Abstract
Background
Urinary tract infection (UTI) is a commonly misdiagnosed infectious syndrome. Diagnostic stewardship interventions can reduce rates of asymptomatic bacteriuria treatment but are often labor intensive, and thus an automated means of reducing unnecessary urine testing is preferred. In this systematic review and meta-analysis, we sought to identify studies describing interventions utilizing clinical decision support (CDS) to optimize UTI diagnosis and to characterize the effectiveness of these interventions.
Methods
We conducted a comprehensive electronic search and manual reference list review for peer-reviewed articles published before July 2, 2021. Publications describing an intervention intending to enhance UTI diagnosis via CDS were included. The primary outcome was urine culture test rate.
Results
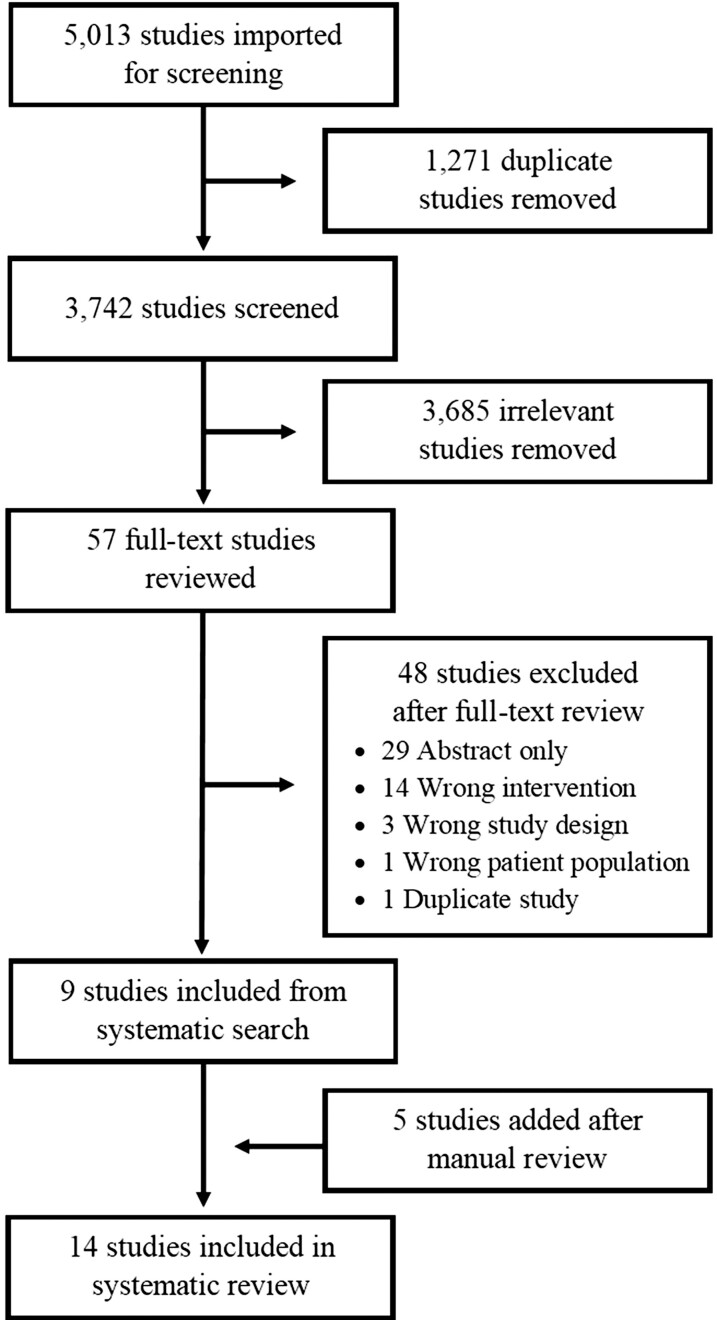
The electronic search identified 5013 studies for screening. After screening and full-text review, 9 studies met criteria for inclusion, and a manual reference list review identified 5 additional studies, yielding a total of 14 studies included in the systematic review. The most common CDS intervention was urinalysis with reflex to urine culture based on prespecified urinalysis parameters. All 9 studies that provided statistical comparisons reported a decreased urine culture rate postintervention, 8 of which were statistically significant. A meta-analysis including 4 studies identified a pooled urine culture incidence rate ratio of 0.56 (95% confidence interval, .52–.60) favoring the postintervention versus preintervention group.
Conclusions
In this systematic review and meta-analysis, CDS appeared to be effective in decreasing urine culture rates. Prospective trials are needed to confirm these findings and to evaluate their impact on antimicrobial prescribing, patient-relevant outcomes, and potential adverse effects.
Keywords: decision support, diagnostic stewardship, electronic health record, urinary tract infection, urine culture
In this systematic review and meta-analysis, the use of electronic clinical decision support for diagnosis of urinary tract infection was associated with decreased urine culture rates. Future studies should evaluate their impact on patient-relevant outcomes.
Urinary tract infection (UTI) represents one of the most common infections in the inpatient setting, with upper and lower UTIs accounting for over 10% of indications for systemic antibiotic use in hospitalized patients, and with a significant portion of antibiotic courses considered inappropriate [1, 2]. Urinary tract infection is also regularly misdiagnosed, typically in patients presenting with asymptomatic bacteriuria (ASB), defined as the presence of bacteria in urine in absence of clinical manifestations of infection [3, 4]. Urinalyses are commonly performed for patients without symptoms of UTI, increasing the probability of inappropriate urine culture and antibiotic prescriptions [5]. Based on evidence demonstrating the lack of benefit and increased risk of adverse outcomes with the treatment of ASB [6–9], the Infectious Diseases Society of America [4] and the US Preventive Services Task Force [10] recommend against testing for or treating ASB in most populations. However, treatment remains frequent, with the proportion of treated cases of ASB ranging between 38% and 75% [5, 8, 11–14].
