Abstract
Introduction: The aim of this study was to review the effect of irreversible electroporation parameter settings on the size of the ablation zone and the occurrence of thermal effects. This insight would help to optimize treatment protocols and effectively ablate a tumor while controlling the occurrence of thermal effects. Methods: Various individual studies report the influence of variation in electroporation parameters on the ablation zone size or occurrence of thermal effects. However, no connections have yet been established between these studies. With the aim of closing the gap in the understanding of and personalizing irreversible electroporation parameter settings, a systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. A quality assessment was performed using an in-house developed grading tool based on components of commonly used grading domains. Data on the electroporation parameters voltage, number of electrodes, inter-electrode distance, active needle length, pulse length/number/protocol/frequency, and pulse interval were extracted. Ablation zone size and temperature data were grouped per parameter. Spearman correlation and linear regression were used to define the correlation with outcome measures. Results: A total of 7661 articles were screened, of which 18 preclinical studies (animal and phantom studies) met the inclusion criteria. These studies were graded as moderate (4/18) and low (14/18) quality. Only the applied voltage appeared to be a significant linear predictor of ablation zone size: length, surface, and volume. The pulse number was moderately but nonlinearly correlated with the ablation zone length. Thermal effects were more likely to occur for higher voltages (≥2000 V), higher number of electrodes, and increased active needle length. Conclusion: Firm conclusions are limited since studies that investigated and precisely reported the influence of electroporation parameters on the ablation zone size and thermal effects were scarce and mostly graded low quality. High-quality studies are needed to improve the predictability of the combined effect of variation in parameter combinations and optimize irreversible electroporation treatment protocols.
Keywords: irreversible electroporation, permeabilization, pulsed electric field, ablation, cancer therapy, temperature, animal model, phantom model
Introduction
Irreversible electroporation (IRE) is increasingly used in clinical practice for local tumor ablation, since the integrity of the vasculature and other vital structures are preserved.1,2 Comparable to electrochemotherapy and radiotherapy, IRE is considered a nonthermal ablation technique, which means that tumor treatment does not depend on a temperature increase. IRE is performed by placement of needle electrodes in and around the tumor. A pulsed electric field with a maximum potential difference of 3000 V is consecutively created between the electrode pairs and gives rise to an electric current of microsecond duration. The electric field strength (V/cm) reached during electroporation determines whether the cell membranes are reversibly or irreversibly permeabilized. In most tissues, 680 V/cm is considered as a target electric field strength threshold to achieve IRE and the desired cell death.3,4 Below this threshold electroporation is considered reversible.5 During IRE treatment, several parameters can be chosen, such as the active needle length, applied voltage, pulse length, and number of pulses to obtain the desired treatment effect. The size and shape of the ablation zone and potential occurrence of unintentional thermal effects and subsequent damage are a net result of these electroporation parameters and tissue interactions, such as tissue heterogeneity and electrical conductivity.6,7 Thermal effects have been observed in simulations, experimental models, and clinical studies on IRE.8–13 The area in the vicinity of the electrodes is most at risk for thermal effects.14 Whether thermal damage occurs (temperatures >50 °C) is dependent on the maximum temperatures reached during electroporation and the time these temperatures are maintained. This is also influenced by heat dissipation through blood vessels and heat diffusion into the surrounding tissue.15–18 Various individual studies reported the influence of variation in electroporation parameters on the ablation zone size or occurrence of thermal effects. However, no connections have yet been established between these studies in a systematic overview. Therefore, the aim of this study was to review the effect of irreversible electroporation parameter settings on the size of the ablation zone and occurrence of thermal effects. These insights would help to optimize IRE treatment protocols and provide for an effective tumor ablation and in terms of safety control the occurrence of thermal effects and damage.
Materials and Methods
Literature Search
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and registered in INPLASY (INPLASY202230161). To achieve high sensitivity, our search strategy was conducted with the help of an experienced librarian and relevant databases were included, PubMed, Embase, Web of Science, Institute of Electrical and Electronics Engineers (IEEE) Xplore Digital Library, and American Society of Mechanical Engineers (ASME) Digital Collection. Studies published from inception up to September 1, 2021, were recorded. A complete overview and description of the search strategy per database is available in Supplementary S1. The search comprised both reversible (RE) and irreversible electroporation studies since the threshold between both is not dichotomous and RE studies could potentially contain relevant data. Duplicates were removed in EndNote X8 following the method described by Bramer et al.19 All de-duplicated titles and abstracts were assessed in Rayyan, a web app developed for systematic review screening, for remaining duplicates and were assessed on eligibility based on predefined in- and exclusion criteria.20 Two authors (A.M.H. and C.G.O) independently reviewed a random sample of 650 articles for eligibility by title and abstract screening. Consensus was reached on discordant judgments. The remaining articles were screened by the first author (A.M.H.) when the inter-rater agreement was >95%. In case of any uncertainty to determine the eligibility, the abstract was independently assessed by and discussed with the second author (C.G.O.). Articles included during the title and abstract screening were assessed in more detail for eligibility during the full-text screening phase. The full-text screening was performed by the first author and randomly verified by the second author. To validate the search, the sensitivity and precision of the search strategy were determined. Precision was defined according to the Cochrane definition, and the sensitivity was determined by the method described by Romeo et al.21,22 Therefore, a library, “seed list,” consisting of potentially relevant articles recommended by the writing group and additional potentially relevant articles retrieved from manual search of the reference lists of these articles was composed . A paper was considered as potentially relevant and included in the seed list, if based on assessment of title and abstract the authors considered that the article should have been included for full-text assessment. This seed list was used to test the sensitivity of the search strategy, by assessing the number of articles from the seed list included in the initial results of the electronic search prior to screening of articles and reference lists. As a general rule, the precision ranges between 1% and 10% for a suitable search strategy.
Eligibility Criteria
Studies were included when all eligibility criteria were met. Inclusion criteria were: (a) ablation zone and/or temperature measurements were performed; (b) during or after IRE or RE; (c) performed on in vivo or ex vivo liver, pancreas, kidneys, or prostate, or on electroporation of potato or tissue phantoms; (d) all electroporation parameter settings (voltage, number of electrodes, inter-electrode distance, active needle length, number of pulses and pulse length); and (e) their exact values used for electroporation (eg, a range, mean or maximum parameter value are not adequate enough) were described; (f) one parameter was consecutively varied during the electroporation, while the other parameters were kept constant and (g) the measured ablation zone sizes and temperatures were described for the consecutive variation per studied electroporation parameter. Exclusion criteria were: (a) needle types other than monopolar electrodes to align with the clinical practice (eg, bipolar electrodes); (b) aberrant needle electrode shapes deviating from a straight needle (eg, plate or flexible endoscopic electrodes (intraluminal electroporation), electrodes used in combination with a grounding pad or other kind of grounding material instead of another electrode); (c) electroporation in vitro, on (single) cell level or performed with the goal to deliver genes, drugs or chemotherapeutics (electrochemotherapy) into the cell. Other phantom models, such as potato tubers and (polyacrylamide) gel phantoms, are eligible for inclusion; (d) combination therapy (eg, electrochemotherapy); (e) pulse forms other than monopolar square wave pulses (eg, bipolar, H-FIRE, exponential decay or sinus formed pulses); (f) simulation models; (g) undefined orientation of the measured ablation zone size with reference to the needle insertion path; (h) metal stents or objects in the electroporation zone; (i) ablation zone and temperature results that were only presented in figures, unable to precisely determine the results; (j) review articles; (k) conference abstracts; and (l) studies published in another language than English, French, or German. Of all conference abstracts of which no full-text article was present in the title and abstract search, a web-based search (Google Scholar, ResearchGate, author, and co-author name(s) in Embase) was done to investigate whether a full-text article was available. The corresponding author was approached by e-mail (in cases where the contact details were available) when the full-text of a relevant abstract (eg, conference abstract) could not be found to verify whether the results were published as full-text. The study was excluded when the full-text could not be found or provided.
The PICO corresponding to this review is presented in Table 1. In vitro studies were beyond the scope of this review since these studies comprise fundamental research on the cell response to IRE.
