Abstract
Glioblastoma (GBM) is the most common malignant adult brain and has a poor prognosis. Routine post-treatment MRI evaluations are required to assess treatment response and disease progression. We present a case of an 83-year-old female who underwent MRI assessment of post-treatment GBM after intravenous iron replacement therapy, ferumoxytol. The brain MRI revealed unintended alteration of MRI signal characteristics from the iron containing agent which confounded diagnostic interpretation and subsequently, the treatment planning. Ferumoxytol injection prior to contrast enhanced MRI must be screened in post-treatment GBM patients to accurately evaluate tumor activity.
Keywords: : adverse events, brain tumor, ferumoxytol, GBM, imaging
Practice points.
Current standard treatment for glioblastoma (GBM) is surgical resection followed by radiation and chemotherapy.
MRI is the study of choice for post-treatment disease monitoring and treatment efficacy evaluation in addition to the initial detection of GBM.
Follow-up MRI evaluation of tumor activity based on routine contrast enhanced imaging protocol can be complicated with treatment related reactions.
Ferumoxytol is an ultrasmall superparamagnetic iron oxide approved for the treatment of iron deficiency anemia.
Ferumoxtyol injection prior to contrast enhanced MRI evaluation can alter enhancement and interfere with diagnostic interpretation of post-treatment GBM.
Providers should screen for patients’ previous ferumoxytol injection prior to MRI evaluations.
Physicians and radiologists must recognize the potential effects of ferumoxytol in MRI exams.
Glioblastoma (GBM) is the most common, deadliest form of malignant tumor in the brain, and even after surgical resection and chemoradiation treatment, the vast majority GBMs recur at some point [1]. MRI evaluation has played a key role in post-treatment GBM to assess efficacy of treatment regimen, determine effective therapeutic dose, monitor disease progression versus therapeutic response and establish further treatment strategy. However, follow-up MRI evaluation of tumor activity based on routine contrast enhanced imaging protocol can be complicated with treatment related reactions (i.e., pseudoprogression and pseudoresponse) [2,3]. Parametric map of dynamic susceptibility contrast perfusion MRI enables us to assess vascularity status distinguished from disrupted blood–brain barrier within enhancing lesions, which offers higher confidence in diagnosing active tumor recurrence [4–6]. This imaging modality relies on paramagnetic susceptibility effect of intravascular gadolinium contrast agent using T2*-weighed MRI sequence.
We present a case of post-treatment GBM assessment with MRI after iron replacement therapy that exhibited T1 shortening and intravascular paramagnetic effects due to this treatment and eventually, confounded MRI interpretation and treatment decision.
Case report
An 83-year-old woman presented to the emergency department with 3-week history of intermittent right sided headaches, left-sided motor weakness, and memory disturbances, necessitating brain CT and MRI exams, which revealed a heterogeneously enhancing intra-axial mass involving the right posterior temporal and occipital areas, measured 3.5 cm in the longest axial diameter, raising a concern of primary CNS malignancy. After stereotaxic biopsy, histopathologic examination confirmed GBM WHO grade IV, MGMT unmethylated and IDH1/2 wild-type. Subsequently, partial tumor resection greater than 50% through a right temporal craniotomy was performed. Post surgical brain MRI demonstrated neither residual enhancing mass nor immediate postsurgical complication. Postoperatively, the patient started fractionated radiation therapy but was not recommended for concurrent chemotherapy with temozolomide (TMZ, Temodar®) given her age and MGMT unmethylated status of the tumor. Follow-up MRI after the fractionated stereotactic radiosurgery revealed absent residual enhancing tumor fraction around the surgical bed.
About 6 months after patient’s initial treatment, she presented with recurrent episode of headaches for 2 weeks, and subsequent brain MRI revealed increased area of contrast enhancing lesions and greater than 25% extent of surrounding T2/fluid-attenuated inversion recovery hyperintensity, suspicious for progressive disease by response assessment in neuro-oncology criteria. Consequently, the patient started to receive adjuvant anti-angiogenic treatment with bevacizumab (Avastin®).
To assess treatment response, second follow-up MRI study (9 months post surgery) was performed at 1.5T scanner (GE Signa HDxt). Study protocol included: axial T1/T2-weighted imaging (WI), fluid-attenuated inversion recovery, susceptibility weighted imaging (TR/TE = 78/47 msec, flip angle = 15°, matrix = 448 × 320, slice thickness = 2 mm, field-of-view = 200 × 200 mm, in-plane voxel size = 0.446 × 0.625 mm, number of slices = 96), diffusion-weighted imaging, sagittal T1 and dynamic susceptibility contrast perfusion-weighted imaging (DSC-PWI) with T2* GRE EPI sequence (TR/TE = 1100/40 msec, flip angle = 30°, matrix = 128 × 128, number of slices = 27) followed by postcontrast sagittal 3D magnetization-prepared rapid grandient-echo (MPRAGE) T1 fat-sat with axial and coronal reformats. Perfusion parametric mapping was provided using a total volume of 0.15 mmol/kg of Gadoteridol (ProHance®, Bracco Diagnostics Inc. NJ, USA) which was administrated intravenously using a power injector with an injection rate of 4 ml per s followed by 20 ml saline flush. Parametric mapping of DSC-PWI included relative cerebral blood volume (rCBV), relative cerebral blood flow and mean transit time, which were computed using IB Neuro™ software (ImagingBiometrics, WI, USA).