Various stewardship interventions such as education, standardized treatment algorithms, and prospective audit and feedback have been successful in reducing rates of ASB treatment; however, these approaches often require significant skilled resources for scaling and maintenance [15–17]. Interventions utilizing clinical decision support (CDS) offer a less resource-intensive means of assisting providers with guideline-directed diagnosis and treatment [18–20]. Rather than attempting to distinguish symptomatic infections from ASB, CDS often capitalizes on the high negative predictive value of a normal urinalysis test for UTI [21, 22]. However, the effectiveness of CDS on improving the diagnostic accuracy of UTI and reducing the rate of unnecessary testing is unknown. In this systematic review and meta-analysis, we aimed to identify all published studies describing the use of CDS to decrease unnecessary diagnostic testing for UTI and to characterize the effectiveness of the various indicated strategies.
METHODS
This systematic review and meta-analysis was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23].
Search Strategy
We conducted a comprehensive search of electronic databases (Ovid MEDLINE, Embase, Web of Science including Science Citation Index, Conference Proceedings Citation Index, and BIOSIS Citation Index, Cochrane Central Register of Controlled Trials, ClinicalTrials.gov) for peer-reviewed articles published from inception of the database to July 2, 2021. The systematic search strategy was developed by a medical librarian with extensive experience in systematic reviews. Search terms included “urinary tract infections,” “urinalysis,” “decision support systems,” “clinical decision support,” “algorithm,” “reflex test,” “electronic medical record,” “alert,” and related terms. A complete electronic search strategy is included in the Supplementary Materials. A supplementary search was also performed using PubMed and Google Scholar with the reference lists of articles included in the full-text review.
Eligibility Criteria
Inclusion criteria included randomized trials or observational studies describing an intervention designed to reduce unnecessary or incorrect diagnosis of UTI through CDS utilization. We defined CDS for UTI as any tool embedded within the electronic medical record intended to support providers in adhering to evidence-based clinical guidelines for UTI diagnosis. Studies were considered for the meta-analysis if they additionally reported data on the number of urine cultures performed pre- and postintervention. Corresponding authors for studies that reported data for urine culture rate without the raw urine culture numbers were contacted in an attempt to obtain the relevant data. Studies in any language that met the inclusion criteria were eligible. Studies were excluded if they were performed in exclusively or primarily pediatric units or hospitals.
Study Selection
Studies meeting the eligibility criteria were imported into COVIDence (www.COVIDence.org) and independently assessed for inclusion by 2 investigators (E.A.S. and L.S.H.). Studies were included only if selected by both reviewers. Disagreements were resolved by discussion between the 2 reviewers and/or a third investigator (A.D.).
Outcomes
The primary outcome was rate of urine culture tests ordered, pre- and postintervention. Additional outcomes of interest included urinalysis testing rate, catheter-associated UTI (CAUTI) diagnoses, antimicrobial use, guideline-concordant treatment, bloodstream infections, Clostridioidesdifficile infection, isolation of multidrug resistant organisms, mortality, costs of patient care, provider acceptance, and accuracy of coding.
Study Quality Assessment
Study quality assessment was performed using the Joanna Briggs Institute (JBI) critical appraisal checklist tool for quasi-experimental studies [24]. The JBI tool evaluates study quality on the basis of 9 questions designed to assess for bias in design, conduct, and analysis of quasi-experimental or nonrandomized experimental study designs. The appraisal was performed independently by 2 investigators (E.A.S. and L.S.H.), with a third investigator (A.D.) serving as a tiebreaker for any disagreements in the 2 assessments. The full checklist tool is included in the Supplementary Materials.
Data Extraction
Two investigators (E.A.S. and L.S.H.) independently extracted relevant data from the full text of the included studies. Data extracted from the studies included study design, setting (eg, acute care, emergency department, intensive care unit [ICU], etc), country, number of hospitals, hospital type (eg, academic, community, Veterans Health Administration, etc), number of participants, inclusion and exclusion criteria, and urinalysis parameters (if applicable), in addition to the aforementioned outcomes.
Data Synthesis
A qualitative description of the studies meeting the inclusion criteria was performed, identifying characteristics common among multiple studies and unique to others. Results from studies with a common study design and outcome measures involving acute care units with complete urine culture volume data pre- and postintervention were aggregated to perform a meta-analysis. The random-effects model and the inverse variance method were used to combine incidence rates (number of cases/person-time), and the effects were expressed as absolute measure (incidence rate difference [IRD] postintervention minus preintervention) and relative measure (incidence rate ratio [IRR] postintervention over preintervention), both with their respective 95% confidence intervals (CIs).
The presence of statistical heterogeneity was evaluated using the Cochran χ2. A P < .1 for χ2 was considered to indicate the presence of heterogeneity. Heterogeneity of effects were quantified with the I2 statistic, with I2 > 60% meaning high heterogeneity and I2 > 75% meaning substantial heterogeneity. The software R 4.2.0 (www.r-project.org) was used for statistical analyses. A P < .05 was considered statistically significant.
