Abstract
A novel injectable pre-exposure prophylaxis, cabotegravir, has greater efficacy and acceptability than oral tenofovir/emtricitabine for prevention of HIV infection. Cabotegravir is currently priced at $22 200 per year, >185 times higher than the $60–$119 estimated cost-effectiveness threshold for middle-income countries (MICs). Following civil society pressure, ViiV provided access to generic versions in 90 countries with the Medicines Patent Pool (MPP), including all African nations. However, several MICs outside Africa have rapidly growing HIV epidemics. We analyzed the ViiV-MPP deal to assess population covered and gross domestic product (GDP) per capita. There were 38 countries excluded from the ViiV-MPP deal despite having a GDP per capita lower than the highest-earning African country. These countries include 2.4 billion people (30% global population), with an incidence of 122 000 (8%). For cabotegravir to have a significant impact on HIV infections, millions will need to be treated at affordable prices in a wide range of countries.
Keywords: PrEP, HIV, access, cabotegravir
Antiretroviral therapy (ART) has reached 28.7 million people, with virologic suppression minimizing transmission risk of HIV [1]. However, inequalities maintain incidence at 1.5 million (2021) [1, 2]. Oral pre-exposure prophylaxis (PrEP; tenofovir/emtricitabine [TDF/FTC]) has been revelatory in combatting incidence; the iPrEx and IPERGAY studies, among many others, have proved efficacy, and PrEP is cheap and readily available [3, 4]. Most governments now recommend its use [5]. However, poor adherence to TDF/FTC in all populations, but particularly in young and heterosexual female populations, limits its effectiveness [6]. Cabotegravir (CAB-LA), the first long-acting injectable PrEP, produced by ViiV, eliminates timely pill-taking and so could improve uptake for many.
CAB-LA has superior efficacy to TDF/FTC, showing 66% risk reduction in the HPTN083 trial in cisgender men and transgender women and 88% in HPTN084 for cisgender women [7, 8]. Both were stopped due to efficacy. CAB-LA has the potential to substantially reduce new HIV infections if used widely, as demonstrated by the 27% reduction in HIV incidence calculated to be possible over the next 20 years in patients using CAB-LA when compared with TDF/FTC in South Africa [9]. This is promising as global incidence currently matches mortality, so that the number of people with HIV (PWH) remains stable, sustaining the pandemic [1].
ViiV markets CAB-LA at US$22 200 per year in the United States and £7100 per year in the United Kingdom [10], vastly outpricing CAB-LA for most people who may benefit from using PrEP. Conversely, generic TDF/FTC is available in the United Kingdom at £168 per year [11], and $55 per year via the Pan-American Health Organisation [12]. From January 2022 to October 2022, there were £20 million CAB-LA sales in the United States, equaling 1059 patient-years when using the market price of US$22 200 [13].
For CAB-LA to reach at-risk populations across low- and middle-income countries (LMICs), significant health system strengthening is needed across infrastructure, training, and primary care. These structural conditions for health are highly politicized, slowing mobilization. As a result, even tiered costs for branded CAB-LA would be untenable. While ViiV suggests a cost price of $1440 per year, a South African cost-effectiveness and threshold analysis suggests that a cost between $60 and $119 per year would need to be achieved for CAB-LA to be as cost-effective as TDF/FTC [9]. Estimates for the lowest possible pricing are around $16 per year [14]. ViiV has responded to civil society pressure [15], agreeing to a voluntary license with the Medicines Patent Pool (MPP) [16].The deal, enabling 90 countries to procure generic CAB-LA, has drawn criticism for its delay and exclusivity, ignoring many MICs [16, 17].
Here, we calculate the number needed to treat to show benefit (NNTB) for cabotegravir compared with oral PrEP, analyze the ViiV-MPP deal in terms of GDP, population, and global incidence, and summarize the latest data on global CAB-LA pricing.
METHODS
To calculate NNTB for CAB-LA, data on infection rates were extracted from the HPTN083/084 studies [7, 8] to give the relative risk of infection for extrapolation to sales figures and ViiV-MPP deal analysis.
We gathered incidence data across 178 countries from the UNAIDS AIDSinfo database; 41 nations did not have 2021 data [18]. All 178 countries were classified by 2021 World Bank income status and inclusion/exclusion from the ViiV-MPP deal [16, 19].
Country-level data on 2021 population and GDP were then extracted from the World Bank database for all 178 countries [20]. GDP per capita was calculated as GDP/population. As the African continent was included in the ViiV-MPP deal (bar Libya), we analyzed the GDP per capita of high-incidence nations excluded from the deal compared with the highest GDP per capita African nations included to assess the appropriateness of exclusion.