Table 1.
Research question according to the PICO format.
| P - Population | Humans, animals (in vivo and ex vivo), and phantom models |
| I - Intervention | Variation in electroporation parameters |
| C- Comparison | Not applicable |
| O - Outcome | Ablation zone size and thermal effects |
Data Extraction
From each article, data on the electroporation equipment (electroporation generator, pulse type, electrode type, and manufacturer), electroporation parameter values (voltage, number of electrodes, inter-electrode distance (IED), active needle length (ANL), pulse length, pulse number, pulse protocol [pulse delivery in a continuous or sequential fashion], pulse frequency [number of pulses per unit of time], and pulse interval [time between cycles of sequential pulses, eg, 45 pulses—30 s delay—45 pulses]), ablation zone size (mm/mm2/mm3), method to assess and measure the ablation zone size (including the time interval between the electroporation procedure and the ablation zone size measurements, the orientation of the electrodes with reference to the ablation zone and number of dimensions in which the ablation zone was measured (length, surface, or volume) as well as data regarding the measured temperatures (°C), method, and location of the temperature measurements were extracted. With respect to temperature data, a distinction was made between (mean) maximum absolute (T) and (mean) maximum relative temperature (ΔT) results. Other collected baseline characteristics include the study model (humane, animal, tissue phantom, gel, or potato) investigated in the study, electroporated organ, ablation of a healthy organ or tumor tissue, electrode location, sample size per investigated set of electroporation parameter combinations and number of ablations performed. Data were only extracted from the individual sections of each study that met all eligibility criteria. The data extraction was performed by the first author (A.M.H.) and randomly verified by the second author (C.G.O.).
Quality Assessment
The large heterogeneity in study designs included in this review meant that existing quality scores did not fully apply. For this reason, we have developed a grading tool, composed of components from the Newcastle-Ottawa scale, Jadad scale, and Cochrane guidelines, for quality assessment.21,23,24 The grading tool comprised of the following domains: the study model, sample size (per investigated set of electroporation parameter combinations), statistical method, the presence of a replicable description of the methods section, ethical approval obtained, availability of the raw data of the ablation zone, and temperature results, whether all hypotheses stated per included article were evaluated and whether the conclusion was justified by the results. An overview of the questions included in the quality assessment and method of judgment is presented in Supplementary S2. The quality assessment tool was only applied to the sections of each study that were eligible for data extraction, with the exception of more overall quality assessment questions. Studies were qualified as high, moderate, or low quality based on their quality assessment score per domain. In accordance with the Cochrane guidelines, the overall quality of the articles was scored as high, moderate, or low when no, up to 2 or more than 2 domains were scored as inadequate (−), respectively. The quality assessment was performed by the first author (A.M.H.) and randomly verified by the second author (C.G.O.).
Data Analysis
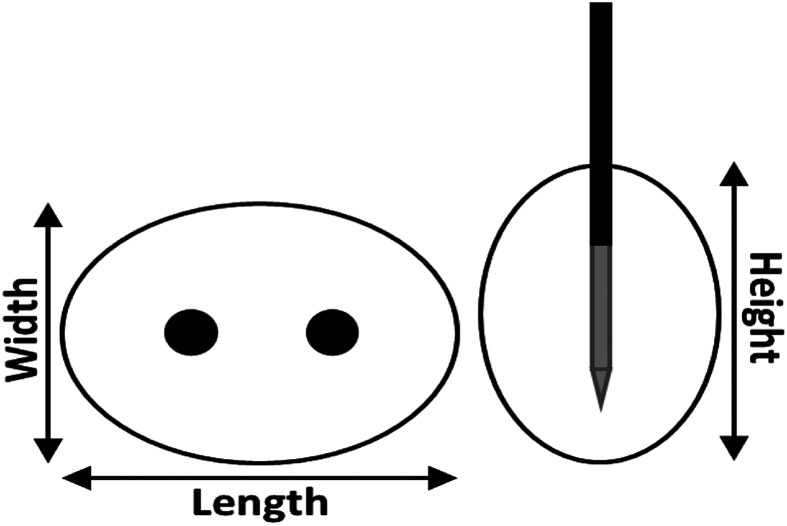
Electroporation outcomes were categorized into ablation zone size (length [Figure 1], surface, and volume) and temperature (T and ΔT) outcomes and separately analyzed and visualized per varied electroporation parameter. SPSS version 25 (SPSS, IBM Corp) and Microsoft Excel were used for data analysis.
Figure 1.
Definition of the length, width, and height of the ablation zone perpendicular (left) and parallel to the needle insertion path (right).
Per graph, the effect of an individual electroporation parameter was investigated. Each individual study, model (animal [in vivo or ex vivo], gel, or potato), and investigated combination of electroporation parameters (“protocol”) are separately visualized by dots of a specific color. An electroporation protocol represents a constant set of parameter values where only the investigated parameter was consecutively varied. However, the standard set of electroporation parameter values differed between consecutive protocol numbers. The standard deviation, as reported in the included studies, was presented as a measure of the dispersion in the ablation zone size or temperature data.
The distribution was assessed based on the study design and sample size per investigated set of electroporation parameter combinations in combination with the Kolmogorov-Smirnov test. In the case of an explorative study character and small number of observations per electroporation parameter combination, a normal distribution could not be assumed so indicating the use of non-parametric tests. The Spearman correlation coefficient (rho) was used to assess the relationship between the electroporation parameters and the ablation zone size and thermal effects, considering the absence of a normal distribution and regarding the small sample size. Electroporation parameters for which ρ > 0.70 (0-0.50 (weak), 0.50-0.80 (moderate), and 0.80-1 (strong)) were presented in the main article. Supplementary S5 and S6 present the electroporation parameters that showed no or weak correlation. Significance was considered as P < .05. In case of a strong correlation and the presence of a trend in data points, linear regression analysis was performed to determine whether a linear model could predict the relation between that electroporation parameter and the ablation zone size or thermal effects. Finally, it was assessed which parameters exceeded the threshold for thermal damage (>50 °C).
Results
Eligible Studies
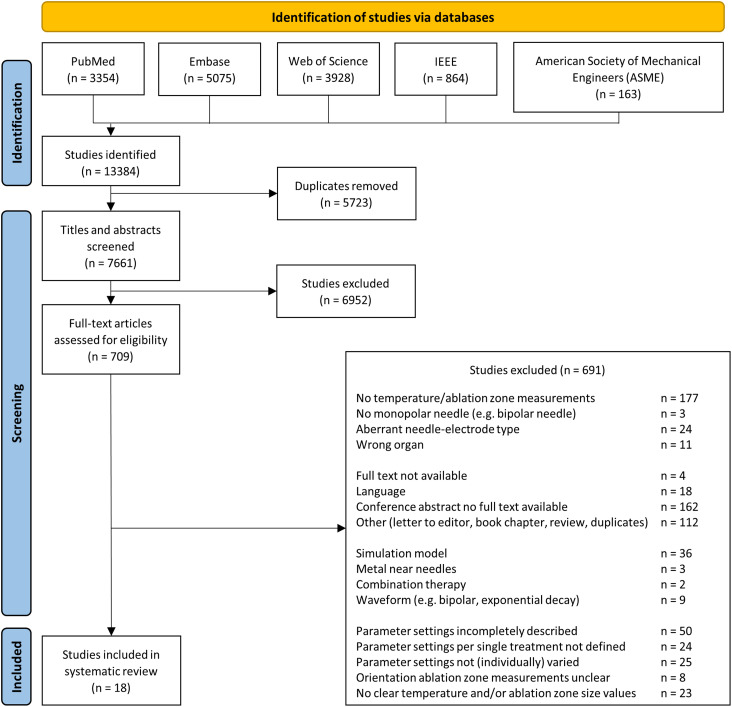
After removal of duplicates and screening of 7661 articles by title, abstract, and full-text, 18 articles were included. A PRISMA flowchart of the article selection process is shown in Figure 2. Ninety-seven percent of the random sample of 650 articles (630/650) were in- or excluded by both authors. Consensus was reached on discordant judgments (20/650, 3%). The precision of the search strategy was 9.3%. A total of 30 articles were recommended for inclusion in the seed list. Upon manual search of the reference lists of these articles, 78 papers were additionally identified as potentially relevant. Except for 1 article, all potentially relevant articles from the seed list were included in the electronic search, suggesting a sensitivity of the search close to 100%.
