Abstract
Background
Understanding the burden of influenza is necessary to optimize recommendations for influenza vaccination. We describe the epidemiology of severe influenza in 50- to 64-year-old residents of metropolitan Toronto and Peel region, Canada, over 7 influenza seasons.
Methods
Prospective population-based surveillance for hospitalization associated with laboratory-confirmed influenza was conducted from September 2010 to August 2017. Conditions increasing risk of influenza complications were as defined by Canada's National Advisory Committee on Immunization. Age-specific prevalence of medical conditions was estimated using Ontario health administrative data. Population rates were estimated using Statistics Canada data.
Results
Over 7 seasons, 1228 hospitalizations occurred in patients aged 50–64 years: 40% due to A(H3N2), 30% A(H1N1), and 22% influenza B. The average annual hospitalization rate was 15.6, 20.9, and 33.2 per 100 000 in patients aged 50–54, 55–59, and 60–64 years, respectively; average annual mortality was 0.9/100 000. Overall, 33% of patients had received current season influenza vaccine; 963 (86%) had ≥1 underlying condition increasing influenza complication risk. The most common underlying medical conditions were chronic lung disease (38%) and diabetes mellitus (31%); 25% of patients were immunocompromised. The average annual hospitalization rate was 6.1/100 000 in those without and 41/100 000 in those with any underlying condition, and highest in those with renal disease or immunocompromise (138 and 281 per 100 000, respectively). The case fatality rate in hospitalized patients was 4.4%; median length of stay was 4 days (interquartile range, 2–8 days).
Conclusions
The burden of severe influenza in 50- to 64-year-olds remains significant despite our universal publicly funded vaccination program. These data may assist in improving estimates of the cost-effectiveness of new strategies to reduce this burden.
Keywords: adult, chronic comorbidity, hospitalization, influenza, mortality
Despite a universal influenza vaccination program, average annual rates of influenza-associated hospitalization and mortality in adults aged 50–64 years were 22.4 and 0.9/100 000/year in our population. Incidence increased with age, and to a greater extent, in the presence of chronic comorbidities.
Annual influenza epidemics impose a substantial burden of illness, particularly in older adults. Between 140 000 and 810 000 hospitalizations and 12 000 to 61 000 deaths are associated with influenza infections in the United States (US) each year, with 50%–70% of the hospitalizations and 70%–85% of the deaths occurring in persons aged ≥65 years [1, 2].
Accordingly, many vaccination programs use age-based recommendations and define those ≥65 years old as a high-risk group [2–5]. However, the incidence of severe illness due to influenza does not change sharply at one age breakpoint. An increasing number of studies have suggested that the burden of influenza in 50- to 64-year-olds may be high enough to warrant implementation of additional strategies to improve protection [6–15]. Our previous rapid literature review demonstrated a very wide range in reported rates of influenza-associated hospitalization in 50- to 64-year-olds, both between jurisdictions and in different years, from a low of 5.7 per 100 000 persons in Portugal during the 2013–2014 season to a high of 112.8 per 100 000 persons in the US during the 2017–2018 season [15]. In this review, we found that adults aged 50–64 years had a median incidence that was 3.0-fold (interquartile range [IQR], 2.4–4.7) that of persons aged 14–49 years and 0.26-fold (IQR, 0.19–0.45) that of those aged ≥65 years. Few studies were performed in jurisdictions with routine influenza testing for hospitalized adults, and data on factors associated with increased risk of hospitalization and death in 50- to 64-year-olds were limited.
We aimed to describe the epidemiology of laboratory-confirmed influenza resulting in hospitalization and death in 50- to 64-year-old residents of metropolitan Toronto and Peel region during the 2010–2011 to 2016–2017 influenza seasons.
METHODS
The Toronto Invasive Bacterial Diseases Network (TIBDN) performed prospective population-based surveillance for hospitalization associated with laboratory-confirmed influenza from September 2010 to August 2017 in residents of metropolitan Toronto and the regional municipality of Peel (combined population ∼4.3 million in 2015) [16–19]. The laboratory-based surveillance involved all hospitals (n = 28) providing care to, and all laboratories (n = 25) processing influenza testing from, hospitalized residents of the population area. Laboratory personnel telephoned or faxed the study office when a positive test for influenza was identified at a participating hospital. Trained study personnel screened reports to identify hospitalized patients and, after obtaining consent, used standardized questionnaires to collect demographic and medical information by chart review and patient and physician interview.
Patient Consent Statement
This surveillance study was approved by the research ethics boards of all participating institutions (eg, Sinai Health System numbers 02-0118-U and 05-0116-C). Written consent or verbal consent by telephone was obtained with consent form and documentation mailed to patients. Consent for chart review was waived for patients who could not be contacted and those who were not competent to consent and for whom there was no substitute decision maker.
Inclusion Criteria
For this analysis, we included persons who (1) were aged ≥18 years when hospitalized, (2) were residents of the population area as defined by postal code of residence or who were homeless/underhoused and admitted to a hospital located within the population area, (3) required hospitalization for an acute illness between September 2010 and August 2017, and (4) had a respiratory specimen (nasopharyngeal swab/aspirate, midturbinate/nasal/throat swab, sputum, endotracheal tube aspirate, or bronchoscopy specimen) obtained within 48 hours of hospital admission that yielded influenza on laboratory testing. Persons whose positive specimen was obtained >48 hours after admission and patients who were admitted with influenza as opposed to for influenza (eg, admitted for induction of labor at 41 weeks) were excluded.
