Abstract
The e-cigarette or vaping product-use-associated lung injury outbreak in the United States has raised concerns about the potential health effects of cannabis vaping, a method of cannabis use that is becoming increasingly popular. We used 2017–2019 Behavioral Risk Factor Surveillance System data to estimate yearly prevalence and trends of past-30-day cannabis use and vaping among US adults. We used multivariable logistic regression to evaluate the associations of cannabis vaping with high-risk behaviors, asthma, and other respiratory symptoms.
Our sample size was 160,209 (53,945–2017; 55,475–2018; and 50,789–2019). Past-30-day cannabis use prevalence increased from 10.0% (95%CI, 9.4%–10.7%) in 2017 to 13.4% (12.8%–12.0%) in 2019. Similarly, past-30-day cannabis vaping prevalence increased from 1.0% (0.8%–1.2%) to 2.0% (1.7%–2.2%) over the same period, with the greatest increase, 1.2% to 3.9%, observed among young adults (18–24years). Individuals who vaped cannabis were more likely to concurrently vape nicotine. Cannabis vaping was associated with increased odds of heavy alcohol use (aOR,1.95; 95%CI,1.45–2.63), binge drinking (aOR,2.82; 95%CI,2.25–3.54), and other high-risk behaviors (aOR,2.47; 95%CI,1.89–3.24). In analyses adjusting for sociodemographic characteristics and body mass index, cannabis vaping was not associated with asthma (aOR,1.03; 95%CI,0.64–1.64) or other respiratory symptoms (aOR,1.08; 95%CI,0.44–2.63). Adjusting for nicotine vaping did not substantively alter these associations.
The prevalence of past-30-day cannabis vaping has increased, particularly among young adults, and was associated with high-risk behaviors. Although there was no association between cannabis vaping and asthma or other respiratory symptoms, the increasing trends of cannabis vaping, particularly among young adults, raise concern and underscore the need for continued surveillance.
Keywords: Cannabis, Trends, Vaping, Asthma, Respiratory Symptoms, High-risk Behaviors
1. Introduction:
E-cigarettes were originally designed to deliver nicotine, without combustion, to users with the intent of limiting exposure to toxic by-products of tobacco combustion (Goniewicz et al., 2014). Although nicotine remains the most commonly used substance in e-cigarette devices (Chadi et al., 2020), an increasing number of individuals now use their e-cigarette devices to vape other substances other than or in addition to nicotine, such as cannabis (Dai, 2020; Kenne et al., 2017). With the increasing legalization across different states in the United States (US), cannabis in various formulations, including those that can be vaporized, has become readily accessible (National Conference of State Legislatures, 2020; Peace et al., 2016). Additionally, the perception that vaping cannabis is associated with fewer health effects than smoking may contribute to the increasing popularity of cannabis vaping among cannabis users (Budney et al., 2015; Malouff et al., 2014).
The e-cigarette or vaping product-use-associated lung injury (EVALI) epidemic, which was reported in August 2019 by the Centers for Disease Control and Prevention (CDC), involved cases of acute, sometimes fatal lung injury that was seen among otherwise healthy individuals who reported e-cigarette use before the onset of symptoms (Centers for Disease Control and Prevention, 2020). As of February 2020, a total of 2,807 EVALI cases and deaths had been reported in all 50 states, the District of Columbia (DC), Puerto Rico, and the US Virgin Islands (Centers for Disease Control and Prevention, 2020). The EVALI epidemic has been strongly linked to the use of e-liquids containing tetrahydrocannabinol (THC)-the principal psychoactive constituent of cannabis, and vitamin E-acetate, an additive commonly used in THC-containing e-liquids (Blount et al., 2020). Therefore, the EVALI outbreak has renewed concerns about the potential health implications of cannabis vaping, particularly among young, healthy adults.
While the association between e-cigarette use, particularly nicotine vaping, with symptoms of asthma and other respiratory conditions has been described, limited data exist on the health effects of cannabis vaping (Bhatta and Glantz, 2020; Entwistle et al., 2020; Osei et al., 2020, 2019; Xie et al., 2020). Emerging evidence from the few studies that examined the impact of cannabis vaping on respiratory health suggests that cannabis vaping, though regarded by many as safe, may significantly impact respiratory health (Boyd et al., 2021; Braymiller et al., 2020). For example, a recent study found that among adolescents aged 12–17 years, lifetime vaping of cannabis was associated with wheezing and dry cough (Boyd et al., 2021). However, there is limited data on the potential association between cannabis vaping and asthma.
Thus, given the increasing legalization of cannabis across different US states, the rising popularity of cannabis vaping, and its potential health effects, updated data are needed on cannabis vaping trends, the association of cannabis vaping with high-risk behaviors, and the adverse respiratory effects associated with cannabis vaping. Therefore, using 2017–2019 data from one of the most comprehensive and continuously conducted health surveys among US adults - the Behavioral Risk Factor Surveillance System (BRFSS), we sought to describe trends in past-30-day cannabis use and vaping and examine the association between cannabis vaping and other high-risk behaviors, as well as the potential association of cannabis vaping with asthma and other respiratory symptoms.
2. Methods:
The BRFSS is an annual nationally representative health-related telephone survey of noninstitutionalized adults (≥18 years) administered by the CDC in all 50 states, DC, and participating territories. The sampling frame and weighting methodology used by the BRFSS to ensure sample representativeness are described elsewhere (Centers for Disease Control and Prevention, n.d.; Iachan et al., 2016). The questionnaire module containing questions on cannabis use in the BRFSS is optional, giving states the flexibility to include this module in their state survey based on their priorities. Therefore, the number of states providing data on cannabis use varied from year to year. We utilized 2017–2019 BRFSS data from the nine (9) states and territories (California, Idaho, Minnesota, New Hampshire, Oklahoma, South Carolina, Tennessee, Wyoming, and Guam) that consistently provided data on cannabis use for all three (3) years (Dataset. Centers for Disease Control and Prevention., 2019, 2018, 2017). Our analytic sample sizes were 53,945 in 2017, 55,475 in 2018, and 50,789 in 2019. The median survey response rate for all states, DC, and participating territories in 2017, 2018, and 2019 was 45.9%, 49.9%, and 49.4%, respectively (Centers for Disease Control and Prevention (CDC), 2019, 2018, 2017). Our study was exempted from an institutional review board review since it used de-identified publicly available BRFSS data. We followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines in reporting our findings (von Elm et al., 2008).
2.1. Cannabis Use and Cannabis Vaping
To assess past-30-day cannabis use, participants were asked, “During the past 30 days, on how many days did you use marijuana or cannabis?” Participants who reported use on at least one day were considered to be using cannabis. Individuals who reported cannabis use were further asked, “During the past 30 days, which one of the following ways did you use cannabis the most often?” Those who chose the option “vaporize it” were considered to be vaping cannabis. Individuals who vaped cannabis were subsequently categorized by their frequency of cannabis use into two groups: less frequent (1–20 days in the past 30 days) and more frequent use (21–30 days in the past 30 days) (Ramadan et al., 2020).
2.2. Combustible Cigarette and Nicotine E-cigarette Use
Participants who responded “yes” to “Have you smoked at least 100 cigarettes in your entire life?” and subsequently responded, “every day” or “some days” to the question “Do you now smoke cigarettes every day, some days, or not at all?” were classified as current combustible cigarette smokers. Those who responded “not at all” were deemed former combustible cigarette smokers. Participants who answered “no” to “Have you smoked at least 100 cigarettes in your entire life?” were classified as never combustible cigarette smokers. Similarly, the use of nicotine e-cigarettes was categorized as never, former, or current, based on these two questions: “Have you ever used an e-cigarette or electronic vaping product even just one time in your entire lifetime?” and “Do you now use an e-cigarette or electronic vaping product every day, some days, or not at all?”