Publication Bias
Funnel plots and the Egger's regression asymmetry test were used to assess publication bias. When asymmetry was detected, the potential impact of publication bias was assessed using Duval and Tweedie trim and fill method.
RESULTS
The search identified 5013 studies to be imported for screening, 1271 of which were excluded due to being duplicates, yielding a total of 3742 studies for screening (Figure 1). After initial screening, full-text review was performed for 57 studies, 9 studies of which [25–33] were identified as meeting criteria for inclusion. Manual review of reference lists for papers included in the full-text review identified an additional 5 studies [34–38] that met inclusion criteria. Interrater agreement (E.A.S. and L.S.H.) was approximately 98%.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram indicating study selection process.
Study Characteristics
Characteristics of the 14 included studies are summarized in Table 1. All studies were observational and quasi-experimental, with most utilizing an interrupted time-series analysis. The study periods ranged between 2011 and 2020. All studies were performed in the United States except for 1 in Hong Kong [35] and 1 in France [27].
Table 1.
Characteristics of Included Studies
| First Author, Year | Study Type | Study Period Years | No. of Hospitals (Unit Type/Hospital Type) | Intervention | Exceptions Allowed |
|---|---|---|---|---|---|
| Claeys, 2021 [25] | Quasi-experimental/interrupted time series | 2013–2018 | 6 (inpatient/VHA) | Reflex urine culture | Provider override; special populations |
| Coughlin, 2020 [26] | Quasi-experimental/interrupted time series | 2015–2017 | 3 (ED/academic; community; free-standing) | Reflex urine culture | Provider override |
| Demonchy, 2014 [27] | Quasi-experimental/treatment versus control versus treatment removal | 2012 | 3 (ED/academic) | EMR soft stop (Pop-up clinical guidelines) | Provider override |
| Epstein, 2016 [34] | Quasi-experimental/interrupted time series | 2011–2013 | 1 (ICU/academic) | Reflex urine culture | Provider override; special populations |
| Eudaley, 2019 [28] | Quasi-experimental/single group pre-post comparison | 2017 | 1 (outpatient/academic) | Test interpretation and treatment guidance | Provider override |
| Howard-Anderson, 2020 [29] | Quasi-experimental/interrupted time series | 2015–2018 | 3 (inpatient/academic; community) | Reflex urine culture | Special populations |
| Keller, 2018 [30] | Quasi-experimental/interrupted time series | 2014–2016 | 1 (inpatient/academic) | Asymptomatic bacteriuria passive guidance | None |
| Lee, 2021 [35] | Quasi-experimental/single group pre-post comparison | 2018–2020 | 12 (inpatient; outpatient/academic; community) | Reflex urine culture | None |
| Lynch, 2020 [36] | Quasi-experimental/interrupted time series | 2016–2018 | 3 (inpatient; ED; LTC/VHA) | Reflex urine culture | None |
| Munigala, 2018 [37] | Quasi-experimental/interrupted time series | 2015 | 1 (ED/academic) | Reflex urine microscopy | Provider override |
| Munigala, 2019 [31] | Quasi-experimental/interrupted time series | 2015–2017 | 1 (inpatient/academic) | Reflex urine culture | Special populations |
| Ourani, 2021 [32] | Quasi-experimental/single group pre-post comparison | 2020 | 1 (inpatient/academic) | Reflex urine culture | None |
| Sarg, 2016 [38] | Quasi-experimental/interrupted time series | 2012–2013 | 1 (ICU/academic) | Reflex urine culture | Provider override; special populations |
| Watson, 2020 [33] | Quasi-experimental/interrupted time series | 2017–2019 | 5 (inpatient/academic; community) | Reflex urine culture | None |
Abbreviations: ED, emergency department; EMR, electronic medical record; ICU, intensive care unit; LTC, long-term care; VHA, Veterans’ Health Administration.
Quality Assessment
The JBI quality assessment identified most studies as being fair quality (Supplementary Table 1). Four studies were deemed to be of poor quality due to poorly described study interventions, poorly defined outcomes, or inclusion of multiple interventions without distinguishing between individual components [28, 30, 32, 35].
Intervention Characteristics
The most common CDS intervention was a reflex urine culture approach (n = 10), which required that the urinalysis possess certain characteristics as a prerequisite for urine culture (Supplementary Table 2). These characteristics typically involved (1) urine white blood cell count (usually more than 10 per high-power fields as the threshold value), (2) presence of leukocyte esterase, and/or (3) presence of nitrites. Ourani et al [32] developed an interesting approach in which they evaluated various combinations of urinalysis parameters in the preintervention phase to determine which yielded the highest positive predictive values and applied these high-yield combinations during the intervention phase [29]. All reflex urine culture protocols allowed for urine culture to proceed if any prespecified parameters were abnormal, aside from Ourani et al [32], which required a combination of abnormal white blood cells plus 1 additional abnormal parameter.
Munigala et al [37] used a similar approach that instead required an abnormal urine dipstick, defined as having blood or greater than trace protein, prior to reflexing to urine microscopy. The remaining studies (n = 3) used reminders to provide diagnostic and treatment guidance, either through pop-up messages [26], passive messages accompanying orders [30], or a nudging order set [28].