Laboratory Testing
From 2010 to 2017, all testing for influenza in participating institutions was performed using reverse-transcription polymerase chain reaction (PCR) in either Public Health Ontario Laboratories (PHOL) or in a clinical laboratory licensed by the Province of Ontario [20]. The exception was the William Osler Health System during the 2010–2011 season only, when all specimens were first tested by enzyme-linked immunosorbent assay (EIA) and all EIA-negative specimens were referred to PHOL for PCR testing. Specimens yielding influenza A in any laboratory were subtyped at PHOL [21]
Definitions
Hospitalization incidence calculations included all eligible patients. For mortality and detailed burden assessment, only those who consented to participate and those for whom a waiver of chart review was granted were included.
Death attributable to influenza was defined as that occurring within 30 days of the positive influenza test. Patients whose last contact was prior to day 30, who were stable or improving at last contact, and who were not readmitted to the same institution were assumed to have survived. Length of hospital stay was defined as the number of nights spent in hospital. Participants admitted and discharged on the same day were assigned a length of stay of 0.5 days.
Intensive care unit (ICU) admissions and mechanical ventilation were attributed to influenza if they occurred during the hospitalization in which influenza was diagnosed and within 15 days of the positive influenza test. Obesity and morbid obesity were defined as body mass index (BMI) of ≥30 kg/m2 and ≥40 kg/m2, respectively. Underlying chronic conditions were as recorded in the patient chart with the exception of anemia, which was defined as a hematocrit below the lower limit of normal on admission.
Influenza vaccination history was obtained from chart review and interviews with patients and/or vaccine providers. Patients were defined as vaccinated for the current season if vaccination was recorded as occurring >14 days before the onset of symptoms of influenza.
Complications of influenza were defined as cardiovascular (myocardial infarction, unstable angina, stroke, new arrhythmia), seizures, complicating secondary infections, and other (eg, Clostridioides difficile colitis, acute renal failure, fractures from falls) as recorded in medical charts. Complicating bloodstream infections were defined as per National Healthcare Safety Network criteria [22]. Possible pneumonia was recorded if the microbiology laboratory reported a pathogen in significant concentrations in sputum or in a specimen obtained at bronchoscopy.
Analysis
All data were analyzed in SAS (version 9.4), with the exception that incidence rate ratios (IRRs) were calculated using Open-Epi (http://www.openepi.com). The incidence of hospitalization, mortality, and ICU admission associated with laboratory-confirmed influenza among adults in different age groups was calculated by dividing the number of cases per season (1 September to 31 August) by the estimated population for the year. Population estimates were obtained from Statistics Canada. As disease incidence was measured in the population rather than a sample of the population, confidence limits were not calculated.
Estimates of Disease Incidence in Population Subgroups
Canada's National Advisory Committee on Immunization (NACI) defines underlying conditions that are associated with a high risk of complications of influenza [23]; information regarding these conditions were collected for all consenting cases. To estimate the prevalence of many of these conditions in the overall population, we applied validated algorithms to data from the Canadian Institute of Health Information's Discharge Abstract Database, the National Ambulatory Reporting System database, the Same-Day Surgery database, and the Ontario Health Insurance Plan database [20, 24]. These datasets were linked using unique encoded identifiers and analyzed at ICES (formerly the Institute for Clinical Evaluative Sciences).
A comparison of NACI and ICES classification of underlying diseases is found in Supplementary Table 1. We calculated the rate of influenza-associated hospitalization in those with underlying chronic illnesses for whom ICES algorithms provided population estimates. We obtained the population prevalence of morbid obesity from Statistics Canada using data from the 2015 to 2020 cycles of the Canadian Community Health Survey for residents of the study area [24].
RESULTS
Patient Characteristics
A total of 7962 eligible episodes of hospitalization due to laboratory-confirmed influenza were identified in adults (≥18 years) during the study period. Of these, 1043 (13%) occurred in 18- to 49-year-olds, 1228 (15%) in 50- to 64-year-olds, and 5691 (71%) in persons aged ≥65 years. Among persons aged 50–64 years, 333 (27%) hospitalizations occurred in 50- to 54-year-olds, 393 (32%) in 55- to 59-year-olds, and 502 (41%) in 60- to 64-year-olds (Table 1). Influenza A(H3N2) was the most common influenza subtype associated with hospitalization (490 cases or 40%) in 50- to 64-year-olds.
Table 1.