Figure 2.
Flowchart article selection.
Study Characteristics
The study characteristics and additional information on the method of ablation zone size and temperature measurements are presented in Supplementary S3. All included studies pertained to IRE and no studies investigated RE. Several studies used multiple models or organ types to investigate the ablation zone size and temperature effects. Of all 12 included animal studies, 11 concerned healthy animals and in 1 study a pancreatic adenocarcinoma tumor located in the flank was ablated.25
Electroporation device
Electroporation pulses were delivered by the NanoKnife IRE generator (AngioDynamics Inc) (n = 7),12,26–31 BTX ECM 830 pulse generator (Harvard Apparatus) (n = 5),25,32–35 custom-made, prototype, or not specified pulse generator (n = 4,36–39 n = 1,40 and n = 1,41 respectively).
Ablation zone measurements
Of the 18 included studies, 13 (72%) investigated the ablation zone size, the ablation zone length (n = 7),26,27,32,34,36,39,40 surface (n = 6),33–35,37,38,41 volume (n = 2),28,39 or a combination (n = 2).34,39 None of the included studies investigated the ablation zone size in humans. Animal models were used in 9 studies to investigate the ablation zone dimensions. Nine studies used an in vivo animal model; porcine (n = 4), canine (n = 2), rabbit (n = 2), and rat (n = 1), and an ex vivo model of undefined animal species was used in 1 study. Consecutively, 7, 2 and 4 studies performed IRE in an in vivo or ex vivo liver, in vivo kidney, or potato model, to determine the effect of the variation in ablation parameters on the ablation zone dimensions. Gross pathology or macroscopic measurements, eventually in combination with CT imaging or in Matlab/ImageJ analyzed photographs were primarily used to determine the ablation zone size. Only 2 studies used histopathologic examination combined with Matlab or ImageJ analysis, with additional MR imaging in 1 of both studies.
Thermal effects
Six studies (33%) performed temperature measurements.12,25,28–31 Temperature experiments were performed in an in vivo animal model; a porcine (n = 3) and mice (n = 1) model of the kidney (n = 2), liver (n = 1), and pancreas (n = 1) in 4 studies, polyacrylamide gel phantom (n = 3) or potato tuber (n = 1). None of the included studies have measured the temperature in human patients during IRE treatment. Fiber-optic temperature sensors (n = 4), a thermal camera (n = 3) and thermopile temperature sensor (n = 1), were used to perform the temperature measurements.
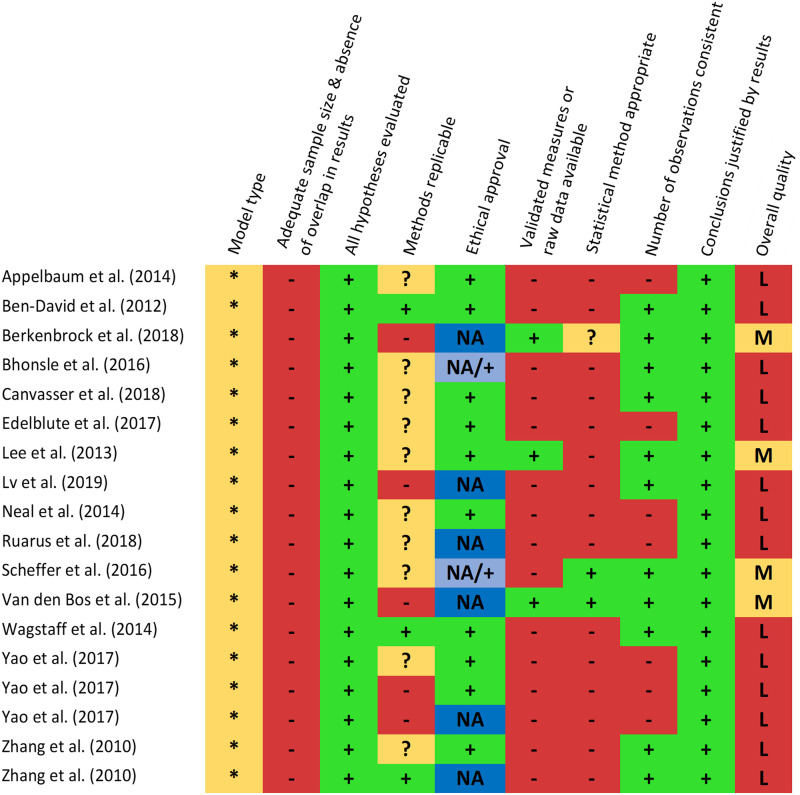
Quality Assessment
The quality assessment scores of the included studies are shown in Figure 3. None of the included studies investigated the ablation zone size or temperature effects in human patients. Additional risks for biases included an inadequate sample size per investigated set of electroporation parameter combinations (n ≤ 10) or absence of a sample size calculation, overlapping study results, inappropriate methods description, and inappropriate statistical methods. The overall quality score was qualified as moderate in 4 studies (22%) and low in 14 studies (78%). All included studies comprised fundamental research on electroporation.
Figure 3.
Overview quality assessment score. ** Human patient, * Validated model including animal (in vivo/ex vivo), tissue phantom, gel, or potato models, + Adequately reported, ? Partially reported: a detail was not reported (eg, pulse interval between sets of consecutive pulses (sequential pulsing)) and - Incompletely reported and hardly replicable: a few details or major point(s) were absent (eg, electroporation generator type, method used to analyze the temperature data, or determine the ablation zone size). The overall quality was qualified as High (H; no domain scored with -), Moderate (M; up to 2 domains scored with -), or Low (L; more than 2 domains scored with -), based on the score per domain.
Effect of Variation in Electroporation Parameters
A summative table of the investigated electroporation parameters and parameter values per study are included in Supplementary S4.
Ablation zone size
Of the 18 included studies, 13 have investigated the relation between variation in an electroporation parameter and the ablation zone size: length, surface, and volume.
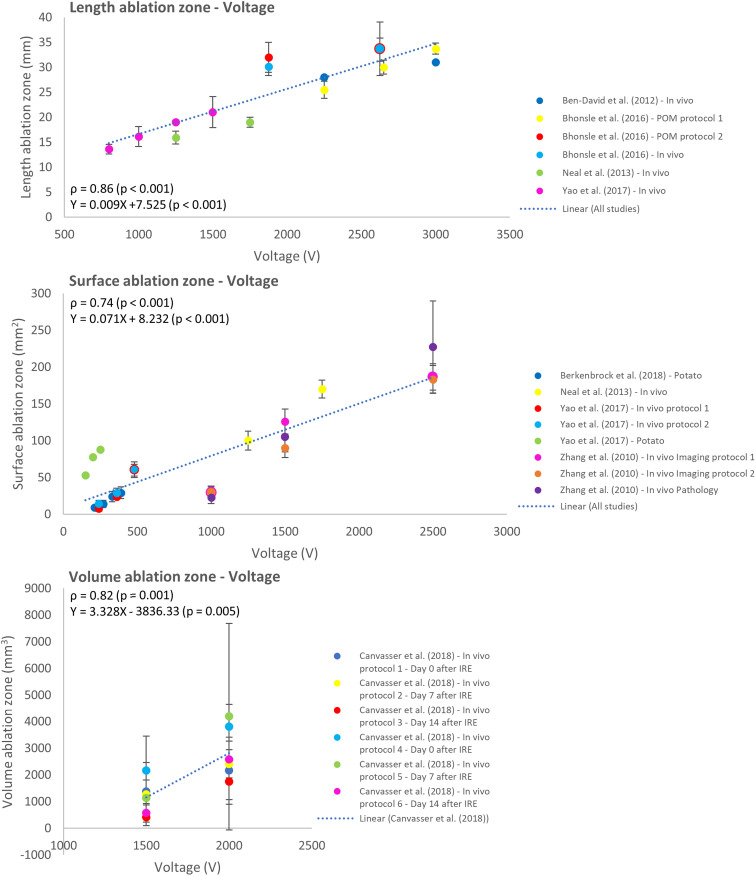
The effect of variation in applied voltage was studied in 9 reports.27,28,32,34–38,41 A significant correlation and positive linear relation were shown between the applied voltage and ablation zone in length (ρ = 0.86; P < .001), surface (ρ = 0.74; P < .001), and volume (ρ = 0.82; P < .001) (Figure 4).