Several of the interventions were not mandatory, in that users could ignore the guidance or circumvent the decision support through overriding the alerts or utilizing a different order (n = 7). In addition, several interventions included exceptions for special populations or circumstances, which included pregnancy [25, 29, 35], urologic procedures [25, 29, 35, 38], nephrostomy or suprapubic catheter specimen [35], neutropenia [29, 31], transplant recipient [29, 34, 35], or otherwise immunocompromised status [38].
Outcomes
Urine Culture Rate
Twelve studies provided data related to the primary outcome, 4 of which had almost identical study designs and reported sufficient data to allow them to be utilized within a meta-analysis [29, 31, 33, 36]. Ten studies reported a percentage change after the intervention, all of which found a decrease postintervention (Table 2). Of the 9 studies that provided a statistical comparison of the urine culture rate pre- and postintervention, 8 noted a statistically significant decrease.
Table 2.
Results of Primary Outcome of Urine Culture Rate by Study
| Author and Year | Preintervention | Postintervention | %Change | P Value |
|---|---|---|---|---|
| Claeys, 2021 [25]a | 35.8/1000 PD | 33.7/1000 PD | −5.9 | .8 |
| Coughlin, 2020 [26] | 15.2/100 ED visits | 9.3/100 ED visits | −38.8 | NR |
| Epstein, 2016 [34] | NR | NR (decreased) | NR | <.001 |
| Eudaley, 2019 [28] | 72% of visits for cystitis | 40% of visits for cystitis | −44.4 (−32 absolute) | .009 |
| Howard-Anderson, 2020 [29] | 35.2/1000 PD | 18.6/1000 PD | −47.2 | <.001 |
| Keller, 2018 [30] | 18.2% of monthly admissions | 11.8% of monthly admissions | −35.2 (−6.4 absolute) | <.001 |
| Lynch, 2020 [36]b | 3.6/100 PD | 1.8/100 PD | −50 | <.001 |
| Munigala, 2018 [37] | 54.3/1000 ED visits | 29.7/1000 ED visits | −45.3 | <.001 |
| Munigala, 2019 [31] | 38.1/1000 PD | 20.9/1000 PD | −45.1 | <.001 |
| Ourani, 2021 [32] | NR | 24.6% of total urine samples | NR | NR |
| Sarg, 2016 [38] | 139/1000 PD | 93/1000 PD | −33.1 | NR |
| Watson, 2020 [33] | 1175.8/10 000 PD | 701.4/10 000 | −40.3 | <.01 |
Abbreviations: CAUTI, catheter-associated urinary tract infection; DOT, days of therapy; ED, emergency department; GN-BSI, Gram-negative bloodstream infection; NR, not reported; PD, patient days.
Comparison of preintervention and postintervention results among intervention sites only.
Acute care results only.
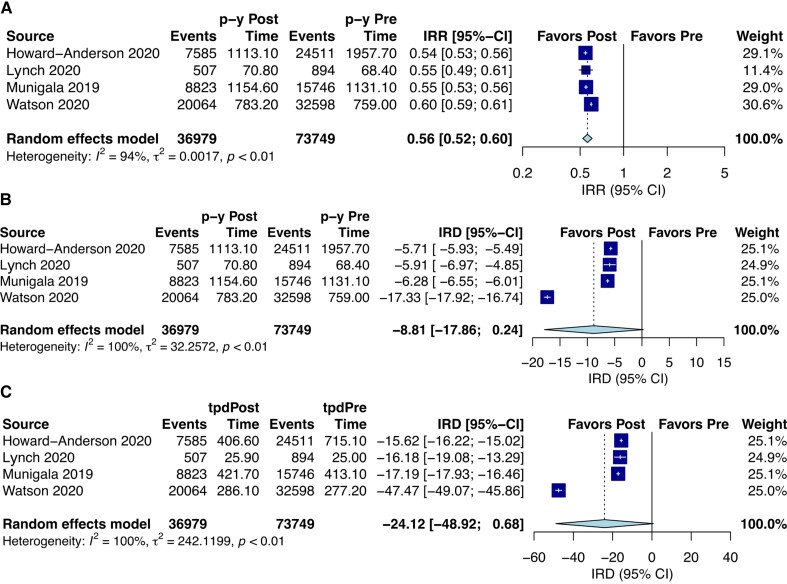
Four studies were included in the meta-analysis [29, 31, 33, 36]. The percentage change in urine culture rate from pre- to postintervention in individual studies ranged from −45.6 to −40.4 (Table 3). An IRR for urine culture performance of 0.56 (95% confidence interval [CI], .52–.60; I2 = 94%) significantly favoring the postintervention group was observed (P < .001) (Figure 2A). In addition, an IRD per person-years of −8.81 (95% CI, −17.86 to .24; I2 = 100%; P = .053) and an IRD per 1000 person-days of −24.12 (95% CI, −48.92 to .68; I2 = 100%; P = .054) were found (Figure 2BandC).
Table 3.
Pre- and Postintervention Urine Culture Rates for Studies Included in Meta-Analysis
| Author and Year | Preintervention Urine Culture Rate (n Urine Cultures/n PD) | Postintervention Urine Culture Rate (n Urine Cultures/n PD) | %Change |
|---|---|---|---|
| Howard-Anderson, 2020 [29] | 35.2/1000 PD (24 511/715 055 PD) | 18.6/1000 PD (7585/406 552 PD) | −45.6 |
| Lynch, 2020 [36]a | 3.58/100 PD (894/24 972 PD) | 1.82/100 PD (507/25 857 PD) | −45.2 |
| Munigala, 2019 [31] | 38.1/1000 PD (15 746/413 137 PD) | 20.9/1000 PD (8823/421 714 PD) | −45.1 |
| Watson, 2020 [33] | 1175.8/10 000 PD (32 598/277 241 PD) | 701.4/10 000 PD (20 064/286 056 PD) | −40.4 |
Abbreviation: PD, patient days.
