Eligible participants were randomized to receive medication/placebo for 7 days. That was followed by 2 laboratory test days, randomly assigned and 2 days apart, where they received either placebo or high dose of alcohol (targeted breath alcohol levels = 100 mg%) intravenously (IV). Study procedures for each test day are outlined in Fig. 1.
Fig. 1.
Test session timeline for infusion, ghrelin collection and self-assessments starting at around 8:00 am and ending at around 3:30 pm
All participants arrived at the Biological Studies Unit at the VA Connecticut Healthcare Center around 8 am. After setup, they were given a standardized breakfast consisting of prepackaged = 43 g cereal (average calories = 238.0), 4 oz of milk (average calories = 85.5) and 4 oz of orange juice (average calories = 71.2) for a total of 394.69 calories. Infusion started ~30 min after breakfast. Both alcohol and placebo doses were infused over ~30 min until targeted breath alcohol level (BrAC) was reached. The targeted dose was maintained using a clamp procedure for 120 min.
All participants were cleared by a physician before being discharged and had a BrAC level of <40 mg%. Healthy controls: All subjects participated in three randomly assigned and counterbalanced test days. They received two doses of alcohol, high dose with a targeted breath alcohol level (BrAC) of 100 mg% (21.7 mmol/L), low dose with BrAC of 40 mg% (8.68 mmol/L) and placebo (saline). Please note that for this study the data from the low dose of alcohol (BrAC of 40 mg%) were not used (for details on study procedures please see Ralevski et al., 2017). The clamp procedure for both studies used a 6% ethanol solution in 0.9% saline. The loading phase rates were based on calculations that used the patient’s age, gender, height and weight. The infusion was conducted by using either a computerized (for details on clamp procedure see (O'Connor et al., 1998, Ramchandani et al., 1999, O'Connor et al., 2000) or manual procedure. Both methods allowed for adjustments to the infusion rate so that the BrACs were maintained within ±5 mg% of the target BrAC for the duration of the clamp (120 min for heavy drinkers and 60 min for healthy controls). Ethanol concentrations in both studies were collected at baseline during the clamped infusion and after infusion ended.
The two studies were very similar in terms of procedures, measures and data collection. The main difference was that for the heavy drinkers, the infusion was set for 2 h (120 min), whereas for the healthy controls the infusion was set for 1 h (60 min).
Ghrelin levels
Blood samples for plasma ghrelin (acyl ghrelin) were collected at four time-points: at baseline (−120 in both studies) when participants came into the laboratory in the morning, just before the infusion (−32), during the infusion (+60 for heavy drinkers and + 50 for healthy controls) and following the infusion (+150 for heavy drinkers and + 80 for healthy controls). Blood samples were collected into 4 ml EDTA chilled vacutainers. A solution (0.384 ml of 4-(2-Aminoethyl) benzenesulfonyl fluoride [AEBSF]) was pipetted into the 4 ml tube and immediately spun for 15 min at 3000 rpm and 4°C (the AEBSF was diluted to a 42 millimole solution and kept in an ice bath throughout the day). It was then pipetted into two separate chilled, preloaded aliquot tubes containing 1 N hydrochloride acid (HCL) in a 5:1 ratio (generally 1 ml of serum was added .2 ml of HCI). The aliquot tubes were frozen at −80°C. Samples were analyzed using Elisa kits for acyl (AG)(#EZGRA-88 K, Millipore) human ghrelin. The results were plotted on a sigmoidal 5-parameter logistic equation to determine the ghrelin concentration (pg/ml) of each sample. In both studies, lunch was served after the last ghrelin collection to avoid the effect of calories on ghrelin concentration.