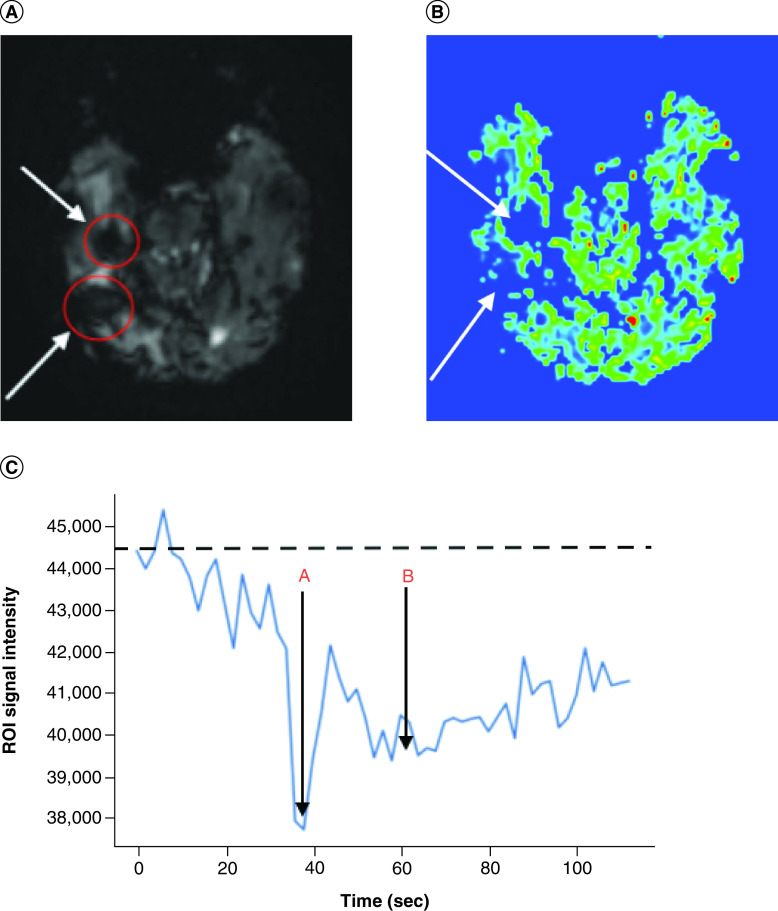
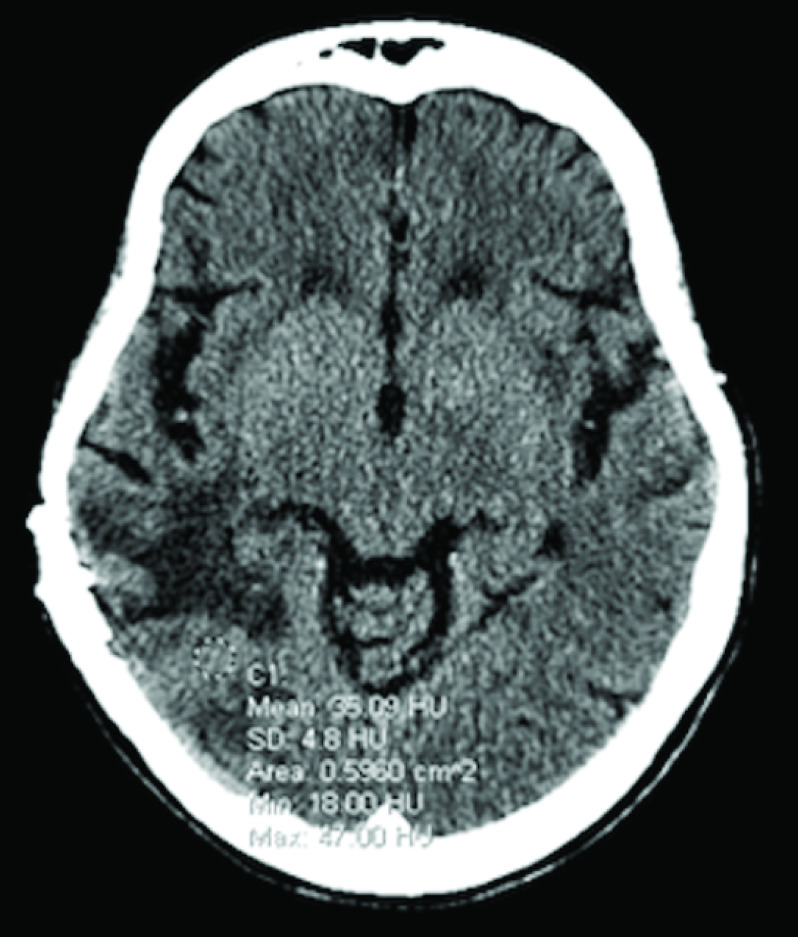
Without having access to patient’s medication history prior to the MRI exam, a neuroradiologist observed unusual appearance of T1 hyperintensity in the right temporal lobe on noncontrast T1-weighted imaging (T1WI) (Figure 1A), and extensive susceptibility signal blooming throughout the entire brain, highlighted in the residual tumor areas (Figure 1C), which were interpreted as a subacute stage blood product within the tumor. T1 signal intensity difference between the pre and postcontrast images within the right temporal tumor was negligible (Figure 1B). Moreover, underlying effect of the strong intravascular para magnetism before intravenous (iv.) administration of gadolinium-based contrast agent (GBCA) precluded hemodynamic interpretation of the DSC-PWI study. Baseline scan of DSC-PWI exhibited preloaded paramagnetic susceptibility effect within the lesions before the first pass bolus arrival of iv. contrast agent (Figure 2A) resulted in rCBV computation error (Figure 2B). Time-signal intensity plot of manually drawn tumor region-of-interest (ROI) on motion corrected DSC-PWI series displayed attenuated T2* effect of the intravascular contrast at the first pass bolus phase compared with the capillary pooling phase (Figure 2C). In fact, the patient