Demographics of Patients 50–64 Years of Age Admitted With Laboratory-Confirmed Influenza, Toronto and Peel Region, 2010–2011 to 2016–2017
| Characteristic | Age Category | Overall | ||
|---|---|---|---|---|
| 50–54 y (n = 333) | 55–59 y (n = 393) | 60–64 y (n = 502) | 50–64 y (N = 1228) | |
| Female sex | 172 (52) | 190 (48) | 253 (50) | 615 (50) |
| Influenza type | ||||
| B | 73 (22) | 87 (22) | 108 (22) | 268 (22) |
| A(H1N1) | 106 (32) | 135 (34) | 129 (26) | 370 (30) |
| A(H3N2) | 127 (38) | 139 (35) | 224 (45) | 490 (40) |
| A, untypeda | 27 (8) | 32 (10) | 42 (8) | 100 (8) |
| Vaccinated, current season (n = 971)b | 70 (28) | 103 (33) | 152 (38) | 325 (33) |
| Underlying chronic conditions (n = 1125)c,d | ||||
| Any | 248 (82) | 306 (84) | 409 (89) | 963 (86) |
| Chronic cardiac disease | 67 (22) | 86 (24) | 141 (31) | 294 (26) |
| Chronic pulmonary disease | 109 (36) | 134 (37) | 180 (39) | 423 (38) |
| Chronic liver disease | 24 (8) | 33 (9) | 34 (7) | 91 (9) |
| Chronic renal disease | 40 (13) | 43 (12) | 51 (11) | 134 (12) |
| Diabetes mellitus | 85 (28) | 111 (30) | 153 (33) | 349 (31) |
| Malignancy | 40 (13) | 65 (18) | 99 (22) | 204 (18) |
| Immunocompromising condition | 72 (24) | 91 (25) | 115 (25) | 278 (25) |
| Neurologic/neurodevelopmental | 29 (10) | 45 (12) | 64 (14) | 137 (12) |
| Anemia or hemoglobinopathy | 85 (28) | 120 (33) | 143 (31) | 348 (31) |
| Charlson score (n = 1125) | ||||
| 0–1 | 83 (28) | 85 (23) | 43 (9) | 211 (19) |
| 2–3 | 150 (50) | 179 (49) | 173 (38) | 502 (45) |
| 4–5 | 39 (13) | 60 (16) | 150 (33) | 249 (22) |
| ≥6 | 29 (10) | 40 (11) | 94 (20) | 163 (14) |
| Smoking status (n = 1079)e | ||||
| Current | 84 (29) | 87 (25) | 108 (24) | 279 (26) |
| Ever | 52 (18) | 66 (19) | 89 (20) | 207 (19) |
| Never | 154 (53) | 201 (57) | 248 (56) | 603 (55) |
| Body mass index (n = 883)f | ||||
| Obesity | 70 (30) | 91 (31) | 110 (30) | 211 (24) |
| Morbid obesity | 21 (9) | 16 (6) | 21 (6) | 54 (6) |
Data are presented as No. (%).
Specimens with low viral load could not be subtyped.
Of 971 cases with data, 252 were aged 50–54, 314 were 55–59, and 405 were 60–64 years.
Of 1125 cases with data, 301 were aged 50–54, 364 were 55–59, and 460 were 60–64 years.
Underlying chronic conditions include conditions that increases the risk of influenza-related complications or hospitalization as per the National Advisory Committee on Immunization [17]. Participants may be identified in >1 disease category.
Of 1079 cases with data, 290 were aged 50–54, 354 were 55–59, and 445 were 60–64 years.
Of 883 cases with known BMI, 233 were aged 50–54, 289 were 55–59, and 361 were 60–64 years.
Of the 1228 persons aged 50–64 years who were hospitalized, 103 (8.4%) declined study participation, yielding 1125 participants with detailed clinical information. Among these, 963 (86%) had at least 1 underlying condition classified by NACI as increasing the risk of influenza-related complications (Table 1). The most common conditions were chronic pulmonary disease (38%), diabetes mellitus (31%), anemia or hemoglobinopathy (31%), and chronic cardiac disease (27%); 25% of patients were immunocompromised by underlying illness or medication. Of the 971 participants with seasonal influenza vaccination status available, 325 (33%) had received the current season influenza vaccine: this included 300 of 522 (37%) of those with a chronic underlying illness and 25 of 124 (17%) of those without.
Rates of Hospitalization, Mortality, and Case Fatality
The annual average rate of influenza-associated hospitalization in 50–64 year-olds during the 7 surveillance seasons was 22.4 per 100 000 per year (Table 2); incidence in individual seasons ranged from 12.4 (2011–2012) to 31.6 per 100 000 (2013–2014). The average annual mortality rate was 0.9 per 100 000, with the lowest season-specific rate of 0.1 per 100 000 in 2011–2012 and the highest rate of 1.6 per 100 000 in 2012–2013. Overall, 215 patients (19%) were admitted to ICU, 139 (12%) required mechanical ventilation, and 49 (4.4%) died. The median length of hospitalization for survivors was 4.0 days (IQR, 2–8 days).
Table 2.
Burden of Laboratory-Confirmed Influenza Stratified by Age Group, Toronto and Peel Region, 2010–2011 to 2016–2017
| Age Group, y | Hospitalizations | Deaths | ICU Admissions | Average Annual Hospitalization Rate (per 100 000) | Average Annual Mortality Rate (per 100 000)a | Case Fatality (% Hospitalized)a | ICU Admissions (% Hospitalized) |
|---|---|---|---|---|---|---|---|
| 18–49 | 1043 | 17 | 144 | 7.5 | 0.1 | 1.8 | 14.9 |
| 50–64 | 1228 | 49 | 215 | 22.4 | 0.9 | 4.4 | 19.1 |
| 50–54 | 333 | 12 | 67 | 15.6 | 0.6 | 4.0 | 22.3 |
| 55–59 | 393 | 19 | 73 | 20.9 | 1.0 | 5.2 | 20.1 |
| 60–64 | 502 | 18 | 75 | 33.2 | 1.2 | 3.9 | 16.3 |
| 65–74 | 1282 | 57 | 174 | 45.9 | 2.0 | 4.8 | 14.6 |
| ≥75 | 4409 | 399 | 373 | 408.7 | 37.0 | 9.8 | 9.1 |
Abbreviation: ICU, intensive care unit.
Mortality, case fatality, and ICU admission rate calculations included only patients for whom clinical data from chart review were available: 18–49 years (n = 966), 50–64 years (n = 1125), 65–74 years (n = 1188), and ≥75 years (n = 4087).