Subjective measures
Main outcome measures included the Biphasic Alcohol Effects Scale (BAES), the Visual Analog Scales (VAS) and the Number of Drinks Scale (NDS). The BAES is a 14-item self-report adjective rating scale used to measure the stimulant and sedative effects of alcohol; The VAS has the following items that evaluated feeling: buzzed, high, drowsy. and tired. The NDS was used to assess how many drinks the subject felt he/she had consumed at a specific timepoint. Craving for alcohol was also evaluated using a single item (rate the desire for an alcoholic beverage right now) from the Yale Craving Scale (YCS).
Data analysis
The main data analysis consisted of using mixed models with time (4 time points) and alcohol dose (high dose of alcohol or placebo) as within subject factors, and ghrelin levels as main outcome variable. Analyses were also performed using the same model and including baseline fasting ghrelin levels as a covariate to evaluate the role of ghrelin in subjective effects of alcohol (measured by the BAES, VAS, NDS, YCS). Additional analysis was performed using ghrelin levels as a main outcome variable but comparing this sample to the sample of healthy subjects (Ralevski et al., 2017). The model used was the same as above except group (heavy drinkers or healthy controls) was added as a between subject factor to examine differences in ghrelin levels and its effects when comparing heavy drinkers to healthy controls. Correlations were performed to test the relationship between craving and ghrelin levels.
RESULTS
Main study with heavy drinkers only
Participant characteristics
The average age of the sample was 31.8 (SD = 8.8) ranging from 21 to 43 years old (Table 1).
Table 1.
Demographic and clinical characteristics of the sample (N = 12)
| Variables | Totals (N = 12) | ||
|---|---|---|---|
| n | % | ||
| Gender | Female | 4 | 33.3 |
| Male | 8 | 66.7 | |
| Race | White | 3 | 25 |
| Black | 5 | 41.7 | |
| Other | 4 | 33.3 | |
| Marital status | Single | 8 | 66.7 |
| Divorced | 1 | 8.3 | |
| Partner | 3 | 25 | |
| Alcohol dependence | None | 3 | 25 |
| Alcohol abuse | 3 | 25 | |
| Alcohol dependence | 6 | 50 | |
| Mean | SD | ||
| Age | 31.8 | 8.82 | |
| Age 1st drink | 20.4 | 4.87 | |
| Age heaviest drinking | 24.2 | 8.55 | |
| Age difficult to stop drinking | 25.9 | 6.99 | |
They (N = 12) were mostly males (66.6%), single (66.6%) and predominantly Black (41.6%). They reported that around age 20.4 (SD = 4.8) they started drinking regularly, by age 24.2 (SD = 8.5) they were drinking heavily and by age 25.9 (SD = 6.9) they found it difficult to stop drinking. As a result, 50% met DSM-IV criteria for alcohol dependence, and 25% met criteria for alcohol abuse. Problem drinking ran in their families with 41.6% having either first or second-degree relatives with drinking problems.
Ghrelin levels
There was a statistically significant main effect for time (F3,9.6 = 5.1, P = 0.02) where ghrelin levels decreased (see Figs 2 and 4 for more detail). There was no main effect of alcohol dose (F1,4.5 = 16, P = 0.26), but a significant alcohol dose × time interaction (F3,8.9 = 4.4, P = 0.03) indicated that alcohol significantly reduced ghrelin levels when compared with placebo over time (Fig. 2).
Fig. 2.
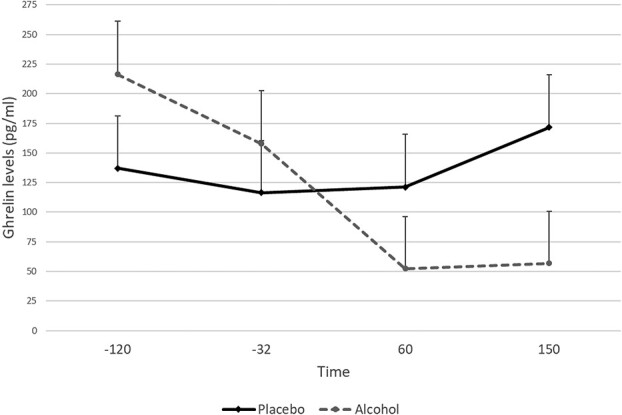
Ghrelin levels following infusion of alcohol or infusion of placebo (N = 12)
Fig. 4.