Yearly pricing figures pertain to 6 doses, bimonthly, whereas an extra dose is given in the first year of treatment.
RESULTS
Population-level data showed that 47% of the global population (3.7 billion people) lives in countries not covered by the ViiV-MPP deal, 31% of which (2.5 billion) lives in MICs.
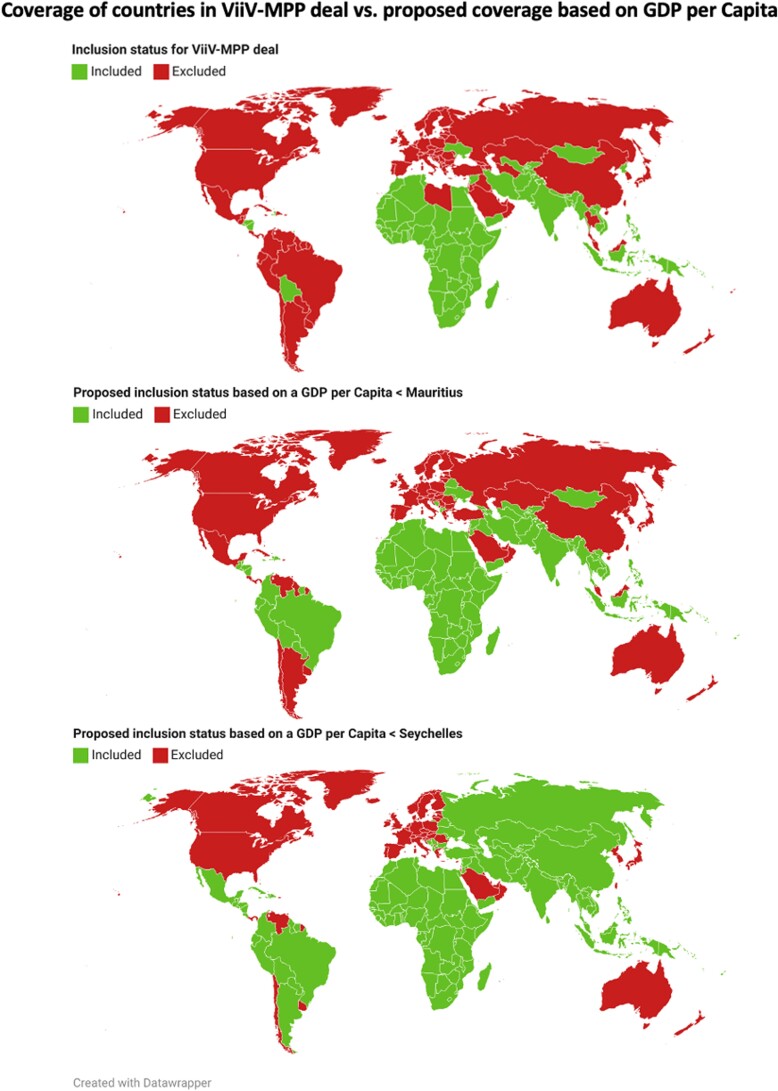
Figure 1 A shows countries that may access generic CAB-LA via ViiV-MPP. Figure 1, B and C, shows the additional countries that would have access to generic versions based on a GDP per capita lower than that of Mauritius ($8800) and the Seychelles ($13 300)—the 2 richest African nations currently included. Mauritius as the decisive GDP per capita includes 24 countries excluded from the ViiV-MPP deal, including Thailand and Brazil. The Seychelles, higher again, includes a further 14 nations currently excluded, for a total of 38, including China, Argentina, and the Russian Federation.
Figure 1.
Choropleth maps comparing the countries currently included in the ViiV-MPP deal (Figure 1a—top) and the proposed countries that would be included in a deal based on GDP per capita of Mauritius (Figure 1b—middle), and Seychelles (Figure 1c—bottom) as the definitive metric for inclusion. On all maps countries in green are included and countries in red are excluded.
Of these 38 countries across 5 continents, 1 was an LMIC, and the remaining 37 were upper middle-income countries. These 38 countries had a total population of 2.4 billion people (30%). There were 122 000 estimated infections across these 38 countries, 8% of global incidence. This estimate does not account for poorly documented rates in Russia and China, home to >1.5 billion people; most recent national estimates of their combined incidence give a conservative 145 000 [21, 22]. Their inclusion makes 267 000 excluded infections, 18% of global incidence. These estimates, however, are difficult to verify and have thus been excluded from the analysis.