underwent treatment for iron deficiency anemia and received normal dose of IV ferumoxytol (Feraheme®, AMAG Pharmaceuticals, MA) treatment within 48 h prior to MRI exam. Later, the patient’s medication history was disclosed to the interpreting neuroradiologist, who recommended to perform repeated MRI exam after withholding iv. ferumoxytol treatment. Noncontrast brain CT obtained following day demonstrated mildly increased attenuation value (35 Hounsfield unit) within the tumor core, which was inconclusive to exclude the possibility of hemorrhage (Figure 3). However, neither continuation of bevacizumab treatment nor additional cytoreduction surgery was considered as a suitable next treatment option given concern of intratumoral hemorrhage as well as indeterminate MRI interpretation for true disease progression. Palliative care was consulted, and the patient was discharged to home with hospice. The patient succumbed to the disease 15 days after the last MRI examination.
Figure 1. . Brain imaging.
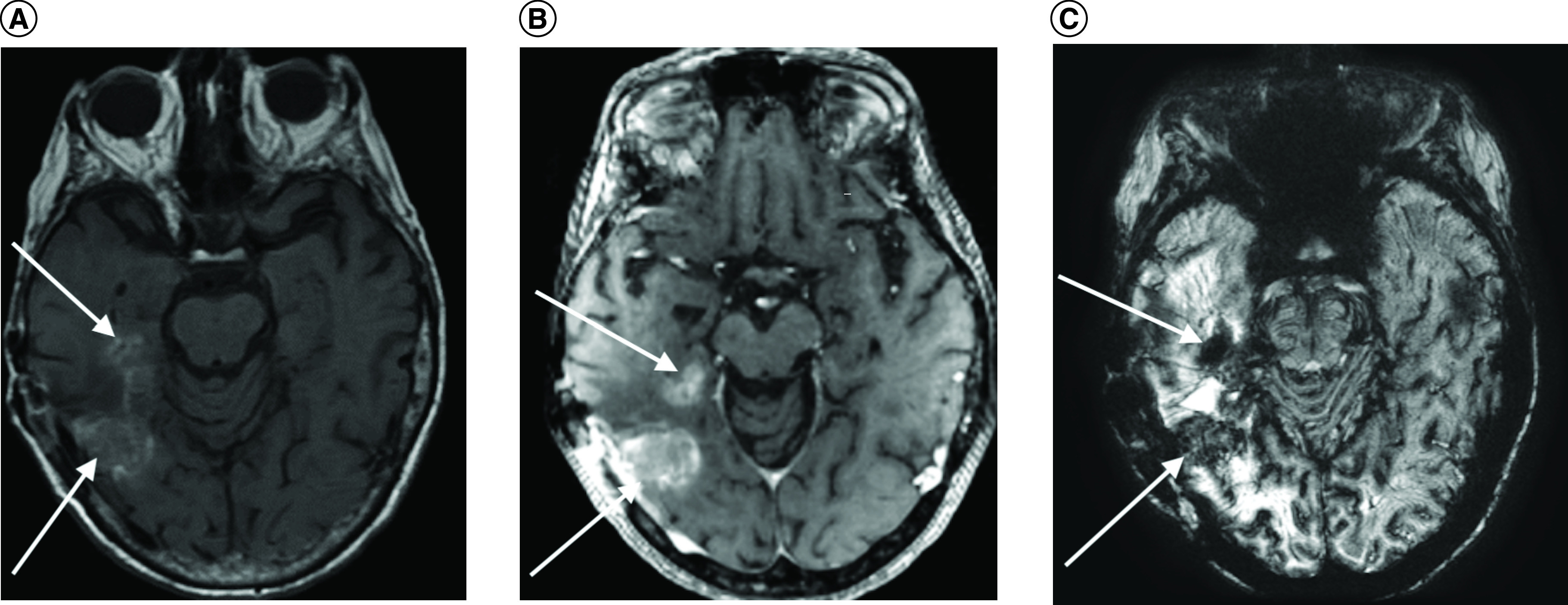
(A) Noncontrast axial T1 hyperintensity in the right hemisphere (arrows), (B) postcontrast T1 intensity (arrows), and (C) susceptibility weighted imaging (SWI) exhibiting iron particle uptakes in the active tumor areas (arrows) and extensive background paramagnetic effect throughout the entire brain.