2.3. Other Covariate Assessment
Sex (male or female), age (18–20, 21–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, or ≥60 years), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, or others), sexual orientation (heterosexual, lesbian/gay, bisexual, or other), marital status (single, married, divorced/separated, or widowed), income, education (less than high school, high school/some college, or college degree), and employment (employed, unemployed, student, or retired) were self-reported. Annual family income was adjusted using federal poverty guidelines and categorized as: below, within 100–200%, or above 200% of the poverty line (US Department of Health and Human Services, n.d.). Body Mass Index (BMI; kg/m2) was calculated based on the participant’s self-reported weight and height and categorized as underweight, normal, overweight, or obese. History of depression was assessed with the question, “Has a doctor, nurse, or other health professionals ever told you that you have a depressive disorder, including depression, major depression, dysthymia, or minor depression?”.
2.4. High-Risk Behaviors
Heavy alcohol use was defined as consuming >14 drinks per week for men or >7 drinks per week for women, and binge drinking as consuming on a single occasion ≥5 drinks for men or ≥4 drinks for women. “Other high-risk behaviors” was a composite of intravenous drug use, treatment for sexually transmitted infections, or exchanging money/drugs for sex in the past year and was assessed with a single question on whether survey participants have been involved in any of these activities.
2.5. Asthma and Respiratory Symptoms
Participants who responded “yes” to the question “Has a doctor, nurse, or other health professionals ever told you that you had asthma?” and subsequently responded “yes” to the question “Do you still have asthma?” were classified as having asthma. Respiratory symptoms, defined as answering “yes” to at least one of these 3 questions: “During the past 3 months, did you have a cough on most days?”, “During the past 3 months, did you cough up phlegm or mucus on most days?” or “Do you have shortness of breath either when hurrying on level ground or when walking up a slight hill or stairs?” was a composite outcome.
2.6. Statistical Analysis
We calculated the pooled and state-level prevalence estimates of past-30-day cannabis use for each year. In addition, the proportion of individuals who used cannabis and vaporized it as their primary method of use was also computed for each year. We then examined trends in cannabis vaping first overall and then stratified by key participant characteristics. Changes in prevalence were obtained using the lincom command and reported as absolute prevalence differences with 95% confidence intervals (CI).
To assess the association of past-30-day cannabis vaping with high-risk behaviors, asthma, and respiratory symptoms, we pooled BRFSS data from all 3 years. We used multivariable logistic regression models to estimate adjusted odds ratios (aOR) for the association between past-30-day cannabis vaping and high-risk behaviors, using individuals who did not use cannabis in any form as reference. The models were adjusted for age, sex, race, marital status, education, poverty level, depression, and combustible cigarette use.
To examine the association of past-30-day cannabis vaping with asthma and other respiratory symptoms, we restricted our analyses to individuals who had never smoked combustible cigarettes and used multivariable logistic regression models adjusted for age, sex, race, education, income, BMI, and nicotine vaping. Individuals who did not use cannabis in any form were used as the reference group. Interaction effects of sex, age, and race were tested, and results were stratified if significant interaction was found. Sensitivity analyses excluding persons with diagnosed respiratory conditions such as chronic obstructive pulmonary disease (COPD) addressed potential reverse causality. Sensitivity analyses additionally adjusting for nicotine vaping (data on nicotine vaping available in only 2017 and 2018) were also done. Complete case analyses were used in all logistic regression models.
All analyses were conducted in 2020 using Stata version 16 (StataCorp, College Station, TX). The svy command was used to account for the weighting methodology used by the BRFSS, and a 2-sided alpha (α) level of <0.05 was used to determine the statistical significance of the results.
3. Results:
3.1. Trends in Cannabis Use and Vaping
The prevalence of past-30-day cannabis use increased from 10.0% (95%CI, 9.4%–10.7%) in 2017 to 13.4% (95%CI, 12.8%–12.0%) in 2019. Similarly, the prevalence of past-30-day cannabis vaping increased from 1.0% (95%CI, 0.8%–1.2%) to 2.0% (95%CI, 1.7%–2.2%) over the same period. Although there was a steep increase in the proportion of individuals who used cannabis and vaporized it as their primary method of use from 9.9% (95%CI, 7.9%–12.2%) in 2017 to 14.9% (95%CI, 13.2%–16.8%) in 2019 (Figure 1), the proportion of those who smoked it as their primary method of use decreased from 76.6% (95%CI, 73.6% – 79.4%) to 66.3% (95%CI, 63.9% – 68.7%) over the same period.
Figure 1:
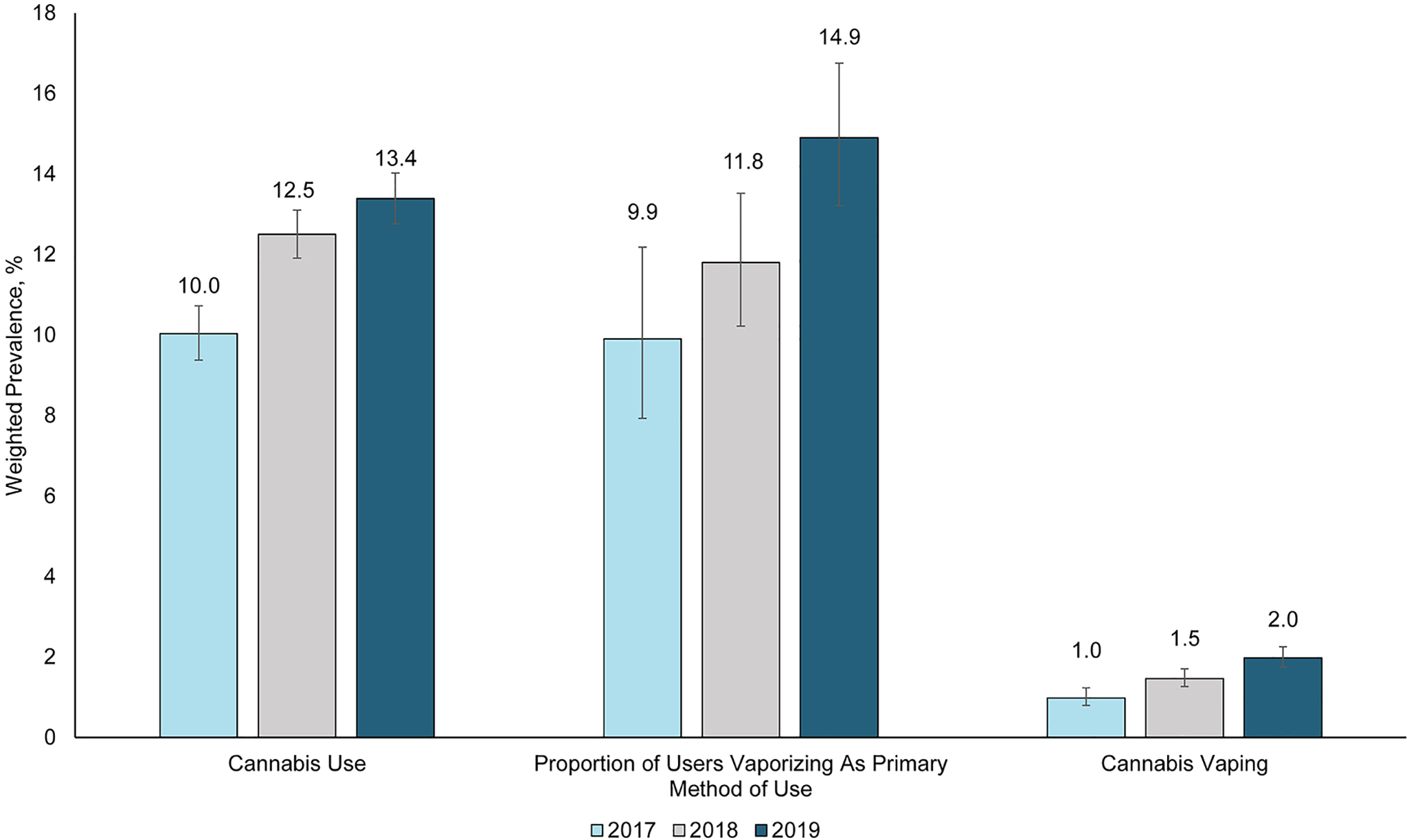
Trends in Cannabis Use, Cannabis Vaping, and the Proportion of Cannabis Users who Vaporize as Primary Method of Use Among US Adults (BRFSS 2017–2019)
The increase in past-30-day cannabis vaping prevalence was observed across various sociodemographic subgroups, with the largest increase observed among young adults (18–24 years)-1.2% in 2017 to 3.9% in 2019 (2.7% absolute and 225% relative increase). Additionally, in each of the years under consideration, males, sexual minority individuals, single persons, students, individuals who previously smoked combustible cigarettes, and those who concurrently vaped nicotine showed a higher prevalence of past-30-day cannabis vaping than their respective comparison groups (Table 1).