Persons aged 50–64 years had a rate of hospitalization that was 3-fold higher than persons aged 18–49 years (22.4 vs 7.54 per 100 000 per year), but 2-fold lower than those aged 65–74 years (45.9 per 100 000 per year) (Table 2). The mortality rate in 50- to 64-year-olds was 9-fold higher than in 18- to 49-year-olds (0.9 vs 0.1 per 100 000 per year) and 2-fold lower than in 65- to 74-year-olds (2.0 per 100 000 per year). The case fatality rate in 50- to 64-year-olds was 3-fold higher than in 18- to 49-year-olds (4.4% vs 1.8%, P < .001, Fisher exact test) and not significantly different from that in 65- to 74-year-olds (4.8%).
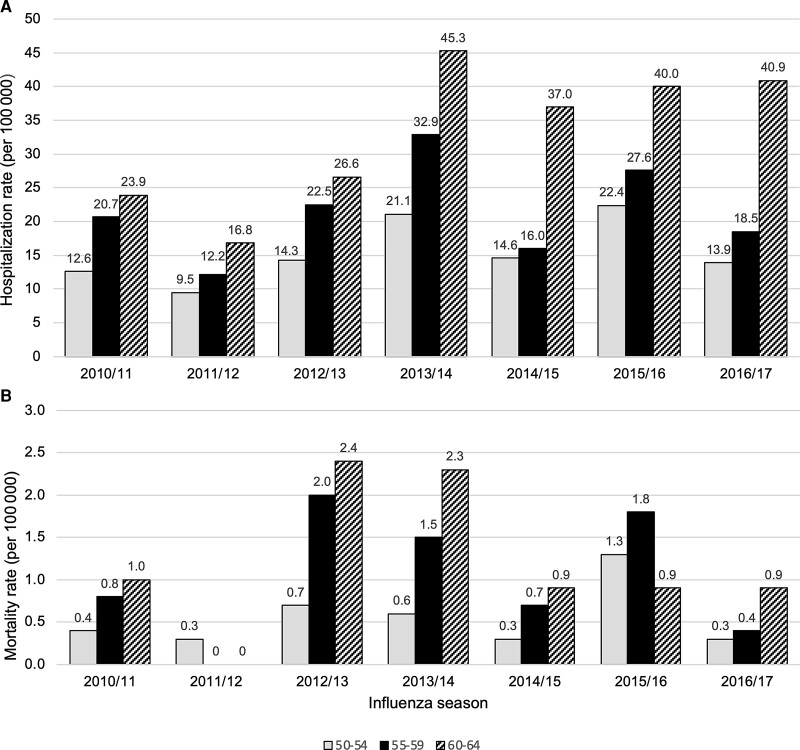
The rate of hospitalization in 60- to 64-year-olds was just over twice that of 50- to 54-year-olds ( IRR, 2.14 [95% confidence interval [CI], 1.86–2.45]), and 1.6 times that of 55- to 59-year-olds (IRR, 1.59 [95% CI, 1.49–1.82]) (Table 2, Figure 1, and Supplementary Table 2). Patients 60–64 years of age also had higher mortality rates than those aged 50–54 years (1.2 vs 0.6 per 100 000; IRR, 2.12 [95% CI, 1.02–4.53]). The case fatality rates and hospital length of stay did not differ significantly between these age subgroups (data not shown).
Figure 1.
Incidence of hospitalization (A) and in-hospital mortality (B) in 50- to 64-year-old residents of Toronto and Peel region with laboratory-confirmed influenza, by age group and influenza season. Incidence for those aged 50-54 years is shown in solid grey bars, for those 55-59 years in solid black bars and for those aged 60-65 years in striped bars.
Burden of Severe Illness in Patients With Underlying Conditions
According to ICES categorization, 926 of the 1125 (82%) study patients with detailed clinical information had at least 1 underlying medical condition associated with an increased risk of influenza complications. This compares to 40% of 50- to 64-year-olds with such conditions in the overall population of the study area. The most common underlying medical conditions in hospitalized patients were anemia (31% [n = 348]), diabetes mellitus (31% [n = 345]), cardiac disease (27% [n = 299]), chronic obstructive pulmonary disease (COPD) (21% [n = 231]), and asthma (19% [n = 215]). Thirty-seven patients had an underlying condition defined by NACI that was not included in any ICES category. Of these, 6 patients had liver disease, 9 had pulmonary disease other than COPD or asthma, 3 had cardiovascular disease with a diagnosis other than those included in ICES definitions, 14 had neurologic/neurodevelopmental disease, and 9 had an immunocompromising condition. There were also 155 patients who were included in non-immunocompromised high-risk groups by ICES definitions, but were categorized as immunocompromised by NACI definitions because they had both a non-immunocompromising condition that was identified by ICES (eg, anemia) and an immunocompromising condition that was not (eg, patient receiving cancer chemotherapy). All morbidly obese patients had other underlying conditions resulting in their inclusion in ICES categories. No patient had an underlying condition defined by ICES that was not included in risk conditions as defined by NACI.
Patients aged 50–64 years who had at least 1 underlying chronic condition had hospitalization and mortality rates (41.0 and 1.8 per 100 000, respectively) that were approximately 6-fold higher than those without underlying conditions (6.1 and 0.3 per 100 000, respectively) (P < .001; Table 3). These hospitalization rates of 6.1 and 41.0 per 100 000 in 50- to 64-year-olds without and with at least 1 underlying illness compare to rates of 14.4 and 83.9 per 100 000 in persons 65–74 years of age without and with underlying illness, respectively.