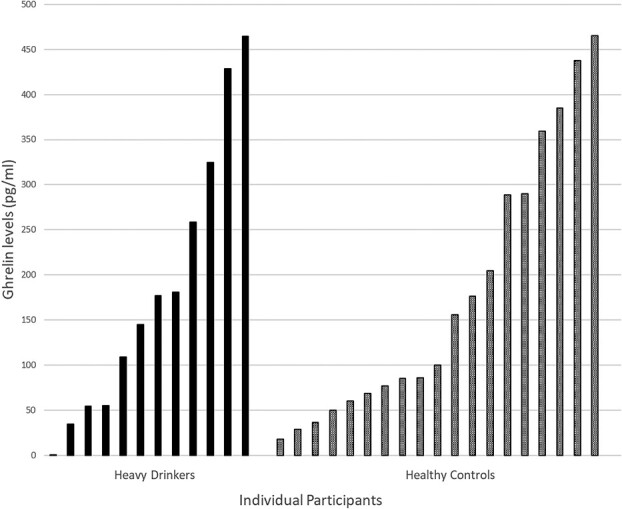
Individual fasting ghrelin levels in heavy drinkers and healthy controls
We also calculated percent change in ghrelin levels (%∆) before alcohol infusion (−32 time point), during (+60) and after alcohol infusion (+150 time point). There was a significant main effect for time (F2,209.3 = 3.1, P = 0.04) but no main effect for alcohol dose (F1,57.8 = 0.79, P = 0.37). Although there was no significant effect for alcohol dose × time, there was a non-significant (trend) alcohol dose × time interaction (F2,144.9 = 2.5, P = 0.08). The changes in ghrelin levels were minimal in the placebo condition (−8.4% during the infusion and 15.7% following the infusion). The alcohol condition produced a greater decrease in ghrelin levels during infusion (−48.1%) and following the infusion (−34.9%).
Fasting ghrelin levels
Participants reported following instructions to fast from the night before. There were no significant differences in fasting ghrelin levels (F1,205.65 = 0.602, P = 0.44) between the two test days. Since there were no significant mean baseline differences, the two baseline values were averaged to create a single baseline fasting ghrelin level for each participant. The distributions of overall fasting ghrelin levels (−120 time point) in this study were wide and were negatively skewed. The average ghrelin level was 186.13 (SD = 182.90) and ranged from 1.87 to 736.38. The average fasting ghrelin levels were used as predictors in all analyses of subjective effects.
Caloric content of alcohol
The calculations for the caloric content of alcohol were based on the following: 100 ml of solution = 6 g; 1 g of pure alcohol = 7 calories. The amount of alcohol for each subject was calculated based on their body weight, height and gender. The average amount of alcohol over the 2 h was 1784.3 (SD = 426.8) and the average amount of calories was 749.4 (SD = 179.3) (range 499.8–1118.2).