Table 1:
Trends in the Prevalence of Past-30-day Cannabis Vaping Stratified by Participant Characteristics (BRFSS 2017–2019)
| Characteristic | Weighted Prevalence, % (95% CI) | |||||
|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2018 vs. 2017 | 2019 vs. 2018 | 2019 vs. 2017 | |
| Sex | ||||||
| Male | 1.1 (0.9–1.5) | 1.8 (1.5–2.1) | 2.5 (2.1–2.9) | 0.7 (0.2–1.1) | 0.7 (0.2–1.2) | 1.4 (0.9–1.9) |
| Female | 0.9 (0.6–1.2) | 1.2 (0.9–1.5) | 1.5 (1.2–1.8) | 0.3 (−0.1–0.8) | 0.3 (−0.1–0.7) | 0.6 (0.2–1.1) |
| Age, years | ||||||
| 18–24 | 1.2 (0.7–2.0) | 2.2 (1.5–3.2) | 3.9 (3.1–4.9) | 1.0 (−0.1–2.0) | 1.7 (0.5–2.9) | 2.7 (1.6–3.8) |
| [18–20] | 1.1 (0.4–2.5) | 2.5 (1.5–4.1) | 3.1 (2.1–4.5) | 1.5 (−0.1–3.0) | 0.6 (−1.1–2.3) | 2.0 (0.5–3.5) |
| [21–24] | 1.3 (0.7–2.5) | 1.8 (1.0–3.4) | 4.6 (3.4–6.2) | 0.6 (−0.9–2.0) | 2.8 (1.0–4.6) | 3.3 (1.7–5.0) |
| 25–29 | 2.2 (1.3–3.7) | 3.8 (2.7–5.4) | 3.8 (2.6–5.6) | 1.6 (−0.2–3.4) | 0.0 (−2.0–2.0) | 1.6 (−0.3–3.5) |
| 30–34 | 1.7 (1.0–2.9) | 1.9 (1.3–2.7) | 2.7 (1.9–3.9) | 0.2 (−1.0–1.3) | 0.8 (−0.4–2.0) | 0.9 (−0.4–2.3) |
| 35–39 | 1.0 (0.5–2.0) | 1.3 (0.8–2.0) | 3.2 [2.2–4.5) | 0.3 (−0.7–1.2) | 1.9 (0.6–3.2) | 2.2 (0.8–3.5) |
| 40–44 | 1.6 (0.7–3.6) | 1.7 (1.1–2.5) | 2.4 (1.6–3.6) | 0.1 (−1.4–1.6) | 0.7 (−0.5–1.9) | 0.8 (−0.8–2.4) |
| 45–49 | 1.2 (0.5–3.0) | 1.4 (0.7–2.7) | 1.7 (1.0–2.7) | 0.1 (−1.3–1.6) | 0.3 (−0.9–1.5) | 0.4 (−0.9–1.8) |
| 50–54 | 0.9 (0.4–1.6) | 1.1 (0.6–2.0) | 1.7 (1.0–2.8) | 0.3 (−0.6–1.1) | 0.6 (−0.5–1.7) | 0.8 (−0.2–1.9) |
| 55–59 | 0.7 (0.2–2.2) | 1.0 (0.5–1.8) | 1.0 (0.6–1.7) | 0.3 (−0.7–1.3) | 0.1 (−0.7–0.9) | 0.3 (−0.6–1.3) |
| >=60 | 0.3 (0.2–0.5) | 0.7 (0.5–1.0) | 0.6 (0.5–0.9) | 0.4 (0.1–0.7) | −0.1 (−0.4–0.3) | 0.3 (0.1–0.6) |
| Race | ||||||
| NH White | 1.0 (0.7–1.3) | 1.5 (1.2–1.8) | 2.1 (1.9–2.5) | 0.5 (0.2–0.9) | 0.7 (0.2–1.1) | 1.2 (0.8–1.6) |
| NH Black | 1.0 (0.4–2.7) | 0.7 (0.3–1.4) | 2.1 (1.3–3.4) | −0.3 (−1.4–0.8) | 1.4 (0.3–2.6) | 1.1 (−0.3–2.6) |
| Hispanic | 1.0 (0.6–1.6) | 1.6 (1.2–2.3) | 1.9 (1.3–2.6) | 0.7 (−0.1–1.4) | 0.2 (−0.6–1.1) | 0.9 (0.1–1.7) |
| Other | 1.0 (0.5–1.9) | 1.5 (1.0–2.2) | 1.5 (1.0–2.3) | 0.5 (−0.4–1.3) | 0.1 (−0.8–1.0) | 0.5 (−0.4–1.4) |
| Sexual Orientation | ||||||
| Heterosexual | 1.0 (0.8–1.3) | 0.5 (0.4–0.6) | 1.4 (1.2–1.7) | −0.5 (−0.8–−0.2) | 0.9 (0.6–1.2) | 0.4 (0.0–0.8) |
| Lesbian/Gay | 6.0 (2.9–12.1) | 1.2 (0.3–4.9) | 4.6 (2.0–10.3) | −4.8 (−9.5–−0.1) | 3.4 (−0.8–7.6) | −1.4 (−7.2–4.4) |
| Bisexual | 2.0 (0.7–5.2) | 1.2 (0.5–2.8) | 2.7 (1.5–5.1) | −0.8 (−2.9–1.4) | 1.5 (−0.5–3.6) | 0.8 (−1.8–3.4) |
| Body Mass Index (BMI, kg/m2) | ||||||
| <18.5 | 0.8 (0.2–4.6) | 3.3 (1.4–7.4) | 3.0 (1.5–6.2) | 2.4 (−0.7–5.5) | −0.2 (−3.7–3.2) | 2.2 (−0.5–4.8) |
| 18.5-<25.0 | 1.6 (1.1–2.2) | 1.8 (1.4–2.3) | 2.5 (2.0–3.1) | 0.2 (−0.4–0.9) | 0.7 (0.0–1.4) | 1.0 (0.2–1.7) |
| 25.0-<30.0 | 0.9 (0.6–1.3) | 1.4 (1.1–1.8) | 2.0 (1.6–2.5) | 0.5 (0.0–1.0) | 0.6 (0.0–1.2) | 1.1 (0.1–1.7) |
| ≥30.0 | 0.7 (0.4–1.1) | 1.3 (0.9–1.8) | 1.6 (1.2–2.2) | 0.6 (0.1–1.1) | 0.3 (−0.3–1.0) | 0.9 (0.4–1.5) |
| Marital Status | ||||||
| Married | 0.9 (0.6–1.2) | 1.2 (0.9–1.4) | 1.3 (1.1–1.6) | 0.3 (−0.1–0.7) | 0.2 (−0.2–0.6) | 0.5 (0.1–0.9) |
| Divorced | 1.0 (0.5–2.1) | 1.2 (0.8–1.7) | 1.9 (1.4–2.7) | 0.1 (−0.7–1.0) | 0.7 (0.0–1.5) | 0.9 (−0.1–1.8) |
| Widowed | 0.3 (0.1–0.8) | 0.6 (0.2–1.9) | 0.3 (0.1–0.7) | 0.4 (−0.4–1.1) | −0.3 (−1.1–0.4) | 0.1 (−0.3–0.4) |
| Single | 1.4 (1.0–1.9) | 2.4 (1.9–3.0) | 3.6 (3.0–4.2) | 1.0 (0.3–1.7) | 1.2 (0.4–2.1) | 2.2 (1.4–3.0) |
| Education | ||||||
| <High school | 0.2 (0.1–0.7) | 0.3 (0.1–0.8) | 0.8 (0.5–1.4) | 0.1 (−0.2–0.5) | 0.5 (0.0–1.0) | 0.7 (0.2–1.1) |
| High school/Some college | 1.0 (0.7–1.4) | 1.5 (1.2–1.9) | 2.1 (1.8–2.5) | 0.5 (0.0–1.0) | 0.6 (0.1–1.1) | 1.1 (0.6–1.6) |
| College graduate | 1.4 (1.0–1.8) | 2.0 (1.6–2.4) | 2.3 (1.9–2.8) | 0.6 (0.1–1.2) | 0.3 (−0.3–0.9) | 1.0 (0.4–1.5) |
| Employment | ||||||
| Employed | 1.1 (0.9–1.5) | 1.8 (1.5–2.1) | 2.6 (2.2–3.0) | 0.7 (0.2–1.1) | 0.8 (0.3–1.3) | 1.5 (1.0–2.0) |
| Unemployed | 1.0 (0.6–1.8) | 1.0 (0.6–1.5) | 1.4 (0.9–2.0) | 0.0 (−0.7–0.6) | 0.4 (−0.3–1.0) | 0.3 (−0.4–1.1) |
| Student | 1.2 (0.5–2.8) | 2.7 (1.7–4.3) | 2.7 (1.8–4.0) | 1.5 (−0.1–3.2) | 0.0 (−1.7–1.6) | 1.5 (0.1–3.0) |
| Retired | 0.5 (0.2–1.1) | 0.6 (0.4–1.0) | 0.5 (0.3–0.9) | 0.1 (−0.4–0.6) | −0.1 (−0.5–0.3) | 0.1 (−0.4–0.6) |
| Income, poverty line | ||||||
| Below | 0.5 (0.2–1.0) | 0.9 (0.6–1.6) | 1.2 (0.8–1.8) | 0.5 (−0.1–1.1) | 0.3 (−0.5–0.9) | 0.7 (0.1–1.3) |
| Within 100–200% | 0.8 (0.4–1.3) | 1.3 (0.9–1.9) | 2.1 (1.6–2.7) | 0.6 (−0.1–1.2) | 0.8 (0.0–1.5) | 1.3 (0.6–2.0) |
| >200% | 1.2 (0.9–1.6) | 1.6 (1.4–1.9) | 2.2 (1.9–2.5) | 0.4 (0.0–0.8) | 0.6 (0.1–1.0) | 1.0 (0.5–1.4) |
| Combustible Cigarette Smoking | ||||||
| Never | 0.8 (0.6–1.1) | 1.3 (1.1–1.6) | 1.5 (1.3–1.8) | 0.5 (0.1–0.8) | 0.2 (−0.2–0.6) | 0.7 (0.3–1.0) |
| Former | 1.5 (1.0–2.2) | 2.1 (1.7–2.7) | 2.9 (2.3–3.6) | 0.6 (−0.2–1.4) | 0.8 (−0.1–1.6) | 1.4 (0.5–2.3) |