Table 3.
Burden of Laboratory-Confirmed Influenza Among 50- to 64-Year-Olds, by Major Underlying Conditions as Defined by ICES, Toronto and Peel Region, 2010–2011 to 2016–2017
| Underlying Conditiona | No. Hospitalized | Average Annual Hospitalization Rate (per 100 000) | Average Annual Mortality Rate (per 100 000) | Case Fatality, No. (%) of Hospitalized | ICU Admission, No (%) of Hospitalized |
|---|---|---|---|---|---|
| None | 200 | 6.1 | 0.3 | 9 (4.5) | 47 (23.5) |
| At least 1 | 926 | 41.0 | 1.8 | 40 (4.3) | 168 (18.2) |
| Specific conditions | |||||
| Asthma | 215 | 35.0 | 0.8 | 5 (2.3) | 31 (14.4) |
| Diabetes mellitus | 345 | 39.8 | 1.4 | 12 (3.5) | 64 (18.6) |
| COPD | 231 | 41.4 | 1.6 | 9 (3.9) | 48 (20.8) |
| Cancer | 204 | 67.3 | 4.3 | 13 (6.4) | 24 (11.8) |
| Cardiac disease | 299 | 72.1 | 3.6 | 15 (5.0) | 52 (17.4) |
| Kidney disease | 123 | 137.5 | 5.6 | 5 (4.1) | 22 (17.9) |
| HIV infection or organ transplant | 114 | 281.2 | 9.9 | 4 (3.5) | 13 (11.4) |
| Anemia | 348 | 129.8 | 7.1 | 19 (5.5) | 81 (23.3) |
| Morbid obesityb | 54 | 34.0 | 0.6 | 1 (1.9) | 14 (26.0) |
Abbreviations: COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; ICU, intensive care unit.
The population prevalence estimates for each underlying conditions (except morbid obesity) and their clinical definitions were as available from ICES (formerly the Institute of Clinical and Evaluative Sciences) (Supplementary Table 2).
The population prevalence estimate for morbid obesity was obtained from Statistics Canada, Canadian Community Health Survey (2015–2020).
The highest rates of hospitalization and mortality occurred in those with human immunodeficiency virus (HIV) infection or living with an organ transplant (281.2 and 9.9 per 100 000, respectively), followed by those with kidney disease (137.5 and 5.6 per 100 000) and anemia (129.8 and 7.1 per 100 000, respectively). The case fatality and ICU admission rates did not differ significantly between those with at least 1 underlying condition and those without (P = .46 and P = .08, respectively), although there was significant variability in ICU admission in those with different underlying conditions (P = .007; Table 3).
Complications of Influenza Episodes
Of 1125 patients with detailed clinical information, 128 (11%) had at least 1 in-hospital complication. Cardiac complications and secondary bacterial/fungal infections were the most common (Table 4). No significant differences were found in the percentage of patients with postinfectious complications by age group (P = .08, χ2 test; Table 4), by vaccination status (P = .16), or by presence of an underlying chronic condition (P = .85) (data not shown).
Table 4.
Incidence of Complications Among Adults Aged 50–64 Years Hospitalized for Influenza, Toronto and Peel Region, 2010–2011 to 2016–2017
| Age Group, y | No. of Hospitalizations | No. (%) Experiencing Complication | ||||
|---|---|---|---|---|---|---|
| Anya | Cardiac Complicationb | Bacterial/Fungal Infectionc | Clostridioides difficile Colitis | Acute Renal Failure | ||
| 50–64 | 1125 | 128 (11) | 87 (8) | 85 (8) | 9 (1) | 29 (3) |
| 50–54 | 301 | 41 (14) | 21 (7) | 31 (10) | 0 (0) | 8 (3) |
| 55–59 | 364 | 45 (12) | 29 (8) | 29 (8) | 3 (1) | 13 (4) |
| 60–64 | 458 | 42 (9) | 37 (8) | 25 (5) | 6 (1) | 8 (2) |
Any complication occurring in the first 30 days of the hospitalization for influenza including cardiac complications, stroke, seizure, C. difficile colitis, acute renal failure, fracture, complicating bacterial/fungal infection.
Cardiac complications include: myocardial infarction, unstable angina, new arrhythmia, and episodes of atrial fibrillation.
Complicating infections included 1 case of endocarditis, 38 other bloodstream infections (14 Staphylococcus aureus, 7 Streptococcus pneumoniae, 6 Escherichia coli, 2 each Haemophilus influenzae, Pseudomonas aeruginosa, viridans group streptococci, and 1 each Streptococcus pyogenes, Klebsiella pneumoniae, Citrobacter koseri, Enterococcus faecalis, and Candida glabrata; 2 cases of empyema; and 44 cases of possible pneumonia (26 S aureus, 11 S pneumoniae, 4 H influenzae, 2 S pyogenes, and 1 Moraxella catarrhalis).
DISCUSSION
In this analysis of population-based surveillance for influenza-associated hospitalization in Toronto, Canada, we assessed the burden of illness in adults aged 50–64 years with and without different underlying conditions known to increase the risk of influenza-related complications. As expected, burden increased steadily with age. The highest incidence of hospitalization was in immunocompromised persons, but any underlying chronic condition increased the rate of hospitalization in 50- to 64-year-olds to between 2.5 and 9 times the rate of hospitalization in healthy 65- to 74-year-olds.