Figure 4.
Effect of variation in voltage on the ablation zone length, surface, and volume.
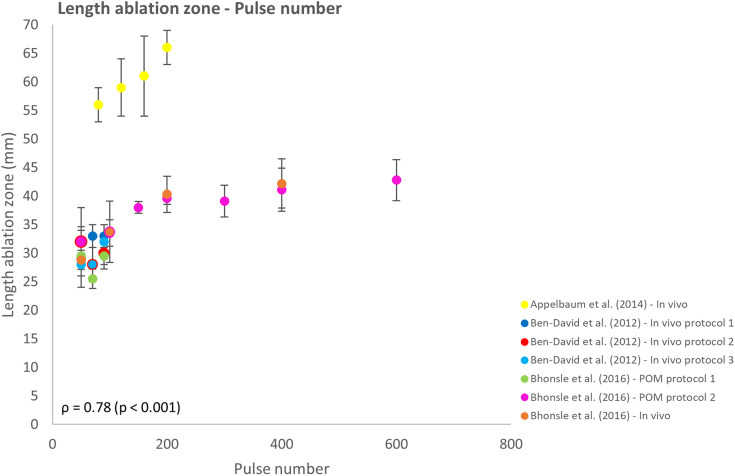
The effect of pulse number on the IRE ablation zone was investigated in 6 reports.26,27,32,33,37,38 With the increasing pulse number, an increase in ablation zone length was observed (Figure 5). However, the rate of this increase was not consistent and varied per study and height of the pulse number. The number of delivered pulses and ablation zone length showed a moderate correlation (ρ = 0.78; P < .001). For pulse numbers >200 pulses, the increase in ablation zone length appears to flatten, deviating from a linear model. Therefore, the effect of pulse number on the length of the ablation zone cannot be predicted unambiguously.
Figure 5.
Effect of variation in pulse number on the ablation zone length.
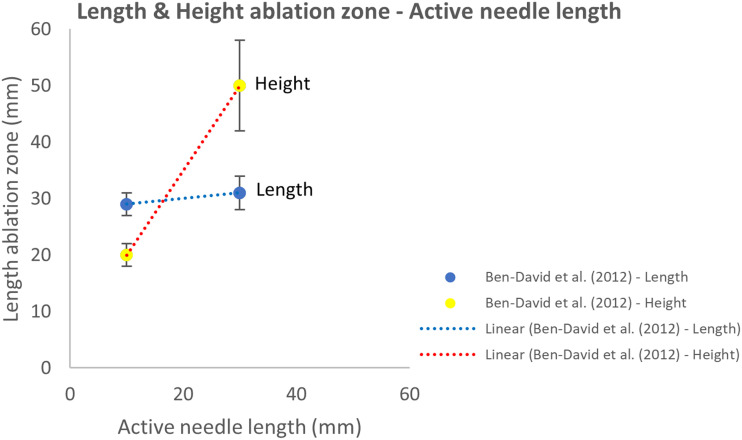
Adjustments in active needle length affected the height of the ablation zone and had a negligible effect on the ablation zone length (Figure 6). Although these findings were based on a single study.26 A weak correlation was present between the ANL and ablation zone volume as shown in Supplementary S5.28
Figure 6.
Effect of variation in active needle length on the length and height of the ablation zone.
Electroporation parameters that showed no or a weak correlation with the ablation zone size are presented in Supplementary S5. Including the effect of the inter-electrode distance40 and pulse length27 on the ablation zone length, relation between pulse number and ablation zone surface,33,37,38 effect of the active needle length on the ablation zone volume28 and influence of variation in pulse protocol, frequency and interval on the ablation zone length and volume.39 No study investigated the relation between the number of electrodes and ablation zone size.
Thermal effects
Of the 18 included studies, 6 have measured the temperature for variation in electroporation parameter values.
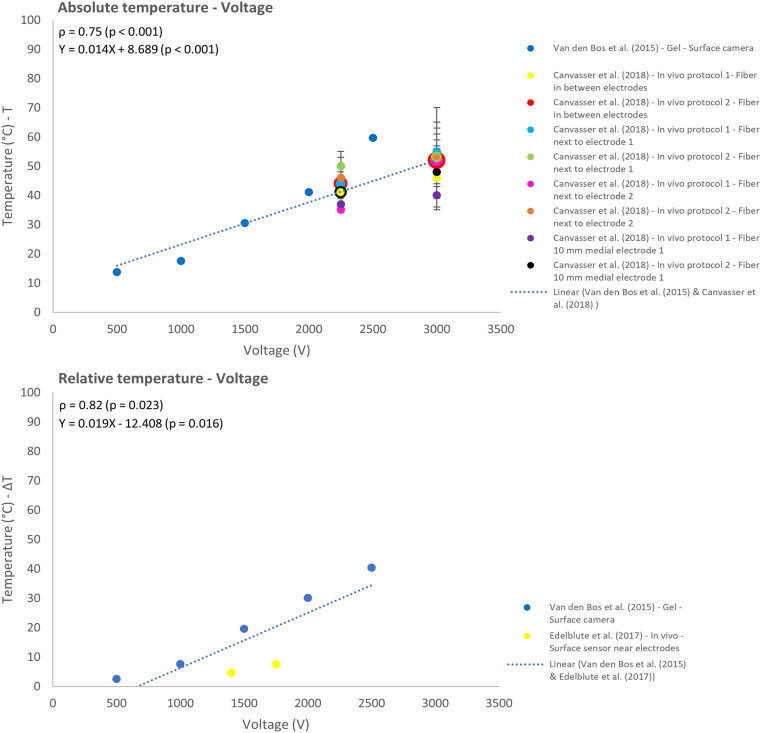
Three reports investigated the effect of the applied voltage on the temperature.25,28,31 Increasing the voltage was moderately correlated with an increase in absolute temperature (ρ = 0.75; P < .001), strongly correlated with the relative temperature (ρ = 0.82; P = .023) and linearly related to both temperature results, T and ΔT (Figure 7).
Figure 7.
Effect of variation in voltage on the absolute (T) and relative (ΔT) temperature.