| Current | 0.8 (0.3–1.9) | 1.2 (0.8–1.7) | 2.6 (2.0–3.4) | 0.4 (−0.5–1.2) | 1.5 (0.6–2.3) | 1.8 (0.9–2.8) |
| Nicotine e-Cigarette Use | ||||||
| Never | 0.6 (0.5–0.9) | 0.9 (0.8–1.2) | - | 0.3 (0.0–0.6) | - | - |
| Former | 2.1 (1.4–3.2) | 3.5 (2.7–4.6) | 1.4 (0.0–2.7) | |||
| Current | 3.8 (1.9–7.3) | 6.5 (4.5–9.2) | 2.7 (−0.7–6.1) | |||
In 2019, there were variations in the prevalence of past-30-day cannabis use by state, ranging from 9.0% in Wyoming to 15.1% in California and New Hampshire. Similarly, the prevalence of past-30-day cannabis vaping varied by state from 0.3% in Guam to 2.4% in New Hampshire. (Supplementary figure).
3.2. Characteristics of Study Participants (Pooled 2017–2019 BRFSS)
In comparison with individuals who did not use cannabis, those who vaped cannabis were more likely males (59.2% vs 47.1%), younger (age <35 years: 49.2% vs 25.8%), lesbian/gay (7.4% vs 1.5%), bisexual (4.6% vs 2.1%), single (47.9% vs 26.1%), student (8.3% vs 5.1%), employed (71.0% vs 56.3%), had a college degree (35.1 vs 28.3), and had income above 200% of the poverty line (72.0% vs 64.4%). Additionally, individuals who vaped cannabis were more likely to report depression (31.2% vs. 16.9%) and combustible cigarette use (13.6% vs. 11.4%). They were also more likely to report current nicotine vaping (15.7% vs. 2.8%) than individuals who did not use cannabis (Table 2).
Table 2:
Characteristics of Study Participants Stratified by Past-30-day Cannabis Use (BRFSS 2017–2019)
| Participant Characteristics | % (95% Confidence Intervals) | ||
|---|---|---|---|
| Non-cannabis users (n=148,171) | Cannabis users | ||
| Vaporize (n=1,239) | Other methods of use* (n=10,682) | ||
| Sex | |||
| Male | 47.1 (46.6–47.7) | 59.2 (54.6–63.6) | 61.2 (59.4–62.9) |
| Female | 52.9 (52.3–53.4) | 40.8 (36.4–45.4) | 38.8 (37.1–40.6) |
| Age, years | |||
| 18–20 | 5.0 (4.7–5.4) | 8.6 (6.5–11.3) | 9.8 (8.7–11.0) |
| 21–24 | 5.3 (5.1–5.6) | 10.7 (8.3–13.6) | 13.1 (11.9–14.5) |
| 25–29 | 6.7 (6.5–7.0) | 16.8 (13.4–20.7) | 13.2 (12.1–14.5) |
| 30–34 | 8.8 (8.4–9.1) | 13.1 (10.4–16.3) | 12.5 (11.4–13.6) |
| 35–39 | 8.0 (7.7–8.3) | 9.8 (7.5–12.8) | 9.2 (8.3–10.2) |
| 40–44 | 8.2 (7.8–8.5) | 10.4 (7.7–13.8) | 7.8 (6.7–9.0) |
| 45–49 | 7.7 (7.4–8.0) | 7.2 (4.9–10.4) | 5.8 (5.1–6.6) |
| 50–54 | 9.2 (8.9–9.5) | 7.2 (5.2–10.0) | 6.4 (5.6–7.3) |
| 55–59 | 8.8 (8.5–9.2) | 5.1 (3.3–7.7) | 6.5 (5.9–7.3) |
| >=60 | 32.2 (31.7–32.7) | 11.2 (9.1–13.7) | 15.7 (14.6–16.9) |
| Race | |||
| NH White | 55.2 (54.6–55.8) | 57.2 (52.5–61.8) | 55.5 (53.7–57.3) |
| NH Black | 7.6 (7.3–7.8) | 6.7 (4.5–9.7) | 10.3 (9.3–11.4) |
| Hispanic | 23.8 (23.3–24.3) | 24.2 (20.0–28.8) | 22.8 (21.3–24.5) |
| Other | 13.5 (12.9–14.0) | 12.0 (9.3–15.4) | 11.4 (10.1–12.8) |
| Sexual Orientation | |||
| Heterosexual | 95.8 (95.5–96.0) | 85.9 (80.2–90.2) | 87.5 (85.6–89.2) |
| Lesbian/Gay | 1.5 (1.3–1.6) | 7.4 (4.2–12.7) | 3.9 (3.1–4.9) |
| Bisexual | 2.1 (1.9–2.3) | 4.6 (2.8–7.6) | 6.7 (5.6–7.9) |
| Others | 0.7 (0.6–0.8) | 2.0 (0.7–5.8) | 2.0 (1.1–3.6) |
| Body Mass Index (BMI, kg/m2) | |||
| <18.5 | 1.8 (1.6–2.0) | 2.8 (1.7–4.7) | 2.4 (2.0–3.0) |
| 18.5-<25.0 | 31.7 (31.1–32.2) | 41.1 (36.7–45.7) | 40.6 (38.9–42.4) |
| 25.0-<30.0 | 36.2 (35.7–36.8) | 32.7 (28.6–37.0) | 33.0 (31.4–34.7) |
| ≥30.0 | 30.4 (29.9–30.9) | 23.4 (19.7–27.5) | 24.0 (22.5–25.5) |
| Marital Status | |||
| Married | 53.8 (53.2–54.3) | 38.4 (34.1–42.9) | 29.9 (28.4–31.5) |
| Divorced | 12.9 (12.5–13.2) | 11.9 (9.3–15.1) | 14.2 (13.2–15.2) |
| Widowed | 7.3 (7.0–7.6) | 1.8 (0.9–3.6) | 3.2 (2.6–3.8) |
| Single | 26.1 (25.6–26.6) | 47.9 (43.4–52.5) | 52.7 (51.0–54.5) |
| Education | |||
| <High school | 15.8 (15.3–16.2) | 4.6 (3.1–6.8) | 11.5 (10.4–12.8) |
| High school/Some college | 55.9 (55.3–56.4) | 60.3 (56.0–64.4) | 68.9 (67.4–70.5) |
| College graduate | 28.3 (27.9–28.8) | 35.1 (31.3–39.2) | 19.5 (18.4–20.7) |
| Employment | |||
| Employed | 56.3 (55.8–56.9) | 71.0 (66.6–74.9) | 61.6 (59.9–63.3) |
| Unemployed | 18.8 (18.3–19.2) | 14.1 (11.1–17.8) | 20.0 (18.7–21.4) |
| Student | 5.1 (4.8–5.4) | 8.3 (6.3–11.0) | 9.3 (8.2–10.5) |
| Retired | 19.8 (19.4–20.3) | 6.6 (4.6–9.3) | 9.1 (8.3–10.1) |
| Income, the poverty line | |||
| Below | 16.5 (16.1–17.0) | 9.9 (7.4–13.0) | 18.4 (17.1–19.8) |
| Within 100–200% | 19.1 (18.7–19.6) | 18.1 (15.0–21.8) | 21.0 (19.7–22.4) |
| >200% | 64.4 (63.8–64.9) | 72.0 (67.8–75.9) | 60.7 (58.9–62.3) |
| Combustible Cigarette Smoking | |||
| Never | 64.9 (64.4–65.4) | 51.2 (46.7–55.7) | 42.4 (40.6–44.1) |
| Former | 23.7 (23.2–24.2) | 35.2 (30.9–39.8) | 26.8 (25.3–28.3) |
| Current | 11.4 (11.1–11.7) | 13.6 (10.9–16.9) | 30.9 (29.3–32.5) |
| Nicotine e-Cigarette Use | |||
| Never | 84.4 (83.8–84.9) | 48.7 (42.2–55.2) | 44.3 (42.0–46.7) |
| Former | 12.8 (12.3–13.3) | 35.6 (29.4–42.2) | 43.8 (41.4–46.1) |
| Current | 2.8 (2.6–3.0) | 15.7 (11.4–21.2) | 11.9 (10.6–13.4) |