Our data support current Canadian recommendations to prioritize influenza vaccination for adults aged <65 years with risk factors for complications.In persons aged 50–64 years, >80% of hospital admissions occurred in persons with risk factors for influenza complications, and the incidence of hospitalization was nearly 7-fold higher in persons with risk factors compared to those without. Cost-effectiveness analyses will be required to determine whether age-based recommendations for 50- to 64-year-olds will be warranted for newer influenza vaccines; these analyses and programmatic decisions should take into consideration the fact that age-based recommendations are easier to implement than risk-based recommendations and may result in overall higher vaccine uptake in risk groups [25]. The degree of increase in incidence associated with underlying chronic conditions in our study is similar to that estimated by modeling studies [26, 27], which suggests that despite the limitations of both types of studies, the relative incidence is useful for parameterizing cost-effectiveness studies.
Our estimated rate of hospitalization (22.4 per 100 000) was within the range of incidence among 50- to 64-year-olds (5.7–112.8 per 100 000) identified in a recent rapid review and similar to that reported from Canadian national surveillance data (26 per 100 000) [8, 15]. It is, however, significantly less than the estimate from the US Influenza Hospitalization Surveillance Network (FluSurv-NET) for the same years (40.8 per 100 000 adults aged 50–64 years) [6]. This difference is most likely due to higher testing rates and fewer missed cases in US surveillance. Other potential factors may include differences in the prevalence of different underlying comorbidities, in healthcare-seeking behavior, or in vaccination coverage between the 2 populations. Data on influenza vaccination coverage are not available for Ontario adults aged 50–64, but national coverage was reported to be 39% in this age group in 2015–2016 [28] compared to US national coverage of 44% in the same year [29]. Thus, differences in vaccination coverage (eg, in those with underlying medical conditions) are an unlikely explanation for the observed differences in influenza hospitalization rates.
Evidence from FluSurv-Net, in which the adjustment of crude hospitalization rates to account for the percentage of admitted patients with respiratory illness tested for influenza and the sensitivity and specificity of laboratory tests, resulted in a 2.5- to 4-fold increase in the estimated incidence of hospitalization [30]. Other studies have found that only 28% of pediatric patients hospitalized for influenza were clinically identified [31], and the degree of underdetection was greater in adults, especially older adults [32]. The burden of severe influenza will be further underestimated because some patients present with complications after viral shedding is controlled and no longer detectable [33] or experience out-of-hospital deaths [34]. These latter complications are often due to cardiovascular events and the extent of this added burden is uncertain. Some recent modeling data suggest that cardiovascular complications of influenza are less common in 50- to 64-year-olds than in those ≥65 years of age [35].
Estimates of burden of illness due to influenza also need to take into consideration the impact of existing vaccination programs. As noted, although vaccine coverage data are not available for 50- to 64-year-olds in Ontario, the seasonal influenza vaccination rate in hospitalized cases (33%) was somewhat lower than Canada's national coverage estimate for adults aged 45–64 years (39%) in 2015–2016, which is compatible with expected vaccine effectiveness in reducing hospitalizations [28]. The existing burden of influenza would be expected to be reduced significantly if coverage could be improved to meet national targets (80% for adults 18–64 years of age with chronic medical conditions) [36].
This analysis has several limitations. As noted, there are several reasons why the estimated incidence of burden described in this analysis is likely significantly lower than the actual burden. We were not able to estimate the extent of undertesting in all of our hospitals in each of the study years, which would have permitted adjustment of our estimates. However, results across age groups should provide unbiased comparisons. Our estimated incidence of hospitalization and mortality are also somewhat underestimated as 8.4% of patients/substitute decision makers declined study participation. Similarly, although in previous case series of influenza <10% of cases were readmitted—and in our population area most would be readmitted to the same hospital—we may have missed an additional small number of deaths [37, 38]. Our identification of underlying illness by chart review may not accurately capture all underlying illnesses. Because ICES risk condition definitions were not available until late in the surveillance period, our definitions of conditions predisposing to influenza-related complications were not identical to ICES categories. This is most evident for anemia where our definition was based on hematocrit at admission while ICES definition was based on anemia being recorded in a health administrative database. ICES was also only able to capture those who were immunocompromised by HIV infection or solid organ/stem cell transplantation, so we were unable to estimate rates of hospitalization for other immunocompromising conditions. Our data on the population prevalence of morbid obesity is based on data available for the years 2015–2020, somewhat later than our surveillance years, and is based on self-report, which underestimates BMI. However, Statistics Canada applies adjustments based on a validation substudy [23, 39].
In conclusion, our analysis provides evidence of the significance of the burden of severe influenza in 50- to 64-year-olds despite the existence of a long-standing universal publicly funded influenza vaccination program in our population area. While improving vaccine coverage would provide the greatest benefit, assessment of the potential benefits of enhanced influenza vaccines is also warranted.
Supplementary Material
Contributor Information
Philip Kim, Department of Microbiology, Sinai Health System, Toronto, Canada.
Brenda Coleman, Department of Microbiology, Sinai Health System, Toronto, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, Canada.
Jeffrey C Kwong, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada; Department of Family and Community Medicine, University of Toronto, Toronto, Canada; ICES, Toronto, Canada; Public Health Ontario, Toronto, Canada.
Agron Plevneshi, Department of Microbiology, Sinai Health System, Toronto, Canada.
Kazi Hassan, Department of Microbiology, Sinai Health System, Toronto, Canada.
Karen Green, Department of Microbiology, Sinai Health System, Toronto, Canada.