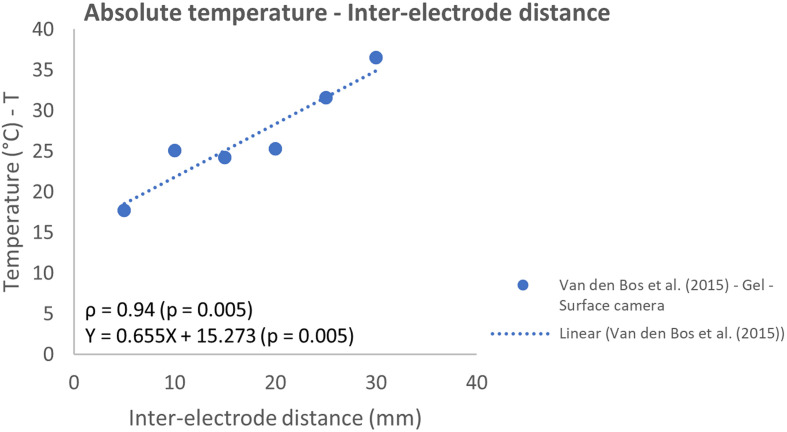
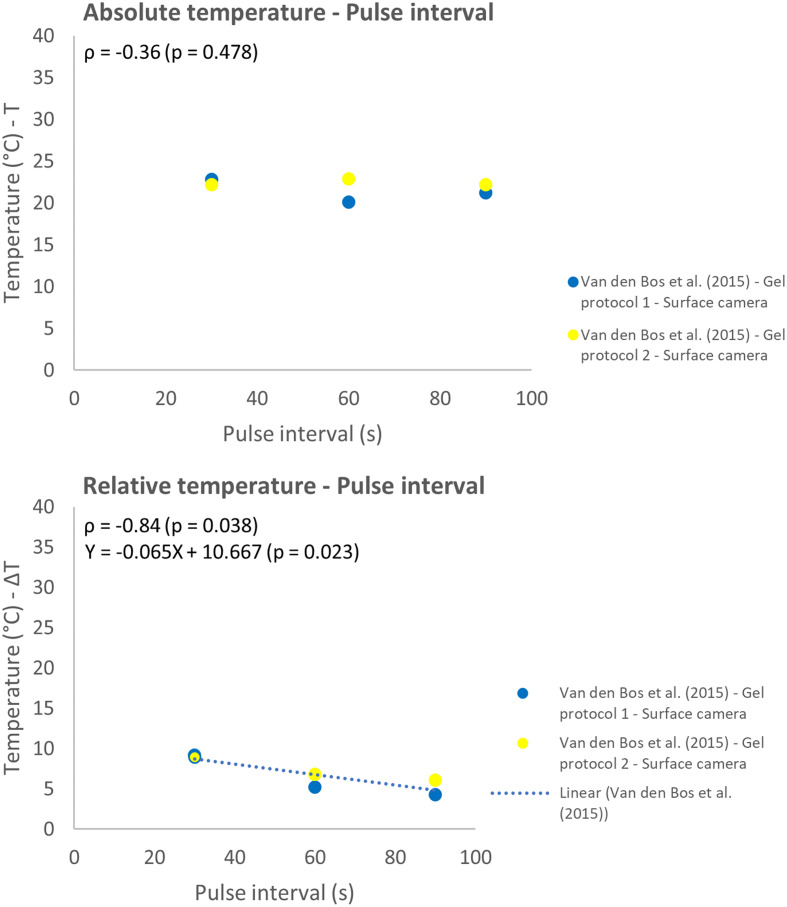
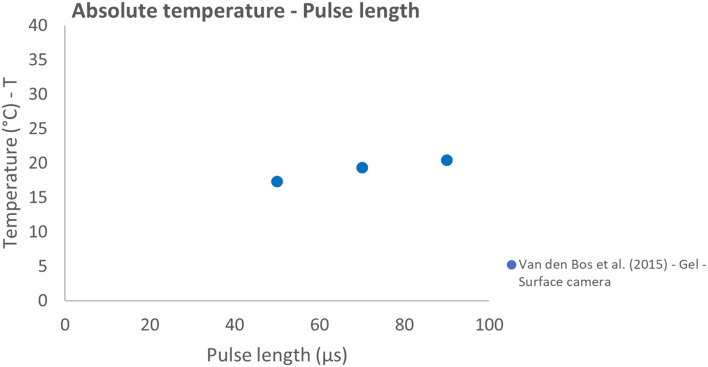
The effect of variation in inter-electrode distance, pulse interval, and pulse length were all studied in 1 report.31 A strong linear correlation was observed between the IED and absolute temperature (ρ = 0.94; P = .005) (Figure 8). The relative temperature and pulse interval showed a strong negative linear correlation (ρ = −0.84; P = .038) (Figure 9). While a weak correlation (ρ = −0.36, P = .478) was observed between the absolute temperature and increased pulse interval. With increasing pulse interval from 30 to 90 µs, the absolute temperature practically remained constant, and the relative temperature slightly decreased. Higher temperatures were measured with increasing pulse length but did not follow a linear model (Figure 10).
Figure 8.
Effect of variation in inter-electrode distance on the absolute temperature.
Figure 9.
Effect of variation in pulse interval on the absolute (T) and relative (ΔT) temperature.
Figure 10.
Effect of variation in pulse length on the absolute temperature.
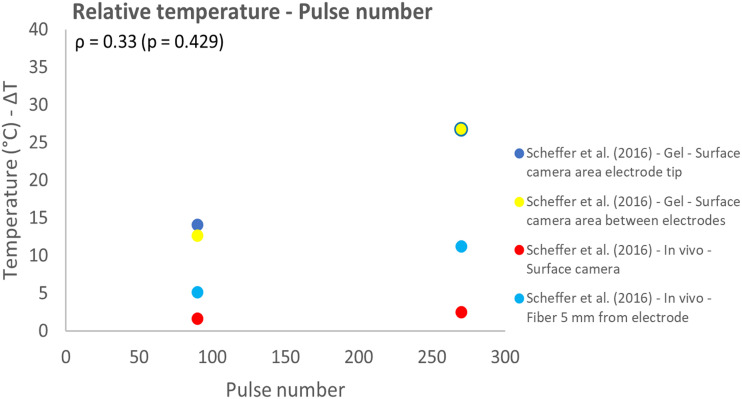
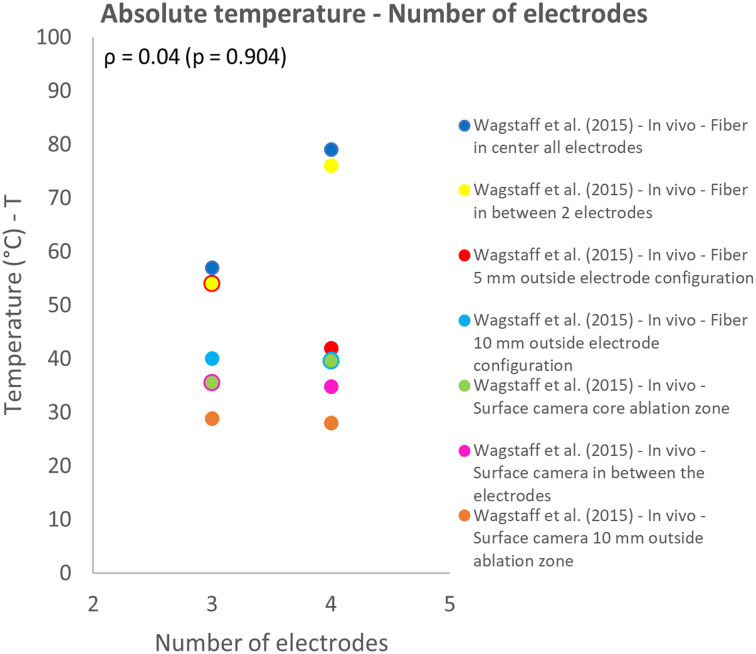
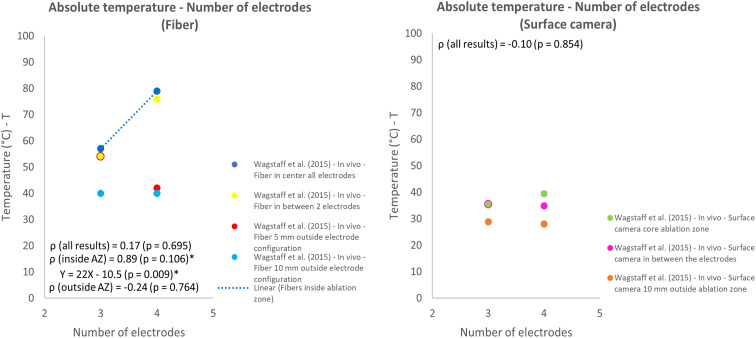
The influence of the number of electrodes and pulse number on thermal effects both were investigated in 1 study.12,30 The measurement technique, location and investigated model (in vivo animal or gel) influenced the measured temperature. This effect was especially observed for the pulse number and number of electrodes used for IRE. The temperature increased for increasing pulse numbers and higher relative temperatures were measured in the gel phantom in comparison to in vivo results, while the same IRE protocol was used (Figure 11). Overall, the effect of pulse number on the relative temperature was represented by a weak correlation (ρ = 0.33; P = .429). An increase in absolute temperature was observed for most protocols when the number of electrodes was increased from 3 to 4 (Figures 12 and 13). Lower temperatures were measured when a surface camera was used and when the temperature outside the ablation zone (AZ) was measured by fiber-optic probes or a surface camera (Figure 13). Temperature results that related to fiber optic probes located in between the electrodes were strongly linearly correlated (ρ = 0.89; P = .106) with the number of electrodes.