| Depression | |||
| No | 83.1 (82.7–83.5) | 68.8 (64.5–72.8) | 69.5 (67.9–71.0) |
| Yes | 16.9 (16.5–17.3) | 31.2 (27.2–35.5) | 30.5 (29.0–32.1) |
All p-values from chi-squared statistic <0.0001
Other methods of use included smoking, eating, drinking, dabbing, and some other way.
3.3. Association of Past-30-day Cannabis Vaping with High-risk Behaviors
After adjusting for sociodemographic status, depression, and combustible cigarette use, individuals who vaped cannabis had higher odds of reporting heavy alcohol use (aOR, 1.95; 95% CI, 1.45–2.63), binge drinking (aOR, 2.82; 95% CI, 2.25–3.54), and other high-risk behaviors (aOR, 2.47; 95% CI, 1.89–3.24) than individuals who did not report cannabis use (Table 3). These associations remain significant after additionally adjusting for nicotine vaping (Supplementary Table 1).
Table 3:
Association of Past-30-day Cannabis Vaping with High-Risk Behaviors among US Adults (BRFSS 2017–2019)
| Odds Ratio (95% Confidence Intervals) | ||||||
|---|---|---|---|---|---|---|
| Heavy Alcohol Use | Binge Drinking | *Other High-risk Behaviors | ||||
| Model 1 (N=146,827) | Model 2 (N=130,921) | Model 1 (N=147,013) | Model 2 (N=131,020) | Model 1 (N=148,246) | Model 2 (N=131,959) | |
| Non-cannabis users (n=148,171) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Cannabis vapers (n=1,239) | 2.55 (1.95–3.34) | 1.95 (1.45–2.63) | 4.18 (3.45–5.05) | 2.82 (2.25–3.54) | 4.45 (3.49–5.68) | 2.47 (1.89–3.24) |
Composite of intravenous drug use, treatment for sexually transmitted diseases, or giving or receiving money/drugs for sex
Model 1: Unadjusted
Model 2: Age, sex, race, marital status, education, income, depression, and combustible cigarette use
3.4. Association of Past-30-day Cannabis Vaping with Asthma and Other Respiratory Symptoms
There was no significant association between cannabis vaping and asthma among individuals who had never smoked combustible cigarettes after accounting for sociodemographic status and BMI (aOR, 1.03; 95%CI, 0.64–1.64) irrespective of the frequency of use. Similarly, there was no significant association between cannabis vaping and respiratory symptoms (aOR, 1.08; 95%CI, 0.44–2.63; Table 4). Further adjustment for nicotine vaping did not change these results qualitatively (Supplementary Table 2). In addition, excluding individuals with COPD diagnoses did not alter the inference of our findings (Table 4). Also, there was no significant interaction of age, sex, and race with these associations.
Table 4:
Association of Past-30-day Cannabis Vaping with Asthma and Other Respiratory Symptoms Among Never Combustible Cigarette Smokers (BRFSS 2017–2019)
| Odds Ratio (95% Confidence Intervals) | ||||||
|---|---|---|---|---|---|---|
| ASTHMA | RESPIRATORY SYMPTOMS* | |||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Entire Sample | (N=87,483) | (N=86,326) | (N=73,674) | (N=19,345) | (N=19,103) | (N=12,945) |
| Non-cannabis users (n=87,369) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Cannabis vapers(n=589) | 0.91 (0.58–1.42) | 0.94 (0.60–1.47) | 1.03 (0.64–1.64) | 0.88 (0.38–2.01) | 1.05 (0.46–2.40) | 1.08 (0.44–2.63) |
| Less frequent (n=448) | 0.78(0.44–1.38) | 0.81 (0.46–1.43) | 0.87(0.48–1.57) | 0.95(0.34–2.65) | 1.11 (0.39–3.14) | 1.00 (0.31–3.21) |
| More frequent (n=141) | 1.25 (0.61–2.57) | 1.31 (0.64–2.70) | 1.49(0.69–3.20) | 0.73(0.23–2.34) | 0.92 (0.29–2.91) | 1.25 (0.42–3.73) |
| Excluding persons with COPD | (N=84,409) | (N=83,272) | (N=71,016) | (N=18,692) | (N=18,456) | (N=12,446) |
| Non-cannabis users (n=84,219) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Cannabis vapers (n=576) | 1.01 (0.63–1.61) | 0.97 (0.61–1.55) | 1.03 (0.63–1.67) | 0.96 (0.42–2.19) | 1.11 (0.48–2.52) | 1.13 (0.46–2.73) |
| Less frequent (n=440) | 0.92 (0.51–1.63) | 0.88 (0.50–1.57) | 0.92 (0.51–1.67) | 1.03 (0.37–2.90) | 1.17 (0.41–3.31) | 1.06 (0.33–3.38) |
| More frequent (n=136) | 1.28 (0.58–2.81) | 1.24 (0.56–2.75) | 1.37 (0.60–3.13) | 0.79 (0.25–2.56) | 0.96 (0.30–3.07) | 1.28 (0.42–3.84) |
Composite of cough, phlegm production, or shortness of breath
Model 1: Unadjusted
Model 2: Age-adjusted
Model 3: Model 2 + sex, race, education, income, body mass index
COPD: Chronic Obstructive Pulmonary Disease
4. Discussion:
Using nationally representative state-based BRFSS data, we report that the prevalence of past-30-day cannabis use and vaping increased significantly between 2017 and 2019, with a more pronounced increase observed among young adults (18–24 years). We also found that compared to individuals who did not use cannabis, those who vaped cannabis were more likely to be younger, members of sexual minority groups, students, and to report concurrent nicotine vaping. Cannabis vaping was also associated with heavy alcohol use, binge drinking, and other high-risk behaviors. Cannabis vaping was, however, not significantly associated with asthma or other respiratory symptoms.