Shelly A McNeil, Department of Medicine, Dalhousie University, Halifax, Canada.
Irene Armstrong, Toronto Public Health, Toronto, Canada.
Wayne L Gold, Department of Medicine, University Health Network, University of Toronto, Toronto, Canada.
Jonathan Gubbay, Public Health Ontario, Toronto, Canada; Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Canada.
Kevin Katz, Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Canada; Department of Microbiology, Sunnybrook Health Sciences Centre, Toronto, Canada.
Stefan P Kuster, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St Gallen, Switzerland.
Reena Lovinsky, Scarborough Health Network, Toronto, Canada.
Larissa Matukas, Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Canada; Division of Microbiology, Unity Health, Toronto, Canada.
Krystyna Ostrowska, Trillium Health Partners, Mississauga, Canada.
David Richardson, Department of Medical Microbiology and Infectious Diseases, William Osler Health System, Brampton, Canada.
Allison McGeer, Department of Microbiology, Sinai Health System, Toronto, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, Canada; Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Canada.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We are grateful to the many staff of microbiology laboratories, infection prevention and control departments, and public health units serving our population who have contributed to this surveillance, and to the participating patients and families without whom this surveillance would not be possible. We thank IQVIA Solutions Canada Inc. for use of their Drug Information File.
Disclaimer. Parts of this material are based on data and/or information compiled and provided by MOH, the Canadian Institute for Health Information (CIHI) Ontario Health (OH) and by IQVIA Solutions Canada Inc. However, the analyses, conclusions, opinions, and statements expressed herein are solely those of the authors, and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Financial support. Toronto Invasive Bacterial Diseases Network (TIBDN) surveillance for influenza was funded by an unrestricted grant from Hoffman LaRoche Ltd. This collaborative analysis was funded by a grant from Sanofi Ltd (to A. M.). This study was also supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the MLTC.
Potential conflicts of interest. S. A. M. reports grant and clinical trials funding from GSK, Merck, Pfizer and Sanofi, and payments from GSK, Pfizer, Sanofi, and Merck outside the submitted work. B. C. reports payments from Seqirus outside the submitted work. A. J. M. reports grant funding from Pfizer, Sanofi and Seqirus, and payments from GSK, Merck, Moderna, Pfizer, and Sanofi outside of the submitted work. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Disease burden of influenza—estimated range of annual burden of flu in the U.S. from 2010 to 2020. 2022. https://www.cdc.gov/flu/about/burden/index.html. Accessed 14 June 2022.
- 2. Centers for Disease Control and Prevention. Who is at high risk for flu complications—flu and people 65 years and older. 2022. https://www.cdc.gov/flu/highrisk/65over.htm. Accessed 14 June 2022.
- 3. European Centre for Disease Prevention and Control . Risk groups for severe influenza. 2022. https://www.ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/vaccines/risk-groupsAccessed 14 June 2022.
- 4. Public Health Agency of Canada . Flu (influenza): for health professionals—risk groups for influenza-related complications.2022. https://www.canada.ca/en/public-health/services/diseases/flu-influenza/health-professionals.html#a5. Accessed 14 June 2022.
- 5. The World Health Organization . Seasonal influenza vaccine, weekly epidemiological record. Weekly Epidemiological Record = Relevé épidémiologique hebdomadaire2012; 87:201–16. Available at: https://apps.who.int/iris/handle/10665/241921. Accessed 14 June 2022.
- 6. Centers for Disease Control and Prevention . Influenza Hospitalization Surveillance Network (FluSurv-NET): fluview interactive.2022. https://gis.cdc.gov/GRASP/Fluview/FluHospRates.html. Accessed 14 June 2022.
- 7. Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66:1511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Public Health Agency of Canada . FluWatch report: FluWatch annual report: 2019–2020 influenza season. 2021. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/fluwatch/2019-2020/annual-report.html. Accessed 6 June 2021.
- 9. Centers for Disease Control and Prevention . Estimated influenza illnesses, medical visits, hospitalizations, and deaths in the United States—2017–2018 influenza season. 2021. https://www.cdc.gov/flu/about/burden/2019-2020.html. Accessed 10 June 2021.