Acute care results only.
Figure 2.
(A) Incidence rate ratio (IRR) postintervention over preintervention. (B) Incidence rate difference (IRD) between postintervention and preintervention in person-years (p-y). (C) Incidence rate difference (IRD) between postintervention and preintervention in 1000 person-days. Abbreviations: CI, confidence interval; tpd, total person-days.
Publication bias was not assessed because of an inadequate number of included studies (<10) for the meta-analysis. It is recommended to have a minimum of 10 studies to properly assess a funnel plot or to assess the use of other more advanced regression-based methods [39].
Catheter-Associated Urinary Tract Infections
Four studies evaluated the impact of CDS on CAUTI rates (Table 4) [31, 33, 34, 36]. Epstein et al [34], utilizing a reflex urine culture protocol in 5 ICU locations, noted a significant downward trend in CAUTI rates during the postintervention period. Although individual rates by unit were not provided, the steepness of the downtrend appeared to be proportional to the preintervention CAUTI rate, with higher preintervention rates being most impacted by the intervention. Lynch et al [36] noted an approximate 10% decrease in CAUTI per 1000 catheter days in acute care units after a reflex culture protocol was initiated. They additionally noted that 29% of preintervention CAUTI cases occurred in patients without pyuria, meaning they would not have been reported had the intervention already been in place. However, 2 other studies that examined CAUTI rates noted no significant changes postintervention [31, 33].
Table 4.
Secondary Outcome Results by Outcome Measure Category and Study
| Author and Year | Outcome Measure | Preintervention | Postintervention | %Change | P Value |
|---|---|---|---|---|---|
| CAUTI | |||||
| Epstein, 2016 [34] | CAUTI rate | NR | NR (decreased) | NR | .04 |
| Lynch, 2020 [36]a | CAUTI rate | 1.82/1000 catheter days | 1.64/1000 catheter days | −9.9 | NR |
| Munigala, 2019 [31] | CAUTI rate | 0.3/1000 PD | 0.3/1000 PD | 0 | .87 |
| Watson, 2020 [33] | CAUTI rate | 11.5/10 000 catheter days | 9.3/10 000 catheter days | −19.1 | .23 |
| Antimicrobial Use | |||||
| Demonchy, 2014 [27] | Guideline-concordant antimicrobial prescription rate | 31.7% | 47.6% | +50.2 (+15.9 absolute) | NR |
| Eudaley, 2019 [28] | Fluoroquinolone use | 42% | 15% | −64.3 (−27 absolute) | <.001 |
| Lee, 2021 [35] | Antimicrobial prescription per normal urinalysis rate | 48.7% | 43.4% | −10.8 (−5.3 absolute) | <.001 |
| Lynch, 2020 [36]a | Antimicrobial prescription per normal urinalysis rate | 35.9% | 31.0% | −13.6 (−4.9 absolute) | NR |
| Ourani, 2021 [32] | Antimicrobial prescription per normal urine culture rate | 45.1% | 9.0% | −80.0 (−36.1 absolute) | <.001 |
| Sarg, 2016 [38] | Antimicrobial DOT | 449/1000 PD | 425/1000 PD | −5.3 | NR |
| Watson, 2020 [33] | Antimicrobial DOT | 102.5/1000 PD | 86.9/1000 PD | −15.2 | <.01 |
Abbreviations: CAUTI, catheter-associated urinary tract infection; DOT, days of therapy; ED, emergency department; NR, not reported; PD, patient days.
Acute care results only.
Antimicrobial Use
The effect of CDS on different measures in relation to antimicrobial use was evaluated in 7 studies (Table 4). Three studies [32, 35, 36] found a decrease in antimicrobial prescriptions for patients with normal urine specimen results, notably with an 80% (45% vs 9%) decrease in rate of prescriptions for normal urine cultures by Ourani et al [32]. Two studies [33, 38] showed a decrease in antimicrobial days of therapy per patient days. In addition, Demonchy et al [27] found a 50% increase in the rate of guideline-concordant antimicrobial prescriptions, and Eudaley et al [28] found a 64% decrease in fluoroquinolone use.
Additional Outcomes
Several additional outcomes were evaluated related to cost-effectiveness, CDS tool utilization, adverse outcomes, and other diagnostic testing measures (Supplementary Table 3). All 3 studies which included cost data noted large savings related to laboratory spending on urine cultures [31, 33, 35]. Watson et al [33] also noted a 30% annual decrease on CAUTI spending. There were no significant differences postintervention in adverse events that could be related to inappropriately omitting a urine culture or inappropriately treating with antimicrobials. These included bloodstream infection [25, 35] or C. difficile infection [35, 38] rates, emergence of multidrug resistant organisms [35], mortality [35], and delayed urine culture ordering [37]. Only 2 studies reported utilization rates, which were 59% [27] and 29% [28], both involving interventions that were optional. Other findings related to diagnostic testing included increased urine culture positivity rates [26, 29], decreased catheter urine culture rates [37], and mixed urinalysis rates results [30, 37].