In agreement with previous reports, we found an increase in the prevalence of cannabis use among US adults (Bose et al., 2018; Uddin et al., 2020). This increase in use may be due to the increasing legalization of cannabis across the US and the nationwide legalization of hemp production through the 2018 Farm Bill, which allowed hemp (with ≤3% THC) cultivation and sale and possession of hemp-derived products (Hall Render, 2019). The rising prevalence may likely continue as more states legalize cannabis, which could potentially be associated with decreased perception of risk and increased use, particularly among adolescents and young adults (Bachman et al., 1998; Mashhoon et al., 2019). The prevalence of cannabis use and vaping among US adults in 2019 was 13.4% and 2.0%, respectively, with variations across states. For example, California, a state with recreational and medical cannabis laws, had a 15.1% prevalence for cannabis use and 2.3% for cannabis vaping. In contrast, Idaho, which has prohibitive cannabis laws, had a prevalence of 9.1% and 0.9% for cannabis use and vaping, respectively. This may reflect the effect of state cannabis laws, as medical and recreational cannabis laws could influence the probability and frequency of cannabis use in a state (Cerdá et al., 2020; Hasin et al., 2017; Martins et al., 2016; Mauro et al., 2019; Wen et al., 2015).
Even though smoking remains the predominant method of cannabis use, as shown in this study and other reports (Schauer et al., 2020), the proportion of individuals who use cannabis and vaporize it as their primary method of use has increased substantially. This increase may be due to the perception that vaping is associated with fewer health effects (Malouff et al., 2014), preference for the discrete nature of vaporizing cannabis (Giroud et al., 2015), and the availability of new devices that can be used to vaporize cannabis, such as modifiable e-cigarettes. The increased use of vaping to deliver cannabis may also be due to the higher blood THC concentrations achieved by vaping or the greater subjective drug effects from vaping relative to smoking cannabis (Spindle et al., 2018). Although in comparison with smoking, vaping might reduce the number of toxicants inhaled, the health effects of chronic cannabis vaping remain unknown (Abrams et al., 2007). Moreover, the perception that vaping is relatively safe may result in increased frequency of use and decreased motivation to quit, leading to misuse and abuse liability (Budney et al., 2015).
Young adults under 35 years, an age group that accounted for 61% of reported EVALI cases, constituted almost half of the individuals who vaped cannabis in our sample (Centers for Disease Control and Prevention, 2020). This is consistent with previous studies showing cannabis vaping to be highly prevalent among younger persons (Steigerwald et al., 2018; Uddin et al., 2020). This is of particular concern because cannabis use in this population could impair learning, produce dependence syndrome, and increase comorbid substance use as well as suicidal ideation (Agrawal et al., 2017; Golub and Johnson, n.d.; Hall and Degenhardt, 2009). In addition, cannabis use could facilitate the initiation of tobacco use and nicotine dependence, a “reverse gateway effect” that could thwart decades-long progress made in reducing nicotine use in youth and young adults (Patton et al., 2005). Equally concerning is the high prevalence of cannabis vaping among individuals who formerly smoked combustible cigarettes because cannabis use has been associated with a higher relapse rate among former smokers or individuals trying to quit smoking (Weinberger et al., 2018).
Nicotine vaping, particularly among the youth, is associated with high-risk behaviors such as using other substances of abuse (Ghosh et al., 2019). Similar to what has been reported for nicotine vaping, we observed cannabis vaping to be associated with heavy alcohol drinking, binge drinking, and other high-risk behaviors. Although this association may not be causal, it suggests that individuals who vape cannabis are more inclined towards a variety of other high-risk behaviors, which may potentially increase their risk of adverse health outcomes. Furthermore, because cannabis vaping appears to cluster with these high-risk behaviors, it may serve as a proxy indicator for these behaviors. Therefore, it seems reasonable to recommend that public health prevention efforts addressing cannabis vaping be targeted at individuals with high-risk behaviors, and opportunities to address such behaviors should be further explored. The association of combustible cigarette use and nicotine vaping with asthma is well known (Coogan et al., 2015; Osei et al., 2019; Wills et al., 2019); however, much less is known about the effects of cannabis vaping. Although the 2017 report by the National Academies of Sciences, Engineering, and Medicine report on the health effects of cannabis did not find conclusive evidence to support a robust association between cannabis smoking and asthma (National Academies of Sciences, 2017), there is growing evidence that chronic cannabis smoking has damaging effects on the airways, causing airway inflammation, which could lead to the development or exacerbation of asthma (Aldington et al., 2007; Roth et al., 1998). Vaping relative to smoking cannabis is associated with reduced exposure to the toxic components of combustible cannabis (Budney et al., 2015). This may account for the lack of an association between cannabis vaping and asthma or other respiratory symptoms in our study. In contrast to this finding, two other recent studies assessing cannabis vaping and respiratory symptoms among adolescents and young adults showed cannabis vaping to be associated with bronchitis symptoms, wheezing, and other respiratory symptoms (Boyd et al., 2021; Braymiller et al., 2020). Several other studies also report that cannabis is associated with pulmonary toxicities (Abeles et al., 2020; Adapa et al., 2020). Though not confirmed in our current study, such associations suggest that even though cannabis vaping is perceived to be safer than cannabis smoking, the likelihood remains that it has a significant impact on respiratory health.
Our findings have important policy implications. With the increasing use of cannabis and cannabis vaping among young individuals, regulations concerning age restrictions on access to cannabis might be warranted. Additionally, as cannabis becomes easily accessible due to its legalization, timely research is needed to explore the health effects of cannabis, particularly cannabis vaping, as many perceive this method of cannabis use to be safe.
4.1. Study Strength and Limitations
A notable strength of our study is the utilization of 3 years of BRFSS data, providing a large sample size for assessing cannabis vaping and its association with high-risk behaviors, asthma, and respiratory symptoms. Nonetheless, our study had some limitations. Data used were self-reported and could have resulted in recall bias and misclassification. Additionally, in assessing the method of cannabis use, study participants reported the method they primarily used in the past month. This implies that individuals who chose other modes of cannabis use could have also vaped cannabis. As a result, our assessment of cannabis vaping prevalence may have underestimated the true prevalence of cannabis vaping among US adults. Also, data from only 9 states were used in this study; therefore, the generalizability of our results may be limited. However, these 9 states have very different cannabis legalization policies (recreational, medical, or prohibitive) and therefore may encompass the extent of cannabis use in other states with similar policies.
Again, the report of asthma was based on health professional diagnosis of asthma. Given that most individuals who vape cannabis are young, and young adults are less likely to be insured or seek healthcare services, asthma may be underdiagnosed in this population. In addition, the study was observational by design; we, therefore, cannot exclude residual confounding and cannot establish causal relations. Finally, due to the cross-sectional nature of this study, we could not explore potential transitions from cannabis smoking to cannabis vaping among study participants or assess the temporality and the direction of the associations, limiting causal inferences.
5. Conclusions:
Cannabis vaping is becoming increasingly popular, particularly among young adults. In addition, the robust association of cannabis vaping with other high-risk behaviors suggests that the clustering of such behaviors may potentially increase the health risks associated with cannabis vaping. These epidemiologic trends are concerning and warrant continued surveillance.