Figure 11.
Effect of variation in pulse number on the relative temperature.
Figure 12.
Effect of variation in number of electrodes on the absolute temperature.
Figure 13.
Effect of variation in number of electrodes on the absolute temperature, subdivided into temperatures measured by a fiber-optic probe and surface camera.
The active needle length28,31 and pulse protocol29,31 showed no or a weak correlation with temperature effects and are presented in Supplementary S6. A decrease in temperature was observed when 90 pulses were delivered in trains of 30 pulses (10-30-30-30) instead of a continuous pulse protocol (10-90). The relative temperature slightly increased when the 120 pulses were delivered in trains of 20 pulses (20-20-20-20-20-20) in comparison to a 30-30-30-30 pulse protocol. Variation in the pulse frequency was not investigated in the studies that reported temperature effects.
The threshold for thermal damage (>50 °C) was exceeded by the electroporation parameters voltage (≥2000 V, Figure 7), number of electrodes (3 and 4 electrodes, fiber, in between or within 5 mm of the ablation zone, Figure 12) and ANL (10 and 15 mm, Supplementary S6). The highest temperatures were reached for the 4-electrode configuration, with reported peak temperatures around 80 °C, measured by a fiber-optic probe in the core of the ablation zone.
Discussion
This work provided an overview of the literature on the influence of electroporation parameters on the ablation zone size and the occurrence of thermal effects. Only the applied voltage appeared to be a significant linear predictor and was most strongly associated with the ablation zone size: length, surface, and volume. The pulse number was moderately but non-linearly correlated with the ablation zone length. An increase in temperature was seen for an increased voltage, inter-electrode distance, pulse length, number of electrodes, pulse number, and active needle length. On the other hand, the temperature decreased when the pulse interval in between pulse trains increased and when the total number of pulses was sequentially delivered instead of continuously. The likelihood of thermal damage is the greatest for high values of the electroporation parameters voltage (≥2000 V), active needle length, and number of electrodes because these induce an increase in electric current and energy release in tissue.
The applied voltage has the strongest correlation with the ablation zone size of all in this review investigated electroporation parameters. An external applied electric field of approximately 680 V/cm is required to change the cell's transmembrane voltage to 1 V, induce rearrangement of the lipid bilayer, and initiate permanent permeabilization of the cell.5,42 When the applied voltage is too low, a dumbbell shaped ablation zone will be formed since the electric field strength is weaker in the center between the electrodes than directly near the electrodes and is not high enough to irreversibly ablate the tissue.14,36 Electroporation parameter settings are complementary to each other. A decrease in applied voltage can be compensated by application of higher pulse numbers or an increased pulse length to electroporate the same cell fraction.43 Thus, adding more pulses or an increase in pulse length will create a larger ablation zone when the applied voltage is unchanged.32,43 The application of multiple pulses shows a cumulative effect and appears to be more effective (results in the lowest percentage of living cells) for a complete ablation in comparison to less pulses delivering the same amount of energy.44,45 Reducing the pulse frequency and using an approach of cycled pulse delivery in multiple small pulse trains with a pause in between the trains, may enlarge the ablated area.26,39,46 The pore exposure time will increase by this approach, leading to a longer lasting imbalance in ions and cell death.46 So, cells respond ingeniously to the application of an external electric field and the application of electric pulses.
The IRE mechanism describes why the applied voltage primarily determines the size of the ablation zone.47 It is important to keep the voltage as high as possible during IRE treatment. However, a balance between ablation zone size and thermal effects should be found since the energy delivery into the surrounding tissue (joule heating) will increase when the applied voltage is increased, resulting in thermal effects.17,48
A temperature increase will occur wherever a current flows that encounters resistance from the surrounding tissue. Therefore, temperature effects are inseparable from irreversible electroporation. The amount of energy required to induce an increase in temperature varies per material and organ. Every tissue has its own specific heat, depending on the tissue's thermal conductivity. The specific heat corresponds to the amount of joule it takes to raise the temperature of 1 kg of that specific material or organ by 1 °C.49 The specific heat of human kidney, human liver, potato, and polyacrylamide gel are 3890, 3600, 3480, and 1750 J/kg °C, respectively.50–52 Higher temperatures are reached when the same amount of energy is delivered in polyacrylamide gels in comparison to kidney tissue. Heat dissipation by blood perfusion also determines the maximum temperature reached during electroporation.17,18 Therefore, the temperature results presented in this review should be interpreted with knowledge of the material or organ of interest, location where the temperature is measured with reference to the core of the ablation zone and the electroporation protocol used.
Mild-hyperthermic temperatures (40-50 °C) and temperatures ≥50 °C are reached in 30% and 5% of the ablation zone, based on a systematic review of mathematical models for thermal effects during IRE treatment.53 No other systematic reviews or meta-analyses were published on the relation between IRE parameters and the ablation zone size and occurrence of thermal effects, besides this study of Agnass et al. Unless temperatures ≥50 °C occur in a relatively small region, care should be taken not to damage critical structures. Especially for an increase in the applied voltage, number of electrodes and amount of delivered pulses, since these parameters are significantly correlated with higher temperatures and potential thermal damage, as investigated in in vivo porcine liver and pancreatic tissue.54 This finding is in line with the results presented in this review. Evidently, awareness and control of the thermal effect are crucial.
Different mathematical models or equations are currently available to predict thermal damage based on the temperatures reached in and around the ablation zone. For example, the Arrhenius equation,11,55,56 a 2-state model,57 3-state model,58 temperature-dependent time delay model,59 and cumulative equivalent minutes model.60,61
The strength of the present work is that the impact of individual electroporation parameters was systematically investigated and pooled, providing an overview to what extent each parameter has been researched and contributes to electroporation effects. The trend of the applied voltage as the main parameter to achieve the desired ablation zone size seen in individual studies was thus confirmed.
This review also has limitations. First, the number of included studies was limited due to the strict eligibility criteria. Incomplete reporting of the electroporation parameter settings used per treatment and the achieved ablation zone size and/or thermal effects per investigated combination of parameter settings was one of the main reasons for exclusion. No studies performing IRE in human patients were eligible for inclusion. Almost all included animal studies comprised IRE of healthy organs, which differs from clinical cases in vessel encasement by tumor tissue, tissue heterogeneity of tumor tissue, and therefore electrical characteristics and electric field distribution.17 The ablation zone size and thermal effects were reported per individual electroporation parameter without a distinction between the investigated organs nor whether an animal or phantom model (gel/potato) was used, since a relatively small number of studies could be included. Nonetheless, the found effects of adjustments in electroporation parameters show general trends. For translation of the results to IRE in human patients, it is important to be aware of the additional effect of tissue characteristics, blood perfusion and potential heat diffusion into surrounding tissue.7
Assessment of the methodological quality of papers is an important aspect of systematic reviews. Another limitation of this study is that previously validated quality scores for systematic reviews did not fully apply to the types of studies included. Therefore, a specific score was developed for use in this study. A few aspects of the quality assessment remained somewhat difficult, in particular the cutoff for an adequate sample size (per investigated set of electroporation parameter combinations). Ideally, this should be based on a sample size calculation. Many included studies, however, were explorative in nature and had no formal sample size calculation. A number of 10 measurements per investigated set of electroporation parameters was considered as adequate for most cases. As it was the ultimate goal of this research to identify IRE settings that impact the ablation size zone and risk for thermal injury in clinical practice, human patients were rated with one extra point on the aspect study model. The type of model used was only one aspect of the quality score and affects 11% of the total score. Collectively, other methodological items have more impact on the study's total score. The formulation of general recommendations for optimal control and personalization of IRE treatment is currently hindered by methodological shortcomings of available literature. Consequently, there is a need for high-quality research with a sufficient sample size, reporting all used electroporation parameter values and including a replicable description of how the ablation zone size and temperature effects are measured.