Fasting ghrelin as predictor of alcohol effects
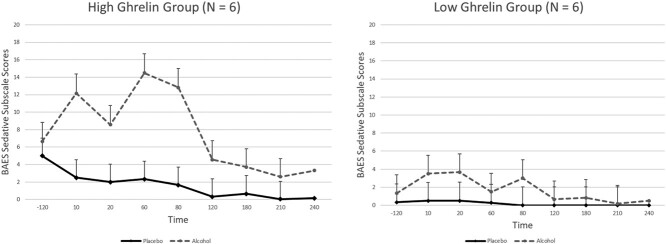
Fasting ghrelin levels were significant predictors of both stimulant (alcohol dose × fasting ghrelin interaction F1,12.8 = 4.5, P = 0.05) and sedative effects (time × alcohol dose × fasting ghrelin F7,38.4 = 4.5, P = 0.001) measured by the BAES, indicating that those with higher ghrelin levels reported stronger stimulant and sedative effects than those with lower ghrelin levels. The same was true for the NDS scale (alcohol dose × fasting ghrelin F1,5144.3 = 11.1, P = 0.001). Those with higher ghrelin levels reported that the amount of alcohol was more like five drinks, whereas those with lower ghrelin levels felt that the amount of alcohol was similar to about 3.5 drinks. The results were similar for the analysis of the VAS scale. Fasting ghrelin levels were significant predictors of feeling ‘buzzed’ (alcohol dose × fasting ghrelin F1,21.6 = 4.9, P = 0.03) and ‘drowsy’ (alcohol dose × fasting ghrelin (F1,263.8 = 5.6, P = 0.01), but not feeling ‘high’ (alcohol dose × fasting ghrelin F1,18.0 = 0.5, P = 0.5) or ‘tired’ (alcohol dose x fasting ghrelin F1,47.8 = 0.2, P = 0.7) (Fig. 3).
Fig. 3.
Sedative subscale of the Biphasic Alcohol Effects Scale (BAES) for the high and low ghrelin groups
Craving
We found no statistically significant relationship between ghrelin levels and craving (r = −0.34, P = 0.30).
COMPARISON OF HEAVY DRINKERS AND HEALTHY CONTROLS
Details for the sample of healthy controls have been published previously (Ralevski et al., 2017). The two groups were compared on: (i) fasting ghrelin levels, (ii) ghrelin levels following high dose of alcohol infusion and placebo and (iii) subjective effects of alcohol. The range of fasting ghrelin levels in both groups was very wide. There was no difference between the two groups on fasting ghrelin levels (mean 186.13 [SD = 182.90]) in heavy drinkers and (mean 176.64 [SD = 138.79]) healthy controls (Fig. 4).
The comparison of ghrelin levels following alcohol infusion revealed that alcohol reduced ghrelin levels similarly in both groups, but the difference was on the placebo day (Fig. S1).
Although in healthy subjects, ghrelin levels increased over time, in heavy drinkers, ghrelin levels remained almost flat (dose by group interaction F1,21.6 = 10.38, P = 0.002). In both groups, fasting ghrelin levels predicted subjective effects and there were no differences between healthy subjects and heavy drinkers on any subjective effects (Fig. S2).
DISCUSSION
The primary objective of this study was to test the effects of high dose of IV alcohol on ghrelin levels and examine if ghrelin levels are related to subjective alcohol effects in heavy drinkers. We found that IV alcohol significantly suppressed ghrelin levels in heavy drinkers. Furthermore, fasting ghrelin levels were related to subjective effects of alcohol (BAES sedative subscale, VAS buzzed, drowsy and tired but not to BAES stimulant subscale or VAS high). Those with higher fasting ghrelin levels were more likely to experience the sedative and stimulant effects of alcohol more intensely. Our findings support results reported by others that alcohol suppresses ghrelin levels in heavy drinkers (Addolorato et al., 2006; Badaoui et al., 2008; Leggio et al., 2012). High dose of IV alcohol in this study suppressed ghrelin by 48.1% compared with placebo that suppressed ghrelin levels by 8.4%. The evidence is consistent in different populations (healthy subjects as well as heavy drinkers with and without AUD) (Addolorato et al., 2006; Leggio et al., 2012; Ralevski et al., 2017), using diverse methods (laboratory as well as population-based studies) (Leggio et al., 2014; Wittekind et al., 2018), and employing a variety of experimental laboratory procedures (oral vs. IV alcohol infusion) (Leggio et al., 2013; Leggio et al., 2014; Ralevski et al., 2017; Farokhnia et al., 2018; Deschaine and Leggio, 2020). The exact mechanism by which alcohol suppresses ghrelin is not well understood but some data suggest that this effect is independent of the caloric value of alcohol indicating a non-direct interaction with the ghrelin system (Deschaine and Leggio, 2020). A recent review (Deschaine and Leggio, 2022) details the complex role of this hormone and suggests that in AUD, as others have proposed (Al Massadi et al., 2019), in alcohol relapse the rewarding effects of alcohol are increased as a result of the increases in ghrelin levels recorded in abstainers.