DISCUSSION
Despite well described harms of ASB treatment, the practice of routine urine testing and treatment based on laboratory results rather than clinical findings persists. Prevention of false UTI diagnoses and subsequent unnecessary antibiotic treatment thus relies upon mindful diagnostic stewardship efforts. In this systematic review and meta-analysis, we identified 14 studies describing interventions utilizing CDS to improve the diagnostic accuracy of urine culture, most of which involved a reflex urine culture approach. All studies that reported the impact on urine culture rate found a decrease, most of which were statistically significant. The meta-analysis of 4 studies showed ∼40% decreased incidence rate of urine cultures in the postintervention group after implementation of CDS, compared to the preintervention group. The uniformity and degree of change noted in these studies confer some degree of confidence that a CDS intervention restricting urine culture based on urinalysis can help decrease urine culture rates. Moreover, the additional studies included only in the systematic review similarly showed decreased urine culture rates after CDS implementation. However, the high heterogeneity among the included studies and the moderate to high level of bias detected could render the results more ambiguous and should thus be interpreted with caution.
More importantly, restricting urine cultures to patients with positive urinalyses does not prevent all patients with ASB from receiving an erroneous diagnosis of UTI and subsequent exposure to unnecessary antimicrobial treatment. Many patients with ASB may have abnormal urinalysis parameters, and special populations that may not require treatment for ASB may nevertheless be excluded from these interventions, such as patients with neutropenia [29, 31] or prior transplant [29, 34, 35]. Interventions designed to assist the provider with recognition of ASB such as that of Keller et al [30] represent one CDS approach that may be helpful in this regard. Other more novel approaches to CDS such as integration of natural language processing tools may be considerations for future studies.
Several studies measured the impact of CDS on antimicrobial use and found decreases in antimicrobial prescriptions for normal urine specimens, antimicrobial days of therapy, use of target antimicrobial classes such as fluoroquinolones, and prescriptions discordant with guidelines. However, there was substantial variation in how antimicrobial use was measured between studies, for example, antimicrobial days of therapy per patient-days versus antimicrobial prescriptions per normal urinalysis or urine culture. Furthermore, antimicrobial use is an intermediate outcome to more relevant clinical and patient-centered outcomes such as antimicrobial-associated adverse events, healthcare utilization, mortality, and patient satisfaction. Only 2 studies [35, 38] evaluated such outcomes, including Lee et al [35] who considered C. difficile infection rate, emergence of multidrug resistance, and mortality, and Sarg [38] et al who considered C. difficile rate only. Neither study noted a meaningful difference in these outcomes between before and after the intervention. Future prospective studies should consider the impact of urine culture CDS on prescription of nonfirst-line or broad-spectrum agents and duration of antimicrobial therapy.
Another outcome of interest is CAUTI rate, which was examined in 4 of the included studies. Although the findings generally pointed toward a decrease in diagnosis rate, only Epstein et al [34] reported a statistically detectable difference. These results are likely limited by small sample size, because the preintervention rate of CAUTI was generally low. Interventions targeting urinalysis may also not be optimal for patients with catheters who are highly likely to have abnormal laboratory parameters regardless of presence of true infection. If the ineffectiveness of CDS for CAUTI reduction is replicated in future studies, this could suggest that inadequate attempts to enhance preventative measures such as reduction in catheter days and aseptic technique are greater contributors to CAUTI rate than misdiagnoses. Alternatively, it could suggest that CDS can be overridden or ignored more facilely in patients with catheter use.
Several key outcomes were insufficiently studied, the most important of which being unintended adverse events related to missed diagnoses. Two studies examined bloodstream infections, whereas Munigala et al [37] evaluated subsequent inpatient urine cultures, and 2 of these 3 studies found a small, nonstatistically significant increase in rates postintervention. This renders the decreased costs identified by several studies difficult to interpret.
Clinical decision support related to any quality measure has several factors that must be considered. “Alert fatigue,” or a failure to accept CDS guidance due to an excessively high volume or frequency of alerts, is a common concern with CDS implementation [40, 41]. Moreover, providers may experience frustration with CDS due to perception of increased workload or hindrance to patient care, risking increased provider burnout. Each of the interventions discussed here included various factors that could impact CDS acceptance, such as ability to override guidance and exceptions for special populations. However, only 2 studies evaluated CDS utilization rate, despite that provider override was an option for at least half of the interventions. Furthermore, provider satisfaction when override was not an option was not studied. The importance of these outcomes is considerable, because the effectiveness of CDS relies upon finding the correct balance between improving the desired outcome and limiting negative impact on provider workload and patient care. With the amount of resources that must be devoted to CDS development, achieving this balance is critical.
Our study has several potential limitations. Our search strategy was limited to full manuscripts; abstracts or posters without an accompanying manuscript were excluded. In addition, the potential exists for data describing no difference in outcomes or other undesirable findings to have been left unpublished. Of the studies that were identified, the total number was small, and although quantitative analysis was performed, only 4 studies had a consistent approach and complete data that allowed for their inclusion into the meta-analysis. It should be noted as well that these 4 studies had slight variations in their urinalysis criteria, although this was thought to be unlikely to have a significant impact on the results. Other factors that may have contributed to the heterogeneity between these studies include differences in hospital setting, number of facilities, inclusion or exclusion of patients seen in the emergency department, and geographical location. Finally, the quality of the data was limited given that none of the studies were randomized controlled trials, although study quality was graded as fair or good in most cases.