Supplementary Material
Highlights.
Past-30-day cannabis use prevalence among US adults increased between 2017–2019
Cannabis vaping is becoming increasingly popular, particularly among young adults
Individuals who vape cannabis are more likely be indulge in high-risk behaviors
Cannabis vaping is not associated with self-reported asthma or respiratory symptoms
Funding
This work was supported, in part, by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) Center for Tobacco Products (CTP) [HL120163 and HL120163]. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Disclosure Statement
The authors have no conflicts of interest to disclose
Credit author statement
Ellen Boakye: Conceptualization, Methodology, Formal analyses, Writing- Original draft preparation
Olufunmilayo H. Obisesan: Methodology, Formal analyses, Writing- Review and editing
S.M. Iftekhar Uddin: Methodology, Formal analyses, Writing- Review and editing
Omar El-Shahawy: Conceptualization, Methodology, Writing- Review and editing
Omar Dzaye: Writing- Review and editing, Visualization
Albert D. Osei: Methodology, Formal analyses, Writing- Review and editing
Emelia J. Benjamin: Writing- Review and editing, Mentoring
Andrew C. Stokes: Conceptualization, Methodology, Writing- Review and editing
Rose Marie Robertson: Conceptualization, Writing- Review and editing, Mentoring, Funding acquisition
Aruni Bhatnagar: Conceptualization, Writing- Review and editing, Mentoring, Funding acquisition
Michael J. Blaha: Conceptualization, Methodology, Writing- Review and editing, Supervision, Mentoring, Funding acquisition
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abeles M, Popofsky S, Wen A, Valsamis C, Webb A, Halaby C, Pirzada M, 2020. Vaping‐associated lung injury caused by inhalation of cannabis oil. Pediatr. Pulmonol 55, 226–228. 10.1002/ppul.24579 [DOI] [PubMed] [Google Scholar]
- Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL, 2007. Vaporization as a Smokeless Cannabis Delivery System: A Pilot Study. Clin. Pharmacol. Ther 82, 572–578. 10.1038/sj.clpt.6100200 [DOI] [PubMed] [Google Scholar]
- Adapa S, Gayam V, Konala VM, Annangi S, Raju MP, Bezwada V, McMillan C, Dalal H, Mandal A, Naramala S, 2020. Cannabis Vaping–Induced Acute Pulmonary Toxicity: Case Series and Review of Literature. J. Investig. Med. High Impact Case Reports 10.1177/2324709620947267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Nelson EC, Bucholz KK, Tillman R, Grucza RA, Statham DJ, Madden PA, Martin NG, Heath AC, Lynskey MT, 2017. Major depressive disorder, suicidal thoughts and behaviours, and cannabis involvement in discordant twins: a retrospective cohort study. The Lancet Psychiatry 4, 706–714. 10.1016/S2215-0366(17)30280-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldington S, Williams M, Nowitz M, Weatherall M, Pritchard A, McNaughton A, Robinson G, Beasley R, 2007. Effects of cannabis on pulmonary structure, function and symptoms. Thorax 62, 1058–1063. 10.1136/thx.2006.077081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman JG, Johnston LD, O’Malley PM, 1998. Explaining recent increases in students’ marijuana use: Impacts of perceived risks and disapproval, 1976 through 1996. Am. J. Public Health 88, 887–892. 10.2105/AJPH.88.6.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatta DN, Glantz SA, 2020. Association of E-cigarette Use With Respiratory Disease Among Adults: A Longitudinal Analysis. Am. J. Prev. Med 58, 182. 10.1016/J.AMEPRE.2019.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount BC, Karwowski MP, Shields PG, Morel-Espinosa M, Valentin-Blasini L, Gardner M, Braselton M, Brosius CR, Caron KT, Chambers D, Corstvet J, Cowan E, de Jesús VR, Espinosa P, Fernandez C, Holder C, Kuklenyik Z, Kusovschi JD, Newman C, Reis GB, Rees J, Reese C, Silva L, Seyler T, Song MA, Sosnoff C, Spitzer CR, Tevis D, Wang L, Watson C, Wewers MD, Xia B, Heitkemper DT, Ghinai I, Layden J, Briss P, King BA, Delaney LJ, Jones CM, Baldwin GT, Patel A, Meaney-Delman D, Rose D, Krishnasamy V, Barr JR, Thomas J, Pirkle JL, 2020. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N. Engl. J. Med 382, 697–705. 10.1056/NEJMoa1916433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J, Hedden SL, Lipari RN, Park-Lee E, Tice P, 2018. Use and Mental Health Indicators in the United States: Results from the 2017 National Survey on Drug Use and Health Recommended Citation Substance Abuse and Mental Health Services Administration
- Boyd CJ, McCabe SE, Evans-Polce RJ, Veliz PT, 2021. Cannabis, Vaping, and Respiratory Symptoms in a Probability Sample of US Youth. J. Adolesc. Heal 69, 149–152. 10.1016/J.JADOHEALTH.2021.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braymiller JL, Barrington-Trimis JL, Leventhal AM, Islam T, Kechter A, Krueger EA, Cho J, Lanza I, Unger JB, McConnell R, 2020. Assessment of Nicotine and Cannabis Vaping and Respiratory Symptoms in Young Adults. JAMA Netw. Open 3, e2030189–e2030189. 10.1001/JAMANETWORKOPEN.2020.30189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Sargent JD, Lee DC, 2015. Vaping cannabis (marijuana): Parallel concerns to e-cigs? Addiction 110, 1699–1704. 10.1111/add.13036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2020. Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products | Electronic Cigarettes | Smoking & Tobacco Use | CDC [WWW Document] URL https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (accessed 7.12.20).
- Centers for Disease Control and Prevention, n.d. Weighting the BRFSS Data [WWW Document] URL https://www.cdc.gov/brfss/annual_data/2017/pdf/weighting-2017-508.pdf (accessed 7.3.20).
- Centers for Disease Control and Prevention (CDC), 2019. Behavioral Risk Factor Surveillance System 2019 Summary Data Quality Report
- Centers for Disease Control and Prevention (CDC), 2018. Behavioral Risk Factor Surveillance System 2018 Summary Data Quality Report
- Centers for Disease Control and Prevention (CDC), 2017. The Behavioral Risk Factor Surveillance System 2017 Summary Data Quality Report
- Cerdá M, Mauro C, Hamilton A, Levy NS, Santaella-Tenorio J, Hasin D, Wall MM, Keyes KM, Martins SS, 2020. Association between Recreational Marijuana Legalization in the United States and Changes in Marijuana Use and Cannabis Use Disorder from 2008 to 2016. JAMA Psychiatry 77, 165–171. 10.1001/jamapsychiatry.2019.3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadi N, Minato C, Stanwick R, 2020. Cannabis vaping: Understanding the health risks of a rapidly emerging trend. Paediatr. Child Health 25, S16–S20. 10.1093/pch/pxaa016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan PF, Castro-Webb N, Yu J, O’Connor GT, Palmer JR, Rosenberg L, 2015. Active and passive smoking and the incidence of asthma in the Black Women’s Health Study. Am. J. Respir. Crit. Care Med 191, 168–176. 10.1164/rccm.201406-1108OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, 2020. Self-reported Marijuana Use in Electronic Cigarettes among US Youth, 2017 to 2018. JAMA - J. Am. Med. Assoc 10.1001/jama.2019.19571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dataset. Centers for Disease Control and Prevention., 2019. CDC - 2019 Behavioral Risk Factor Surveillance System Survey Data and Documentation [WWW Document] URL https://www.cdc.gov/brfss/annual_data/annual_2019.html (accessed 4.19.21).
- Dataset. Centers for Disease Control and Prevention., 2018. CDC - 2018 BRFSS Survey Data and Documentation [WWW Document] URL https://www.cdc.gov/brfss/annual_data/annual_2018.html (accessed 6.2.21).
- Dataset. Centers for Disease Control and Prevention., 2017. CDC - 2017 BRFSS Survey Data and Documentation [WWW Document] URL https://www.cdc.gov/brfss/annual_data/annual_2017.html (accessed 6.2.21).