It is valuable to gain more insight into clinical IRE in human patients on a large scale. This can be realized by systematically and precisely reporting the electroporator (eg, applied voltage) and electrode settings (eg, number of electrodes and configuration) per individual patient in a database in combination with ablation outcomes, the developed ablation zone size, and thermal effects. Relevant patient specific electroporation characteristics should be reported as well, such as the location of the electrodes with reference to nearby critical structures, (average) heart rate and the amperage reached during pulse delivery. Based on the amperage, an indication of the energy release in tissue and occurrence of thermal effects during treatment can be made. All this information provides valuable insights to develop a model that may guide and personalize clinical electroporation procedures.
Conclusion
Firm conclusions are limited since studies that investigated and precisely reported the influence of electroporation parameters on the ablation zone size and thermal effects were scarce and their overall quality was low to moderate. Only the applied voltage appeared to be a significant linear predictor and most strongly associated with the ablation zone size: length, surface, and volume. The pulse number was moderately but non-linearly correlated with the ablation zone length. Thermal effects are inseparable from IRE and were more likely to occur for higher voltages (≥2000 V), higher number of electrodes, and increased active needle length. High-quality studies are needed to improve the predictability of the combined effects of variation in parameter combinations and optimize IRE treatment protocols.
Supplemental Material
Supplemental material, sj-pdf-1-tct-10.1177_15330338221125003 for The Influence of Irreversible Electroporation Parameters on the Size of the Ablation Zone and Thermal Effects: A Systematic Review by Annemiek M Hogenes, MSc, Christiaan G Overduin, PhD, Cornelis H Slump, PhD, Cornelis J H M van Laarhoven, MD, PhD, Jurgen J Fütterer, MD, PhD, Richard P G ten Broek, MD, PhD, and Martijn W J Stommel, MD, PhD in Technology in Cancer Research & Treatment
Acknowledgments
Special thanks to OnYing Chan for her support in the development of the search strategy and Jacqueline Evans for a native English proofread of the manuscript.
Abbreviations
- AZ
ablation zone
- ANL
active needle length
- ASME
American Society of Mechanical Engineers
- IED
inter-electrode distance
- IRE
irreversible electroporation
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RE
reversible electroporation
- T
temperature.
Author Contributions: A.M.H.: Conceptualization, Methodology, Investigation, Formal Analysis, Visualization, Writing – Original Draft, Project Administration C.G.O.: Methodology, Investigation, Visualization, Writing – Review & Editing C.H.S.: Methodology, Visualization, Writing – Review & Editing, Supervision C.J.H.M.L.: Conceptualization, Writing – Review & Editing J.J.F.: Conceptualization, Writing – Review & Editing, Supervision R.P.G.B.: Methodology, Formal Analysis, Visualization, Writing – Review & Editing M.W.J.S.: Conceptualization, Methodology, Writing – Review & Editing, Supervision. All authors approved the version to be published and agreed to be accountable for all aspects of the work. The data that support the findings of this study are available from the corresponding author upon reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Annemiek M Hogenes https://orcid.org/0000-0002-4337-2803
Supplemental Material: Supplementary material for this article is available online.
References
- 1.Bower M, Sherwood L, Li Y, Martin R. Irreversible electroporation of the pancreas: definitive local therapy without systemic effects. J Surg Oncol. 2011;104(1):22‐28. [DOI] [PubMed] [Google Scholar]
- 2.Vogel J, Van Veldhuisen E, Agnass Pet al. Time-dependent impact of irreversible electroporation on pancreas, liver, blood vessels and nerves: a systematic review of experimental studies. PLoS One. 2016;11(11):e0166987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davalos RV, Mir L, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33(2):223‐231. [DOI] [PubMed] [Google Scholar]
- 4.Miklavčič D, Šemrov D, Mekid H, Mir LM. A validated model of in vivo electric field distribution in tissues for electrochemotherapy and for DNA electrotransfer for gene therapy. Biochim Biophys Acta. 2000;1523(1):73‐83. [DOI] [PubMed] [Google Scholar]
- 5.Neu WK, Neu JC. Theory of electroporation. In: Efimov IR, Kroll MW, Tchou PJ, eds. Cardiac Bioelectric Therapy. Springer; 2009:133‐161. [Google Scholar]
- 6.Edd JF, Davalos RV. Mathematical modeling of irreversible electroporation for treatment planning. Technol Cancer Res Treat. 2007;6(4):275‐286. [DOI] [PubMed] [Google Scholar]
- 7.Garcia PA, Neal R, Davalos RV. Non-thermal irreversible electroporation for tissue ablation. In: Spugnini EP, Baldi A. eds. ,Electroporation in Laboratory and Clinical Investigations. Nova Science; 2010:63-84. [Google Scholar]
- 8.Daniels C, Rubinsky B. Electrical field and temperature model of nonthermal irreversible electroporation in heterogeneous tissues. J Biomech Eng. 2009;131(7):071006. [DOI] [PubMed] [Google Scholar]
- 9.Dunki-Jacobs E, Philips P, Martin Ii R. Evaluation of thermal injury to liver, pancreas and kidney during irreversible electroporation in an in vivo experimental model. Br J Surg. 2014;101(9):1113‐1121. [DOI] [PubMed] [Google Scholar]
- 10.Faroja M, Ahmed M, Appelbaum Let al. Irreversible electroporation ablation: is all the damage nonthermal? Radiology. 2013;266(2):462‐470. [DOI] [PubMed] [Google Scholar]
- 11.Garcia PA, Davalos RV, Miklavcic D. A numerical investigation of the electric and thermal cell kill distributions in electroporation-based therapies in tissue. PLoS One. 2014;9(8):e103083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagstaff PG, de Bruin DM, van den Bos Wet al. Irreversible electroporation of the porcine kidney: temperature development and distribution. Urol Oncol. 2015;33(4):168.e1‐168.e7. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Moser M, Zhang E, Zhang W, Zhang B. Optimization of electrode configuration and pulse strength in irreversible electroporation for large ablation volumes without thermal damage. J Eng Sci Med Diagn Ther. 2018;1(2):021002. [Google Scholar]
- 14.Hogenes AM, Slump CH, te Riet og Scholten GAet al. Effect of irreversible electroporation parameters and the presence of a metal stent on the electric field line pattern. Sci Rep. 2020;10(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moritz AR, Henriques F, Jr. Studies of thermal injury: II. The relative importance of time and surface temperature in the causation of cutaneous burns. Am J Pathol. 1947;23(5):695‐720. [PMC free article] [PubMed] [Google Scholar]
- 16.Diller KR. Modeling of bioheat transfer processes at high and low temperatures. In: Cho YI, ed. Advances in Heat Transfer. 22. Harcourt Brace Jovanovich; 1992:157‐357. [Google Scholar]
- 17.Davalos RV, Garcia PA, Edd JF. Thermal aspects of irreversible electroporation. In: Rubinsky B, ed. Irreversible Electroporation. Springer; 2010:123‐154. [Google Scholar]
- 18.Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol. 1948;1(2):93‐122. [DOI] [PubMed] [Google Scholar]
- 19.Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(210):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thomas J, Chandler Jet al. et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. John Wiley & Sons; 2019. [Google Scholar]
- 22.Romeo S, Zeni O, Sannino A, Lagorio S, Biffoni M, Scarfì MR. Genotoxicity of radiofrequency electromagnetic fields: protocol for a systematic review of in vitro studies. Environ Int. 2021;148:106386. [DOI] [PubMed] [Google Scholar]
- 23.Jadad AR, Moore RA, Carroll Det al. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
- 24.Wells GA, Shea B, O’Connell Det al. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. 2000.