The results from this study add to the growing evidence that ghrelin modulates the alcohol reward pathway. Our group was the first to report that fasting ghrelin levels were related to stimulant and sedative effects of alcohol in healthy social drinkers (Ralevski et al., 2017). Those findings were replicated in this study with heavy drinkers, many of whom met criteria for AUD. Those with higher ghrelin levels were more likely to report stronger sedative and stimulant effects of alcohol. One possible mechanism for this relationship is through the mesolimbic dopamine pathway since ghrelin is directly implicated in the release of dopamine and has a role in reward for both food and drugs of abuse (Jerlhag et al., 2007).
In this study, we found no relationship between ghrelin levels and craving among the heavy drinkers. This is consistent with studies that measured ghrelin in current drinkers (Kraus et al., 2005, Akkişi Kumsar and Dilbaz, 2015). This is contrasted with studies with abstainers (minimum 6 days), which report a positive relationship between ghrelin and craving (Leggio et al., 2012; Koopmann et al., 2018). Therefore, differences in drinking status (abstainers vs. non-abstainers) may be one possibility for this discrepancy in findings. Another could be differences in craving measurement (we used a single item scale vs longer questionnaires used in most studies). Finally, differences in ghrelin measurement (acylated vs. total plasma ghrelin) could also account for discrepancy in findings.
Additional analysis was performed to compare heavy drinkers to healthy controls on fasting ghrelin levels, and the role of ghrelin in subjective effects of alcohol. There was no difference in fasting ghrelin levels between heavy drinkers and healthy controls in this study. This is contrary to findings reported by others that show significantly lower (Badaoui et al., 2008) or significantly higher fasting ghrelin levels in active drinkers with AUD and controls (Kim et al., 2005; Kraus et al., 2005). In addition to a small sample size in our study, not everyone met criteria for AUD and this may have contributed to differences in findings.
In both groups, high dose of IV alcohol significantly reduced ghrelin levels. Interestingly, the levels of alcohol-induced ghrelin suppression were similar in heavy drinkers and controls. However, the placebo-induced ghrelin levels were very different. Although the ghrelin levels among the heavy drinkers remained almost unchanged, placebo-induced increases in ghrelin levels in the control group were more than double. This is an interesting finding that suggests a possible dysregulation of the ghrelin system in heavy drinkers.
There were no differences between the two groups in terms of subjective effects. High dose of IV alcohol resulted in significantly higher and similar reports in both groups of stimulant and sedative effects when compared with placebo. This is not consistent with the literature that generally shows heavy drinkers report greater stimulant and lower sedation when compared with light social drinkers (Holdstock et al., 2000; Marczinski et al., 2007; Roche et al., 2014; Boyd et al., 2017). The mixed and small sample of heavy drinkers with and without AUD may have contributed to the diverse findings. As we reported previously in healthy controls (Ralevski et al., 2017), ghrelin levels in this study predicted the response to alcohol among heavy drinkers. The findings that fasting ghrelin levels predicted the subjective effects of alcohol in both groups support the notion that ghrelin has a role in reward mechanisms. The role of dopamine in reward for food and alcohol (Weafer et al., 2018) has been well documented, as is the role of ghrelin in dopamine release (Jerlhag et al., 2007).
Because of the small sample size in the study of heavy drinkers, the findings from this study should be interpreted with caution. Using data from two different studies is not optimal but the lab procedures for the two comparison groups were similar overall but not identical (clamp was longer in the heavy drinker group) although having identical procedures may have produced different results. Despite this difference, the consistency in results in both groups is important to underline.