- Entwistle MR, Valle K, Schweizer D, Cisneros R, 2020. Electronic cigarette (e-cigarette) use and frequency of asthma symptoms in adult asthmatics in California https://doi-org.proxy1.library.jhu.edu/10.1080/02770903.2020.1805751. 10.1080/02770903.2020.1805751 [DOI] [PubMed] [Google Scholar]
- Ghosh TS, Tolliver R, Reidmohr A, Lynch M, 2019. Youth Vaping and Associated Risk Behaviors — A Snapshot of Colorado. N. Engl. J. Med 380, 689–690. 10.1056/NEJMc1900830 [DOI] [PubMed] [Google Scholar]
- Giroud C, de Cesare M, Berthet A, Varlet V, Concha-Lozano N, Favrat B, 2015. E-cigarettes: A review of new trends in cannabis use. Int. J. Environ. Res. Public Health 10.3390/ijerph120809988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub A, Johnson BD, n.d. The Shifting Importance of Alcohol and Marijuana as Gateway Substances among Serious Drug Abusers* [DOI] [PubMed]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, Benowitz N, 2014. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 23, 133–139. 10.1136/tobaccocontrol-2012-050859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Render, 2019. FDA Clarifies Position on CBD After Passage of 2018 Farm Bill | Hall Render [WWW Document]
- Hall W, Degenhardt L, 2009. Adverse health effects of non-medical cannabis use. Lancet 10.1016/S0140-6736(09)61037-0 [DOI] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Cerdá M, Keyes KM, Stohl M, Galea S, Wall MM, 2017. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991–1992 to 2012–2013. JAMA Psychiatry 74, 579–588. 10.1001/jamapsychiatry.2017.0724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iachan R, Pierannunzi C, Healey K, Greenlund KJ, Town M, 2016. National weighting of data from the Behavioral Risk Factor Surveillance System (BRFSS). BMC Med. Res. Methodol 16, 1–12. 10.1186/s12874-016-0255-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenne DR, Fischbein RL, Tan ASL, Banks M, 2017. The Use of Substances Other Than Nicotine in Electronic Cigarettes Among College Students. Subst. Abus. Res. Treat 11, 1–8. 10.1177/1178221817733736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouff JM, Rooke SE, Copeland J, 2014. Experiences of Marijuana-vaporizer users. Subst. Abus 35, 127–128. 10.1080/08897077.2013.823902 [DOI] [PubMed] [Google Scholar]
- Martins SS, Mauro CM, Santaella-Tenorio J, Kim JH, Cerda M, Keyes KM, Hasin DS, Galea S, Wall M, 2016. State-level medical marijuana laws, marijuana use and perceived availability of marijuana among the general U.S. population. Drug Alcohol Depend 169, 26–32. 10.1016/j.drugalcdep.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Sagar KA, Gruber SA, 2019. Cannabis Use and Consequences. Pediatr. Clin. North Am 10.1016/j.pcl.2019.08.004 [DOI] [PubMed] [Google Scholar]
- Mauro CM, Newswanger P, Santaella-Tenorio J, Mauro PM, Carliner H, Martins SS, 2019. Impact of Medical Marijuana Laws on State-Level Marijuana Use by Age and Gender, 2004–2013. Prev. Sci 20, 205–214. 10.1007/s11121-017-0848-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, E. and M., 2017. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research, Psychiatria National Academies Press, Washington, DC: 10.17226/24625 [DOI] [PubMed] [Google Scholar]
- National Conference of State Legislatures, 2020. State Medical Marijuana Laws [WWW Document] URL https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx (accessed 11.24.20).
- Osei AD, Mirbolouk M, Orimoloye OA, Dzaye O, Uddin SMI, Benjamin EJ, Hall ME, DeFilippis AP, Bhatnagar A, Biswal SS, Blaha MJ, 2020. Association Between E-Cigarette Use and Chronic Obstructive Pulmonary Disease by Smoking Status: Behavioral Risk Factor Surveillance System 2016 and 2017. Am. J. Prev. Med 58, 336–342. 10.1016/J.AMEPRE.2019.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei AD, Mirbolouk M, Orimoloye OA, Dzaye O, Uddin SMI, Dardari ZA, Defilippis AP, Bhatnagar A, Blaha MJ, 2019. The association between e-cigarette use and asthma among never combustible cigarette smokers: Behavioral risk factor surveillance system (BRFSS) 2016 & 2017. BMC Pulm. Med 19. 10.1186/s12890-019-0950-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M, 2005. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction 100, 1518–1525. 10.1111/j.1360-0443.2005.01220.x [DOI] [PubMed] [Google Scholar]
- Peace MR, Butler KE, Wolf CE, Poklis JL, Poklis A, 2016. Evaluation of two commercially available cannabidiol formulations for use in electronic cigarettes. Front. Pharmacol 7. 10.3389/fphar.2016.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan MM, Banta JE, Bahjri K, Montgomery SB, 2020. Frequency of cannabis use and alcohol-associated adverse effects in a representative sample of US adolescents and youth (2002–2014) a cross-sectional study. J. Cannabis Res 2, 38. 10.1186/s42238-020-00043-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MD, Arora A, Barsky SH, Kleerup EC, Simmons M, Tashkin DP, 1998. Airway Inflammation in Young Marijuana and Tobacco Smokers. Am. J. Respir. Crit. Care Med 157, 928–937. 10.1164/ajrccm.157.3.9701026 [DOI] [PubMed] [Google Scholar]
- Schauer GL, Njai R, Grant-Lenzy AM, 2020. Modes of marijuana use – smoking, vaping, eating, and dabbing: Results from the 2016 BRFSS in 12 States. Drug Alcohol Depend 209, 107900. 10.1016/j.drugalcdep.2020.107900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, Hayes E, Vandrey R, 2018. Acute Effects of Smoked and Vaporized Cannabis in Healthy Adults Who Infrequently Use Cannabis: A Crossover Trial. JAMA Netw. open 1, e184841. 10.1001/jamanetworkopen.2018.4841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigerwald S, Wong PO, Cohen BE, Ishida JH, Vali M, Madden E, Keyhani S, 2018. Smoking, vaping, and use of edibles and other forms of marijuana among US adults. Ann. Intern. Med 10.7326/M18-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services, n.d. Poverty Guidelines | ASPE [WWW Document] URL https://aspe.hhs.gov/poverty-guidelines (accessed 12.17.20).
- Uddin SMI, Osei AD, Obisesan OH, El-Shahawy O, Dzaye O, Cainzos-Achirica M, Mirbolouk M, Orimoloye OA, Stokes A, Benjamin EJ, Bhatnagar A, DeFilippis AP, Henry TS, Nasir K, Blaha MJ, 2020. Prevalence, trends, and distribution of nicotine and marijuana use in E-cigarettes among US adults: The behavioral risk factor surveillance system 2016–2018. Prev. Med. (Baltim) 139, 106175. 10.1016/j.ypmed.2020.106175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, 2008. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol 61, 344–349. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Platt J, Copeland J, Goodwin RD, 2018. Is cannabis use associated with increased risk of cigarette smoking initiation, persistence, and relapse? longitudinal data from a representative sample of US adults. J. Clin. Psychiatry 79. 10.4088/JCP.17m11522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Hockenberry JM, Cummings JR, 2015. The effect of medical marijuana laws on adolescent and adult use of marijuana, alcohol, and other substances. J. Health Econ 42, 64–80. 10.1016/j.jhealeco.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Wills TA, Pagano I, Williams RJ, Tam EK, 2019. E-cigarette use and respiratory disorder in an adult sample. Drug Alcohol Depend 194, 363–370. 10.1016/j.drugalcdep.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Kathuria H, Galiatsatos P, Blaha MJ, Hamburg NM, Robertson RM, Bhatnagar A, Benjamin EJ, Stokes AC, 2020. Association of Electronic Cigarette Use With Incident Respiratory Conditions Among US Adults From 2013 to 2018. JAMA Netw. Open 3, e2020816–e2020816. 10.1001/JAMANETWORKOPEN.2020.20816 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