CONCLUSIONS
The results of this systematic review and meta-analysis suggest that CDS appears to be an effective means of reducing urine culture rate for adults in a wide variety of settings. However, the impact of urine culture CDS on additional outcomes such as CAUTI rate, antimicrobial use, C. difficile infection rate, development of antimicrobial resistance, and mortality has not been established. Moreover, important negative outcomes including effects of delayed UTI diagnosis and provider dissatisfaction are largely unstudied in this context. Regardless, the magnitude of the reduction in urine culture rate identified in our study combined with the known association between ASB treatment and negative outcomes provide a basis for future clinical trials evaluating CDS in the context of urine culture diagnostic stewardship.
Supplementary Material
Acknowledgments
Potential conflicts of interest. E.A.S. serves as a consultant for Janssen. C.J.D. has received research funding from Clorox, PDI, and Pfizer. A.D. has received funding from Clorox and Seres Therapeutics and serves as a consultant for Merck. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Leila S Hojat, Department of Medicine, Division of Infectious Diseases, Case Western Reserve University, Cleveland, Ohio, USA; Division of Infectious Diseases and HIV Medicine, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Elie A Saade, Department of Medicine, Division of Infectious Diseases, Case Western Reserve University, Cleveland, Ohio, USA; Division of Infectious Diseases and HIV Medicine, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Adrian V Hernandez, Health Outcomes, Policy, and Evidence Synthesis (HOPES) Group, Department of Pharmacy Practice, University of Connecticut School of Pharmacy, Storrs, Connecticut, USA; Unidad de Revisiones Sistemáticas y Meta-análisis (URSIGET), Vicerrectorado de Investigación, Universidad San Ignacio de Loyola (USIL), Lima, Peru.
Curtis J Donskey, Department of Medicine, Division of Infectious Diseases, Case Western Reserve University, Cleveland, Ohio, USA; Geriatric Research, Education, and Clinical Center, Louis Stokes Cleveland Veterans’ Affairs Medical Center, Cleveland, Ohio, USA.
Abhishek Deshpande, Center for Value Based Care Research, Cleveland Clinic Community Care, Cleveland Clinic, Cleveland, Ohio, USA; Department of Infectious Diseases, Respiratory Institute, Cleveland Clinic, Cleveland, Ohio, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Versporten A, Zarb P, Caniaux I, et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health 2018; 6:e619–29. [DOI] [PubMed] [Google Scholar]
- 2. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. Morb Mortal Wkly Rep 2014; 63:194–200. [PMC free article] [PubMed] [Google Scholar]
- 3. Shpunt Y, Estrin I, Levi Y, et al. Antimicrobial use for asymptomatic bacteriuria—first, do no harm. Infect Control Hosp Epidemiol 2021; 42:37–42. [DOI] [PubMed] [Google Scholar]
- 4. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis 2019; 68:e83–110. [DOI] [PubMed] [Google Scholar]
- 5. Yin P, Kiss A, Leis JA. Urinalysis orders among patients admitted to the general medicine service. JAMA Intern Med 2015; 175:1711–3. [DOI] [PubMed] [Google Scholar]
- 6. Asscher AW, Sussman M, Waters WE, et al. Asymptomatic significant bacteriuria in the non-pregnant woman. Br Med J 1969; 1:804–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cai T, Mazzoli S, Mondaini N, et al. The role of asymptomatic bacteriuria in young women with recurrent urinary tract infections: to treat or not to treat? Clin Infect Dis 2012; 55:771–7. [DOI] [PubMed] [Google Scholar]
- 8. Dufour AB, Shaffer ML, D’Agata EMC, Habtemariam D, Mitchell SL. Survival after suspected urinary tract infection in individuals with advanced dementia. J Am Geriatr Soc 2015; 63:2472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Das R, Towle V, Van Ness PH, Juthani-Mehta M. Adverse outcomes of increasing episodes of observed bacteriuria in nursing home residents. Infect Control Hosp Epidemiol 2011; 32:84–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. U.S. Preventive Services Task Force . Screening for asymptomatic bacteriuria in adults: U.S. Preventive services task force reaffirmation recommendation statement. Ann Intern Med 2008; 149:43–7. [DOI] [PubMed] [Google Scholar]
- 11. Cope M, Cevallos ME, Cadle RM, Darouiche RO, Musher DM, Trautner BW. Inappropriate treatment of catheter-associated asymptomatic bacteriuria in a tertiary care hospital. Clin Infect Dis 2009; 48:1182–8. [DOI] [PubMed] [Google Scholar]
- 12. Spivak ES, Burk M, Zhang R, et al. Management of bacteriuria in veterans affairs hospitals. Clin Infect Dis 2017; 65:910–7. [DOI] [PubMed] [Google Scholar]
- 13. Hartley S, Valley S, Kuhn L, et al. Overtreatment of asymptomatic bacteriuria: identifying targets for improvement. Infect Control Hosp Epidemiol 2015; 36:470–3. [DOI] [PubMed] [Google Scholar]
- 14. Grein JD, Kahn KL, Eells SJ, et al. Treatment for positive urine cultures in hospitalized adults: a three medical center survey of prevalence and risk factors. Infect Control Hosp Epidemiol 2016; 37:319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hecker MT, Fox CJ, Son AH, et al. Effect of a stewardship intervention on adherence to uncomplicated cystitis and pyelonephritis guidelines in an emergency department setting. PLoS One 2014; 9:e87899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter–associated asymptomatic bacteriuria. JAMA Intern Med 2015; 175:1120–7. [DOI] [PubMed] [Google Scholar]
- 17. Kelley D, Aaronson P, Poon E, McCarter YS, Bato B, Jankowski CA. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol 2014; 35:193–5. [DOI] [PubMed] [Google Scholar]
- 18. Rawson TM, Moore LSP, Hernandez B, et al. A systematic review of clinical decision support systems for antimicrobial management: are we failing to investigate these interventions appropriately? Clin Microbiol Infect 2017; 23:524–32. [DOI] [PubMed] [Google Scholar]
- 19. Borbolla D, Ficheur G. Clinical decision support systems and computerized provider order entry: contributions from 2020. Yearb Med Inform 2021; 30:172–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Willis VC, Thomas Craig KJ, Jabbarpour Y, et al. Digital health interventions to enhance prevention in primary care: scoping review. JMIR Med Inform 2022; 10:e33518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kayalp D, Dogan K, Ceylan G, Senes M, Yucel D. Can routine automated urinalysis reduce culture requests? Clin Biochem 2013; 46:1285–9. [DOI] [PubMed] [Google Scholar]
- 22. Stovall RT, Haenal JB, Jenkins TC, et al. A negative urinalysis rules out catheter-associated urinary tract infection in trauma patients in the intensive care unit. J Am Coll Surg 2013; 217:162–6. [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62:1006–12. [DOI] [PubMed] [Google Scholar]
- 24. Joanna Briggs Institute . Checklist for quasi-experimental studies. Available at: https://jbi.global/critical-appraisal-tools. Accessed 28 August 2022.