In sum, in heavy drinkers, IV alcohol significantly suppressed ghrelin levels, and ghrelin levels were related to subjective effects of alcohol. There was no difference in fasting ghrelin levels or subjective effects between heavy drinkers and healthy controls. Although in both groups high dose of IV alcohol significantly and similarly reduced ghrelin levels, on the placebo day, ghrelin levels in heavy drinkers remained almost flat, whereas in healthy subjects, placebo-induced increases in ghrelin levels were more than double. Our findings support the notion that ghrelin has a role in reward mechanisms and suggest that there is a dysregulation of the ghrelin system in heavy drinkers.
Supplementary Material
ACKNOWLEDGEMENTS
This study was conducted with the invaluable help of Jane Weiner, RN, Diana DeNegre, BA, Jessica Dasher, BA and the nurses of the VA Connecticut Healthcare System Neurobiological Studies Unit.
Contributor Information
Elizabeth Ralevski, Department of Psychiatry, Yale University School of Medicine, New Haven, CT 06520, USA; Department of Veteran Affairs, VA Connecticut Healthcare System, West Haven, CT, USA; Mental Illness Research and Clinical Center, VA Connecticut Healthcare System, West Haven, CT, USA.
Tamas L Horvath, Program of Integrative Cell Signaling and Neurobiology of Metabolism, Section of Comparative Medicine, Yale University School of Medicine, New Haven 06520, CT, USA; Department of Obstetrics/Gynecology and Reproductive Sciences, Yale University School of Medicine, New Haven 06520, CT, USA.
Marya Shanabrough, Program of Integrative Cell Signaling and Neurobiology of Metabolism, Section of Comparative Medicine, Yale University School of Medicine, New Haven 06520, CT, USA.
Jenelle Newcomb, Department of Psychiatry, Yale University School of Medicine, New Haven, CT 06520, USA; Department of Veteran Affairs, VA Connecticut Healthcare System, West Haven, CT, USA; Mental Illness Research and Clinical Center, VA Connecticut Healthcare System, West Haven, CT, USA.
Emily Pisani, Department of Psychiatry, Yale University School of Medicine, New Haven, CT 06520, USA; Department of Veteran Affairs, VA Connecticut Healthcare System, West Haven, CT, USA; Mental Illness Research and Clinical Center, VA Connecticut Healthcare System, West Haven, CT, USA.
Ismene Petrakis, Department of Psychiatry, Yale University School of Medicine, New Haven, CT 06520, USA; Department of Veteran Affairs, VA Connecticut Healthcare System, West Haven, CT, USA; Mental Illness Research and Clinical Center, VA Connecticut Healthcare System, West Haven, CT, USA.
SOURCE OF FUNDING
Veterans Affairs VISN I Mental Illness Research Education and Clinical Center (MIRECC), National Center for PTSD, Clinical Neurosciences Division (NCPTSD) and National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grant 1R21AA023150-01A1. The funding sources had no role in the study design, collection, analysis, or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
CONFLICT OF INTEREST
Dr Petrakis has received study drug donated in-kind from Alkermes, Inc. and BioXcel Therapeutics, Inc.
DATA AVAILABILITY STATEMENT
The raw data supporting the conclusions of this article may be made available by the authors upon request.