Table 1 shows that the ViiV-MPP deal does not discriminate by national wealth; 6 African nations have a higher GDP per capita than excluded high-incidence nations. The 10 richest African nations included show a similar average GDP per capita as that of 10 high-incidence excluded MICs (mean, $6936 vs $8418) and a total incidence of 240 000 vs 109 000. However, South Africa is a major outlier in incidence, bearing 87% of infections out of the 10 richest African nations (210 000). Excluding South Africa, there are only 30 000 infections across these countries. Furthermore, the top 5 high-incidence nations excluded have a caseload of 88 600 compared with just 15 000 for the 5 highest-GDP African nations.
Table 1.
Population, PWH, and Incidence of HIV Infection in the Richest African Nations Included in the Deal and Highest-Incidence Nations Excluded From the Deal With a Lower GDP per Capita Than the Seychelles, Ranked by GDP per Capita
| Countrya | Population, Millions | PWH | Incidence | GDP per Capita, US$ |
|---|---|---|---|---|
| Seychelles | 0.1 | 600 | 75 | 13 307 |
| Malaysia | 32.8 | 82 000 | 5500 | 11 371 |
| Argentina | 45.8 | 140 000 | 5000 | 10 729 |
| Kazakhstan | 19.0 | 35 000 | 3500 | 10 041 |
| Mexico | 13.0 | 360 000 | 18 000 | 9926 |
| Mauritius | 1.3 | 12 000 | 1000 | 8812 |
| Dominican Republic | 11.0 | 78 000 | 4200 | 8603 |
| Equatorial Guinea | 1.5 | 66 000 | 5200 | 8462 |
| Gabon | 2.3 | 47 000 | 1700 | 8017 |
| Brazil | 214.0 | 960 000 | 50 000 | 7521 |
| Botswana | 2.4 | 360 000 | 7200 | 7348 |
| Thailand | 70.0 | 520 000 | 6500 | 7233 |
| South Africa | 60.0 | 7 500 000 | 210 000 | 6994 |
| Peru | 33.4 | 98 000 | 5500 | 6692 |
| Colombia | 51.3 | 170 000 | 8600 | 6131 |
| Ecuador | 17.9 | 47 000 | 2000 | 5935 |
| Namibia | 2.6 | 220 000 | 6600 | 4729 |
| Eswatini | 1.1 | 220 000 | 6900 | 4215 |
| Tunisia | 11.9 | 5400 | 500 | 3924 |
| Morocco | 37.3 | 23 000 | 1000 | 3554 |
| Highest GDP per capita of African nations | ||||
| Total | 120 573 480 | 845 400 | 240 175 | |
| Mean | 6936 | |||
| Median | 3450 | 7171 | ||
| Top incidence of nations excluded from ViiV-MPP dealb | ||||
| Total | 625 201 470 | 2 490 000 | 108 800 | |
| Mean | 8419 | |||
| Median | 5500 | 8062 | ||
Abbreviations: GDP, gross domestic product; PWH, people with HIV.
Non-African nations in bold.
Bar China and Russia, as incidence figures are out of date.
With the most up-to-date numbers from HPTN083/084, there were 95/4797 infections on TDF/FTC (1.98% incidence) vs 19/4818 on CAB-LA (0.39% incidence) (Table 2), an incidence reduction of 1.59%. Applying this incidence reduction of 1.59% to the 1059 patient-years calculated from the $20 million CAB-LA sales in the United States and the market price of US$22 200 per patient per year, at this price just 16 infections would have been prevented using CAB-LA compared with the same population receiving oral PrEP.
Table 2.
Data From HPTN 083/084 Trials on Infection Rate by Treatment Arm, Extrapolated Across to Give Risk Reduction on Cabotegravir vs TDF/FTC, and Number Needed to Treat to Cause Benefit as a Result
| Infection Rate, No. (%) | Risk Reduction, % | NNTB | ||
|---|---|---|---|---|
| Trial | TDF/FTC | Cabotegravir | ||
| HPTN 083 | 39/3187 (1.22) | 13/3205 (0.41) | 0.81 | 124 |
| HPTN 084 | 56/1610 (3.48) | 6/1613 (0.37) | 3.11 | 33 |
| Both | 95/4797 (1.98) | 19/4818 (0.39) | 1.59 | 63 |
Abbreviations: NNTB, number needed to treat to cause benefit (prevention of 1 infection), calculated by 100 divided by risk reduction; TDF/FTC, tenofovir/emtricitabine.
This incidence reduction of 1.59% equates to an NNTB of 63, that is, 63 patients on CAB-LA to prevent 1 infection compared with TDF/FTC (Table 2). Subsequently, to prevent 1.5 million annual infections, CAB-LA must reach 94 million people. Furthermore, to cover 122 000 infections across nations excluded from the ViiV-MPP deal (excluding China and Russia), nearly 7.7 million people would have to be treated annually at branded prices.