- 25. Claeys KC, Zhan M, Pineles L, et al. Conditional reflex to urine culture: evaluation of a diagnostic stewardship intervention within the veterans’ affairs and Centers for Disease Control and Prevention practice-based research network. Infect Control Hosp Epidemiol 2021; 42:176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coughlin RF, Peaper D, Rothenberg C, et al. Electronic health record–assisted reflex urine culture testing improves emergency department diagnostic efficiency. Am J Med Qual 2020; 35:252–7. [DOI] [PubMed] [Google Scholar]
- 27. Demonchy E, Dufour JC, Gaudart J, et al. Impact of a computerized decision support system on compliance with guidelines on antibiotics prescribed for urinary tract infections in emergency departments: a multicentre prospective before-and-after controlled interventional study. J Antimicrob Chemother 2014; 69:2857–63. [DOI] [PubMed] [Google Scholar]
- 28. Eudaley ST, Mihm AE, Higdon R, Jeter J, Chamberlin SM. Development and implementation of a clinical decision support tool for treatment of uncomplicated urinary tract infections in a family medicine resident clinic. J Am Pharm Assoc 2019; 59:579–85. [DOI] [PubMed] [Google Scholar]
- 29. Howard-Anderson J, Ashraf S, Overton E, Reif L, Murphy DJ, Jacob JT. Sustained decrease in urine culture utilization after implementing a reflex urine culture intervention: a multicenter quasi-experimental study. Infect Control Hosp Epidemiol 2020; 41:369–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keller SC, Feldman L, Smith J, Pahwa A, Cosgrove SE, Chida N. The use of clinical decision support in reducing diagnosis of and treatment of asymptomatic bacteriuria. J Hosp Med 2018; 13:392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Munigala S, Rojek R, Wood H, et al. Effect of changing urine testing orderables and clinician order sets on inpatient urine culture testing: analysis from a large academic medical center. Infect Control Hosp Epidemiol 2019; 40:281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ourani M, Honda NS, MacDonald W, Roberts J. Evaluation of evidence-based urinalysis reflex to culture criteria: impact on reducing antimicrobial usage. Int J Infect Dis 2021; 102:40–4. [DOI] [PubMed] [Google Scholar]
- 33. Watson KJ, Trautner B, Russo H, et al. Using clinical decision support to improve urine culture diagnostic stewardship, antimicrobial stewardship, and financial cost: a multicenter experience. Infect Control Hosp Epidemiol 2020; 41:564–70. [DOI] [PubMed] [Google Scholar]
- 34. Epstein L, Edwards JR, Halpin AL, et al. Evaluation of a novel intervention to reduce unnecessary urine cultures in intensive care units at a tertiary care hospital in Maryland, 2011–2014. Infect Control Hosp Epidemiol 2016; 37:606–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee ALH, Leung ECM, Lee MKP, Lai RWM. Diagnostic stewardship programme for urine culture: impact on antimicrobial prescription in a multi-centre cohort. J Hosp Infect 2021; 108:81–9. [DOI] [PubMed] [Google Scholar]
- 36. Lynch CS, Appleby-Sigler A, Bork JT, et al. Effect of urine reflex culturing on rates of cultures and infections in acute and long-term care. Antimicrob Resist Infect Control 2020; 9:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Munigala S, Jackups RR Jr, Poirier RF, et al. Impact of order set design on urine culturing practices at an academic medical center emergency department. BMJ Qual Saf 2018; 27: 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sarg M, Waldrop GE, Beier MA, et al. Impact of changes in urine culture ordering practice on antimicrobial utilization in intensive care units at an academic medical center. Infect Control Hosp Epidemiol 2016; 37:448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Page MJ, Higgins JPT, Sterne JAC. Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Ed. Chichester, (UK): John Wiley & Sons; 2019: 349–74. [Google Scholar]
- 40. van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc 2006; 13:138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ancker JS, Edwards A, Nosal S, et al. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak 2019; 19:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.