References
- Addolorato G, Capristo E, Leggio L, et al. (2006) Relationship between ghrelin levels, alcohol craving, and nutritional status in current alcoholic patients. Alcohol Clin Exp Res 30:1933–7. [DOI] [PubMed] [Google Scholar]
- Akkisi Kumsar N, Dilbaz N. (2015) Relationship between craving and ghrelin, adiponectin, and resistin levels in patients with alcoholism. Alcohol Clin Exp Res 39:702–9. [DOI] [PubMed] [Google Scholar]
- Al Massadi O, Nogueiras R, Dieguez C, et al. (2019) Ghrelin and food reward. Neuropharmacology 148:131–8. [DOI] [PubMed] [Google Scholar]
- Badaoui A, De Saeger C, Duchemin J, et al. (2008) Alcohol dependence is associated with reduced plasma and fundic ghrelin levels. Eur J Clin Invest 38:397–403. [DOI] [PubMed] [Google Scholar]
- Boyd SJ, Corbin WR, Morean ME, et al. (2017) Alcohol stimulation and sedation: a critical review of the biphasic alcohol effects scale. Curr Addict Rep 4:209–20. [Google Scholar]
- De Timary P, Cani PD, Duchemin J, et al. (2012) The loss of metabolic control on alcohol drinking in heavy drinking alcohol-dependent subjects. PLoS One 7:e38682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschaine SL, Leggio L. (2020) Understanding plasma treatment effect on human acyl-ghrelin concentrations. Eur Rev Med Pharmacol Sci 24:1585–9. [DOI] [PubMed] [Google Scholar]
- Deschaine SL, Leggio L. (2022) From "Hunger Hormone" to "It's Complicated": ghrelin beyond feeding control. Physiology (Bethesda) 37:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhnia M, Grodin EN, Lee MR, et al. (2018) Exogenous ghrelin administration increases alcohol self-administration and modulates brain functional activity in heavy-drinking alcohol-dependent individuals. Mol Psychiatry 23:2029–38. [DOI] [PubMed] [Google Scholar]
- Farokhnia M, Faulkner ML, Piacentino D, et al. (2019) Ghrelin: from a gut hormone to a potential therapeutic target for alcohol use disorder. Physiol Behav 204:49–57. [DOI] [PubMed] [Google Scholar]
- Holdstock L, King AC, De Wit H. (2000) Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res 24:789–94. [PubMed] [Google Scholar]
- Jerlhag E. (2019) Gut-brain axis and addictive disorders: a review with focus on alcohol and drugs of abuse. Pharmacol Ther 196:1–14. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, et al. (2007) Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol 12:6–16. [DOI] [PubMed] [Google Scholar]
- Kerfoot K, Pittman B, Ralevski E, et al. (2013) Effects of family history of alcohol dependence on the subjective response to alcohol using the intravenous alcohol clamp. Alcohol Clin Exp Res 37:2011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Yoon SJ, Choi B, et al. (2005) Increased fasting plasma ghrelin levels during alcohol abstinence. Alcohol Alcohol 40:76–9. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim SJ, Lee WY, et al. (2013) The effects of alcohol abstinence on BDNF, ghrelin, and leptin secretions in alcohol-dependent patients with glucose intolerance. Alcohol Clin Exp Res 37:E52–8. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3:760–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmann A, Von Der Goltz C, Grosshans M, et al. (2012) The association of the appetitive peptide acetylated ghrelin with alcohol craving in early abstinent alcohol dependent individuals. Psychoneuroendocrinology 37:980–6. [DOI] [PubMed] [Google Scholar]
- Koopmann A, Lippmann K, Schuster R, et al. (2017) Drinking water to reduce alcohol craving? A randomized controlled study on the impact of ghrelin in mediating the effects of forced water intake in alcohol addiction. Psychoneuroendocrinology 85:56–62. [DOI] [PubMed] [Google Scholar]
- Koopmann A, Schuster R, Kiefer F. (2018) The impact of the appetite-regulating, orexigenic peptide ghrelin on alcohol use disorders: a systematic review of preclinical and clinical data. Biol Psychol 131:14–30. [DOI] [PubMed] [Google Scholar]