DISCUSSION
Our analysis reveals that access to CAB-LA by the ViiV-MPP deal is arbitrary, motivated by neither incidence nor national wealth. African nations have been included indiscriminately (bar Libya), while 38 MICs were excluded from the deal despite similar GDP per capita as included African nations and often higher incidence of new HIV infections (Table 1). Examples include Thailand, home to nearly half a million PWH, of whom 1 in 4 are untreated, and Brazil, where CAB-LA clinical trials were conducted (Table 1) [23, 24].
While GDP per capita is useful for ascertaining the industrial wealth of nations, it does not correlate to health expenditure or well-being. Neither are inequalities in health care accounted for; these are rife in many excluded nations despite high GDP per capita. Databases for alternative measures are unfortunately incomplete, outdated, or difficult to analyze [25, 26]. In summary, GDP per capita is used as a basic measure of national wealth correlating roughly to healthcare expenditure, to question justification of the deal.
Branded CAB-LA will not be cost-effective. High NNTB means that CAB-LA will only be a feasible alternative to TDF/FTC if prices are dramatically cut. ViiV currently claims a lowest price of $1440 per year, 16 times higher than cost-effectiveness estimates and 100 times higher than the lowest possible pricing estimates [9, 14]. ViiV is seeking regulatory approval in several excluded countries where clinical trials were conducted, such as Brazil, spiking further ethical questions around post-trial access.
Overall, arbitrary decisions on licensing cause unmet PrEP needs in nations that are no more able to afford CAB-LA than included nations. Within excluded MICs, populations structurally disempowered from achieving suppression on ART due to inequitable services and stigma will be unable to use injectable PrEP, despite it being more effective and tolerable, which will worsen existing inequalities in exposure and propensity to infection [2].
The structures that limit adherence to ART and oral PrEP will reduce access to CAB-LA, more so where high prices limit capacity or will to address important determinants of access beyond procurement. The necessity for advocacy on unfair licensing detracts from a global need and responsibility to address structural causes of sustained HIV incidence for progress toward the 95-95-95 goals.
CONCLUSIONS
Despite greater efficacy and higher reported acceptability in at-risk populations, 63 people must be treated with CAB-LA instead of oral PrEP to prevent 1 HIV infection, so only cheap generic production can unlock any benefit. The current ViiV-MPP deal must be widened; 2.4 billion (30%) people live in countries excluded from the deal despite having a lower GDP per capita than the Seychelles, the highest-earning African nation included, so that 122–267 thousand (8%–18% global) new cases of HIV are missed. Truly equitable public procurement must be coupled with improvement on wider health determinants like infrastructure, primary care, and social protection. Overall, there is limited justification for the ViiV-MPP deal on CAB-LA, which should be expanded to all MICs.
Acknowledgments
Financial support. This work was supported by the International Treatment Preparedness Coalition, Make Medicines Affordable campaign.
Patient consent. This paper does not include factors necessitating patient consent.
Contributor Information
Toby Pepperrell, School of Medicine and Veterinary Medicine, University of Edinburgh, Edinburgh, United Kingdom.
Samuel Cross, School of Medicine, Imperial College London, London, United Kingdom.
Andrew Hill, Department of Translational Medicine, Liverpool University, Liverpool, United Kingdom.
References
- 1. UNAIDS . Global HIV & AIDS statistics: fact sheet. 2021. Available at: https://www.unaids.org/en/resources/fact-sheet. Accessed August 30, 2022.
- 2. World Health Organisation . Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. 2016. Available at: https://apps.who.int/iris/bitstream/handle/10665/246200/9789241511124-eng.pdf. Accessed August 30, 2022.
- 3. Rosenberg Z. iPrEx clinical trial demonstrates 44% protection against HIV | International Partnership for Microbicides. 2010. Available at: https://www.ipmglobal.org/publications-media/iprex-clinical-trial-demonstrates-44-protection-against-hiv. Accessed August 30, 2022.
- 4. Molina J-M, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373:2237–46. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organisation . Global data shows increasing PrEP use and widespread adoption of WHO PrEP recommendations. 2021. Available at: https://www.who.int/news-room/feature-stories/detail/global-data-shows-increasing-prep-use-and-widespread-adoption-of-who-prep-recommendations. Accessed August 30, 2022.