- 10. Matias G, Taylor RJ, Haguinet F, Schuck-Paim C, Lustig R, Fleming DM. Modelling estimates of age-specific influenza-related hospitalisation and mortality in the United Kingdom. BMC Public Health 2016; 16:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gil A, Gil R, Oyagüez I, Carrasco P, Lez AG. Hospitalization by pneumonia and influenza in the 50–64 year old population in Spain (1999–2002). Hum Vaccin 2006; 2:181–4. [DOI] [PubMed] [Google Scholar]
- 12. Shah NS, Greenberg JA, McNulty MC, et al. Severe influenza in 33 US hospitals, 2013–2014: complications and risk factors for death in 507 patients. Infect Control Hosp Epidemiol 2015; 36:1251–60. [DOI] [PubMed] [Google Scholar]
- 13. Arriola C, Garg S, Anderson EJ, et al. Influenza vaccination modifies disease severity among community-dwelling adults hospitalized with influenza. Clin Infect Dis 2017; 65:1289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arriola CS, Anderson EJ, Baumbach J, et al. Does influenza vaccination modify influenza severity? Data on older adults hospitalized with influenza during the 2012–2013 season in the United States. J Infect Dis 2015; 212:1200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim DK, McGeer A, Uleryk E, Coleman BL. Burden of severe illness associated with laboratory confirmed influenza in adults aged 50–64 years: a rapid review. Influenza Other Respir Viruses 2022; 16:632–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coleman BL, Hassan K, Green K, et al. Pre-and post-pandemic trends in antiviral use in hospitalized patients with laboratory-confirmed influenza: 2004/05–2013/14, Toronto, Canada. Antiviral Res 2017; 140:158–63. [DOI] [PubMed] [Google Scholar]
- 17. McNeil S, Shinde V, Andrew M, et al. Interim estimates of 2013/14 influenza clinical severity and vaccine effectiveness in the prevention of laboratory-confirmed influenza-related hospitalisation, Canada, February 2014. Euro Surveill 2014; 19:20729. [DOI] [PubMed] [Google Scholar]
- 18. Kuster SP, Katz KC, Blair J, et al. When should a diagnosis of influenza be considered in adults requiring intensive care unit admission? Results of population-based active surveillance in Toronto. Crit Care 2011; 15:R182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuster SP, Drews S, Green K, et al. Epidemiology of influenza-associated hospitalization in adults, Toronto, 2007/8. Eur J Clin Microbiol Infect Dis 2010; 9:835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kwong JC, Buchan SA, Chung H, et al. Can routinely collected laboratory and health administrative data be used to assess influenza vaccine effectiveness? Assessing the validity of the flu and other respiratory viruses research (FOREVER) cohort. Vaccine 2019; 37:4392–400. [DOI] [PubMed] [Google Scholar]
- 21. Skowronski DM, Leir S, Sabaiduc S. Influenza vaccine effectiveness by A(H3N2) phylogenetic subcluster and prior vaccination history: 2016–2017 and 2017–2018 epidemics in Canada. J Infect Dis 2022; 225:1387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention . National Healthcare Safety Network—patient safety component manual, chapter 4: bloodstream infection event (central line-associated bloodstream infection and non-central line associated bloodstream infection). 2022. https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf. Accessed 4 July 2022.
- 23. Public Health Agency of Canada. Canadian immunization guide chapter on influenza and statement on seasonal influenza vaccine for 2021–2022. 2022. https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2021–2022.html. Accessed 14 June 2022.
- 24. Statistics Canada, Canadian Community Health Survey. 2022. https://www.canada.ca/en/health-canada/services/food-nutrition/food-nutrition-surveillance/health-nutrition-surveys/canadian-community-health-survey-cchs.html. Accessed 4 July 2022.
- 25. Jiménez-García R, Rodríguez-Rieiro C, Hernández-Barrera V, et al. Effectiveness of age-based strategies to increase influenza vaccination coverage among high risk subjects in Madrid (Spain). Vaccine 2011; 29:2840–5. [DOI] [PubMed] [Google Scholar]
- 26. Cromer D, van Hoek AJ, Jit M, Edmunds WJ, Fleming D, Miller E. The burden of influenza in England by age and clinical risk group: a statistical analysis to inform vaccine policy. J Infect 2014; 68:363–71. [DOI] [PubMed] [Google Scholar]
- 27. Ontario Agency for Health Protection and Promotion (Public Health Ontario) . The relationship between influenza medical risk factors and age.2017. https://www.publichealthontario.ca/-/media/Documents/I/2017/influenza-risk-factors-age-technical.pdf? sc_lang=en. Accessed 20 July 2022.
- 28. Public Health Agency of Canada . Influenza vaccine uptake: results from the 2015/16 national influenza immunization coverage survey in Canada.2022. https://www.canada.ca/en/public-health/services/publications/healthy-living/vaccine-uptake-results-2015-16-national-influenza-immunization-coverage-survey.html. Accessed 14 July 2022.
- 29. Centers for Disease Control and Prevention . Flu vaccination coverage, United States, 2020–21 influenza season. 2021. https://www.cdc.gov/flu/fluvaxview/coverage-2021estimates.htm. Accessed 21 July 2022.
- 30. Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66:1511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med 2016; 355:31–40. [DOI] [PubMed] [Google Scholar]
- 32. Reed C, Chaves SS, Daily Kirley P, et al. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One 2015; 10:e0118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med 2018; 378:345–53. [DOI] [PubMed] [Google Scholar]
- 34. Moa A, Tan T, Wei J, Hutchinson D, MacIntyre CR. Burden of influenza in adults with cardiac arrest admissions in Australia. Int J Cardiol 2022; 361:109–15. [DOI] [PubMed] [Google Scholar]
- 35. Moa AM, Menzies RI, Yin JK, MacIntyre CR. Modelling the influenza disease burden in people aged 50–64 and ≥65 years in Australia. Influenza Other Respir Viruses 2022; 16:132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Public Health Agency of Canada . Vaccination coverage goals and vaccine preventable disease reduction targets by 2025.2022.https://www.canada.ca/en/public-health/services/immunization-vaccine-priorities/national-immunization-strategy/vaccination-coverage-goals-vaccine-preventable-diseases-reduction-targets-2025.html. Accessed 15 July 2022.
- 37. Wallemacq S, Danwang C, Scohy A, et al. A comparative analysis of the outcomes of patients with influenza or COVID-19 in a tertiary hospital in Belgium. J Infect Chemother 2022; 28:1489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verma AA, Hora T, Jung HY, et al. Characteristics and outcomes of hospital admissions for COVID-19 and influenza in the Toronto area. CMAJ 2021; 193:E410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sherry B, Jefferds ME, Grummer-Strawn LM. Accuracy of adolescent self-report of height and weight in assessing overweight status: a literature review. Arch Pediatr Adolesc Med 2007; 161:1154–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.