- Kraus T, Schanze A, Gröschl M, et al. (2005) Ghrelin levels are increased in alcoholism. Alcohol Clin Exp Res 29:2154–7. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, et al. (2012) Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving. Addict Biol 17:452–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Schwandt ML, Oot EN, et al. (2013) Fasting-induced increase in plasma ghrelin is blunted by intravenous alcohol administration: a within-subject placebo-controlled study. Psychoneuroendocrinology 38:3085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, Fricchione SR, et al. (2014) Intravenous ghrelin administration increases alcohol craving in alcohol-dependent heavy drinkers: a preliminary investigation. Biol Psychiatry 76:734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, Zigman JM. (2017) Ghrelin as a survival hormone. Trends Endocrinol Metab 28:843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, Combs SW, Fillmore MT. (2007) Increased sensitivity to the disinhibiting effects of alcohol in binge drinkers. Psychol Addict Behav 21:346–54. [DOI] [PubMed] [Google Scholar]
- Meyer RM, Burgos-Robles A, Liu E, et al. (2014) A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol Psychiatry 19:1284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris LS, Voon V, Leggio L. (2018) Stress, motivation, and the gut-brain axis: a focus on the ghrelin system and alcohol use disorder. Alcohol Clin Exp Res 42:1378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'connor S, Morzorati S, Christian J, et al. (1998) Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res 22:202–10. [PubMed] [Google Scholar]
- O'connor S, Ramchandani VA, Li TK. (2000) PBPK modeling as a basis for achieving a steady BrAC of 60 +/− 5 mg% within ten minutes. Alcohol Clin Exp Res 24:426–7. [PubMed] [Google Scholar]
- Petrakis IL, Ralevski E, Gueorguieva R, et al. (2019) Targeting neuroinflammation with minocycline in heavy drinkers. Psychopharmacology (Berl) 236:3013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevski E, Horvath TL, Shanabrough M, et al. (2017) Ghrelin is supressed by intravenous alcohol and is related to stimulant and sedative effects of alcohol. Alcohol Alcohol 52:431–8. [DOI] [PubMed] [Google Scholar]
- Ralevski E, Shanabrough M, Newcomb J, et al. (2018) Ghrelin is related to personality differences in reward sensitivity and impulsivity. Alcohol Alcohol 53:52–6. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, O'connor S, Blekher T, et al. (1999) A preliminary study of acute responses to clamped alcohol concentration and family history of alcoholism. Alcohol Clin Exp Res 23:1320–30. [PubMed] [Google Scholar]
- Roche DJO, Palmeri MD, King AC. (2014) Acute alcohol response phenotype in heavy social drinkers is robust and reproducible. Alcohol Clin Exp Res 38:844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha L, Dey P, Khess CR, et al. (2021) The association of plasma acyl ghrelin level with alcohol craving in early abstinent alcohol dependent patients. J Postgrad Med 67:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LA, Harmatz ES, Goosens KA. (2020) Ghrelin as a stress hormone: implications for psychiatric illness. Biol Psychiatry 88:531–40. [DOI] [PubMed] [Google Scholar]
- Weafer J, Ross TJ, O’connor S, et al. (2018) Striatal activity correlates with stimulant-like effects of alcohol in healthy volunteers. Neuropsychopharmacology 43:2532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittekind DA, Kratzsch J, Mergl R, et al. (2018) Alcohol consumption is positively associated with fasting serum ghrelin in non-dependent adults: results from the population-based LIFE-Adult-Study. Psychoneuroendocrinology 97:143–8. [DOI] [PubMed] [Google Scholar]
- Wurst FM, Graf I, Ehrenthal HD, et al. (2007) Gender differences for ghrelin levels in alcohol-dependent patients and differences between alcoholics and healthy controls. Alcohol Clin Exp Res 31:2006–11. [DOI] [PubMed] [Google Scholar]
- Zallar LJ, Farokhnia M, Tunstall BJ, et al. (2017) The role of the ghrelin system in drug addiction. Int Rev Neurobiol 136:89–119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article may be made available by the authors upon request.