- 6. Yun K, Xu J-J, Zhang J, et al. Female and younger subjects have lower adherence in PrEP trials: a meta-analysis with implications for the uptake of PrEP service to prevent HIV. Sex Transm Infect 2018; 94:163–8. [DOI] [PubMed] [Google Scholar]
- 7. Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med 2021; 385:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delany-Moretlwe S, Hughes JP, Bock P, et al. Long acting injectable cabotegravir: updated efficacy and safety results from HPTN 084. 2022. Available at: https://www.hptn.org/sites/default/files/inline-files/220803IAS2022HPTN084forHPTNwebsiterevised_0.pdf. Accessed August 30, 2022.
- 9. Jamieson L, Johnson LF, Nichols BE, et al. The relative cost-effectiveness of long-acting injectable cabotegravir versus oral pre-exposure prophylaxis a modelled economic evaluation and threshold analysis in South Africa based on the HPTN 083 and 084 trials HE RO 2. 2022. Available at: https://thembisa.org. Accessed August 30, 2022.
- 10.. NICE. BNF medicinal forms, cabotegravir—prolonged release suspension for injection. Available at: https://bnf.nice.org.uk/drugs/cabotegravir/medicinal-forms/#prolonged-release-suspension-for-injection. Accessed September 8, 2022.
- 11.. Department of Health and Social Care. Drugs and pharmaceutical electronic market information tool (eMIT). Published March 16, 2011. Updated November 1, 2022. Available at: https://www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit. Accessed August 31, 2022.
- 12.PAHO. PAHO strategic fund long term agreements price list. Available at: https://www.paho.org/en/documents/paho-strategic-fund-long-term-agreements-price-list-updated-4212022. Accessed September 8, 2022.
- 13.. GSK. Press release third quarter 2022. Available at: https://www.gsk.com/media/9632/q3-2022-results-announcement.pdf. Accessed August 31, 2022.
- 14.. Basset J. Long-acting PreP is a necessity, not a luxury: ViiV's greed is still blocking global access to injection that could transform HIV prevention and help end the pandemic. Health GAP. April 4, 2022. Available at: https://healthgap.org/press/long-acting-prep-is-a-necessity-not-a-luxury-viivs-greed-is-still-blocking-global-access-to-injection-that-could-transform-hiv-prevention-and-help-end-the-pandemic/. Accessed August 30, 2022.
- 15.Médecins Sans Frontières Access Campaign. Cabotegravir: what are we waiting for? Available at: https://msfaccess.org/cabotegravir-what-are-we-waiting. Accessed August 30, 2022.
- 16.. MPP. Cabotegravir long-acting (LA) for HIV pre-exposure prophylaxis (PrEP). 2022. Available at: https://medicinespatentpool.org/licence-post/cabotegravir-long-acting-la-for-hiv-pre-exposure-prophylaxis-prep. Accessed August 30, 2022.
- 17.. González LL. ViiV Healthcare to allow 90 countries to access generic versions of HIV prevention shot. AIDSmap. July 28, 2022. Available at: https://www.aidsmap.com/news/jul-2022/viiv-healthcare-allow-90-countries-access-generic-versions-hiv-prevention-shot. Accessed August 30, 2022.
- 18. UNAIDS, AIDSinfo . UNAIDS data 2021. Available at: https://aidsinfo.unaids.org/. Accessed August 30, 2022.
- 19. World Bank . WDI—the world by income and region. 2021. Available at: https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html. Accessed August 30, 2022.
- 20. World Bank. Gross domestic product 2021. 2021. Available at: https://databankfiles.worldbank.org/data/download/GDP.pdf. Accessed August 30, 2022.
- 21.Federal Scientific and Methodological Center for the Prevention and Control of AIDS. Information on HIV infection in Russia. Available at: http://www.hivrussia.info/dannye-po-vich-infektsii-v-rossii/. Accessed September 10, 2022.
- 22. National Bureau of Statistics of China . National HIV statistics. China statistical yearbook. 2017. Available at: http://www.stats.gov.cn/tjsj/ndsj/2017/indexeh.htm. Accessed September 10, 2022.
- 23. UNAIDS, AIDSinfo . Focus on: Thailand. 2021. Available at: https://www.unaids.org/en/20200117_country_focus_Thailand. Accessed September 10, 2022.
- 24. Zhang C, Liu Y, Moyo E, Murewanhema G, Musuka G, Dzinamarira T. Long-acting injectable drugs for HIV-1 pre-exposure prophylaxis: considerations for Africa. Trop Med Infect Dis 2022; 7:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. WHO . Global health expenditure database. 2021. Available at: https://apps.who.int/nha/database/Home/Index/en. Accessed August 30, 2022.
- 26. OECD . Health expenditure. 2021. Available at: https://www.oecd.org/health/health-expenditure.htm. Accessed August 30, 2022.