- 25.Edelblute CM, Hornef J, Burcus NIet al. Controllable moderate heating enhances the therapeutic efficacy of irreversible electroporation for pancreatic cancer. Sci Rep. 2017;7(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appelbaum L, Ben-David E, Faroja M, Nissenbaum Y, Sosna J, Goldberg SN. Irreversible electroporation ablation: creation of large-volume ablation zones in in vivo porcine liver with four-electrode arrays. Radiology. 2014;270(2):416‐424. [DOI] [PubMed] [Google Scholar]
- 27.Ben-David E, Appelbaum L, Sosna J, Nissenbaum I, Goldberg SN. Characterization of irreversible electroporation ablation in in vivo porcine liver. Am J Roentgenol. 2012;198(1):W62‐WW8. [DOI] [PubMed] [Google Scholar]
- 28.Canvasser NE, Lay AH, Koseoglu Eet al. et al. Effect of differing parameters on irreversible electroporation in a porcine model. J Endourol. 2018;32(4):338‐343. [DOI] [PubMed] [Google Scholar]
- 29.Ruarus AH, Vroomen LG, Puijk RS, Scheffer HJ, Faes TJ, Meijerink MR. Conductivity rise during irreversible electroporation: true permeabilization or heat? Cardiovasc Intervent Radiol. 2018;41(8):1257‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheffer HJ, Vogel JA, Van Den Bos Wet al. The influence of a metal stent on the distribution of thermal energy during irreversible electroporation. PLoS One. 2016;11(2):e0148457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bos W VD, Scheffer HJ, Vogel JAet al. Thermal energy during irreversible electroporation and the influence of different ablation parameters. J Vasc Interv Radiol. 2016;27(3):433‐443. [DOI] [PubMed] [Google Scholar]
- 32.Bhonsle S, Bonakdar M, Neal RE, IIet al. Characterization of irreversible electroporation ablation with a validated perfused organ model. J Vasc Interv Radiol. 2016;27(12):1913‐1922.e2. [DOI] [PubMed] [Google Scholar]
- 33.Lv Y, Yao C, Rubinsky B. A conceivable mechanism responsible for the synergy of high and low voltage irreversible electroporation pulses. Ann Biomed Eng. 2019;47(7):1552‐1563. [DOI] [PubMed] [Google Scholar]
- 34.Neal RE, Garcia PA, Kavnoudias Het al. In vivo irreversible electroporation kidney ablation: experimentally correlated numerical models. IEEE Trans Biomed Eng. 2014;62(2):561‐569. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Guo Y, Ragin ABet al. MR Imaging to assess immediate response to irreversible electroporation for targeted ablation of liver tissues: preclinical feasibility studies in a rodent model. Radiology. 2010;256(2):424‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao C, Dong S, Zhao Yet al. et al. Bipolar microsecond pulses and insulated needle electrodes for reducing muscle contractions during irreversible electroporation. IEEE Trans Biomed Eng. 2017;64(12):2924‐2937. [DOI] [PubMed] [Google Scholar]
- 37.Yao C, Lv Y, Dong S, Zhao Y, Liu H. Irreversible electroporation ablation area enhanced by synergistic high-and low-voltage pulses. PLoS One. 2017;12(3):e0173181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao C, Lv Y, Zhao Y, Dong S, Liu H, Ma J. Synergistic combinations of short high-voltage pulses and long low-voltage pulses enhance irreversible electroporation efficacy. Sci Rep. 2017;7(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B, Moser MA, Zhang EM, Xiang J, Zhang W. An in vitro experimental study of the pulse delivery method in irreversible electroporation. J Eng Sci Med Diagn Ther. 2018;1(1), 014501. [Google Scholar]
- 40.Lee YJ, Lu DS, Osuagwu F, Lassman C. Irreversible electroporation in porcine liver: acute computed tomography appearance of ablation zone with histopathologic correlation. J Comput Assist Tomogr. 2013;37(2):154‐158. [DOI] [PubMed] [Google Scholar]
- 41.Berkenbrock JA, Pintarelli GB, Júnior A, Suzuki DOH. Verification of electroporation models using the potato tuber as in vitro simulation. J Med Biol Eng. 2019;39(2):224‐229. [Google Scholar]
- 42.Weaver JC, Chizmadzhev YA. Theory of electroporation: a review. Bioelectrochem Bioenerg. 1996;41(2):135‐160. [Google Scholar]
- 43.Pucihar G, Krmelj J, Reberšek M, Napotnik TB, Miklavčič D. Equivalent pulse parameters for electroporation. IEEE Trans Biomed Eng. 2011;58(11):3279‐3288. [DOI] [PubMed] [Google Scholar]
- 44.Miller L, Leor J, Rubinsky B. Cancer cells ablation with irreversible electroporation. Technol Cancer Res Treat. 2005;4(6):699‐705. [DOI] [PubMed] [Google Scholar]
- 45.Rubinsky J, Onik G, Mikus P, Rubinsky B. Optimal parameters for the destruction of prostate cancer using irreversible electroporation. J Urol. 2008;180(6):2668‐2674. [DOI] [PubMed] [Google Scholar]
- 46.Jiang C, Shao Q, Bischof J. Pulse timing during irreversible electroporation achieves enhanced destruction in a hindlimb model of cancer. Ann Biomed Eng. 2015;43(4):887‐895. [DOI] [PubMed] [Google Scholar]
- 47.Rols M-P, Teissie J. Electropermeabilization of mammalian cells. Quantitative analysis of the phenomenon. Biophys J. 1990;58(5):1089‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ivorra A. Tissue electroporation as a bioelectric phenomenon: basic concepts. Irreversible electroporation: Springer; 2010. p. 23–61.
- 49.Giancoli DC. Heat and the first law of thermodynamics. In: Giancoli D, ed. Physics for Scientists and Engineers With Modern Physics. 4th ed. Pearson Education International; 2009:499‐500. [Google Scholar]
- 50.Cooper TE, Trezek GJ. A probe technique for determining the thermal conductivity of tissue. J Heat Transfer. 1972;94(2):133‐140. [Google Scholar]
- 51.Fellows PJ. Part I Basic Principles: Properties of Foods and Processing Theory. Food Processing Technology: Principles and Practice. 2nd ed. Woodhead Publishing Limited and CRC Press; 2000. [Google Scholar]
- 52.Hirata Y, Kato Y, Andoh N, Fujiwara N, Ito R. Measurements of thermophysical properties of polyacrylamide gel used for electrophoresis. J Chem Eng Jpn. 1993;26(2):143‐147. [Google Scholar]
- 53.Agnass P, van Veldhuisen E, van Gemert MJet al. Mathematical modeling of the thermal effects of irreversible electroporation for in vitro, in vivo, and clinical use: a systematic review. Int J Hyperthermia. 2020;37(1):486‐505. [DOI] [PubMed] [Google Scholar]
- 54.Agnass P, van Veldhuisen E, Vogel JAet al. Thermodynamic profiling during irreversible electroporation in porcine liver and pancreas: a case study series. J Clin Transl Res. 2020;5(3):109. [PMC free article] [PubMed] [Google Scholar]
- 55.Arrhenius S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Z Phys Chem. 1889;4(1):226‐248. [Google Scholar]
- 56.Henriques FC. Studies of thermal injury; the predictability and the significance of thermally induced rate processes leading to irreversible epidermal injury. Arch Pathol. 1947;43(5):489‐502. [PubMed] [Google Scholar]
- 57.Feng Y, Oden JT, Rylander MN. A two-state cell damage model under hyperthermic conditions: theory and in vitro experiments. J Biomech Eng. 2008;130(4):041016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Neill DP, Peng T, Stiegler Pet al. et al. A three-state mathematical model of hyperthermic cell death. Ann Biomed Eng. 2011;39(1):570‐579. [DOI] [PubMed] [Google Scholar]
- 59.Pearce JA. Improving accuracy in Arrhenius models of cell death: adding a temperature-dependent time delay. J Biomech Eng. 2015;137(12):121006. [DOI] [PubMed] [Google Scholar]
- 60.Pearce JA. Comparative analysis of mathematical models of cell death and thermal damage processes. Int J Hyperthermia. 2013;29(4):262‐280. [DOI] [PubMed] [Google Scholar]
- 61.Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10(6):787‐800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tct-10.1177_15330338221125003 for The Influence of Irreversible Electroporation Parameters on the Size of the Ablation Zone and Thermal Effects: A Systematic Review by Annemiek M Hogenes, MSc, Christiaan G Overduin, PhD, Cornelis H Slump, PhD, Cornelis J H M van Laarhoven, MD, PhD, Jurgen J Fütterer, MD, PhD, Richard P G ten Broek, MD, PhD, and Martijn W J Stommel, MD, PhD in Technology in Cancer Research & Treatment