Figure 2. . Brain images and ROI curve.
(A) Dynamic susceptibility contrast (DSC) perfusion MR image of right temporal tumor (arrows) with ROI mask (red circle) at the baseline. (B) Relative cerebral blood volume (rCBV) map of tumor region (arrows). (C) ROI signal-intensity curve across the acquisition time (A: intravascular peak and B: capillary pooling phase).
ROI: Region of interest.
Figure 3. . Noncontrast brain CT exhibiting mildly increased attenuation value (35 Hounsfield unit) at the area of tumor core.
Discussion
GBM has a poor prognosis due to its high recurrence rate with a median survival of 15 months [7]. Current standard treatment is surgical resection followed by radiation and chemotherapy with TMZ. Multiple factors including age, performance status and tumor type are considered in individual treatment management [8].
MRI is the study of choice for post-treatment disease monitoring and treatment efficacy evaluation in addition to the initial detection of GBM. Specifically, post-treatment MRI evaluation is important in distinguishing treatment induced inflammation (pseudoprogression) and true tumor progression. However, imaging features between tumor progression and pseudoprogression significantly overlap often leading to diagnostic dilemma and difficulty in determining therapeutic strategies, which can result in unnecessary delays of otherwise effective treatment for patients with actual true tumor progression [2,9]. One of the practically useful diagnostic tools for distinguishing tumor growth from treatment response is contrast enhanced MRI and rCBV map derived from DSC-PWI [4,5,10].
Ferumoxytol is an iv. agent for treatment of iron deficiency anemia [11]. It consists of ultrasmall superparamagnetic iron oxide particles covered with a carbohydrate shell that provides a long intravascular half-life of approximately 14–15 h. The superparamagnetic properties and long half-life of the ferumoxtyol make it an ideal contrast agent, especially for perfusion weighted MRI studies [12,13]. Studies have shown that ferumoxytol produces T1 shortening effect enhances signal on T1WI and strong susceptibility effect dephases signal on T2*-weighted DSC-PWI MRI [13,14]. However, the administration of ferumoxytol prior to contrast enhanced MRI studies may alter pattern of intracranial contrast enhancement from the iv. GBCA administration. This alteration of MRI studies may last up to 3 months following the last dose and vascular MRI alterations may be evident for 1–2 days following injection [15].
In our case, T1 shortening and extensive susceptibility effects from iv. ferumoxytol administration hampered diagnostic utility of contrast enhanced MRI with DSC-PWI. The enhancing lesions were interpreted as hematomas, precluding the option for anti-angiogenic treatment, bevacizumab, which is used as a second-line treatment for recurrent GBM [1]. Furthermore, due to the confounding interpretation and increased risk of intracranial hemorrhage, our patient was discouraged from continuing the bevacizumab treatment. However, patient’s treatment with iron replacement therapy prior to MRI studies raises suspicion for iron particle uptake as a possible contributing factor to the high signal intensity in the tumor bed on T1WI. Additionally, the superparamagnetic effect of ferumoxytol may have masked the expected GBCA related enhancement, and distorted hemodynamic signal of the DSC-PWI that could have reflected tumor activity, leading to devoid rCBV map.
Although ferumoxytol injection can cause confounding effects on contrast enhanced MRI as we demonstrated in this case report, this treatment alone may be used as an inflammation imaging agent. The ultrasmall superparamagnetic iron oxide nanoparticles in ferumoxytol are taken up by the macrophages in the lesions with disrupted blood–brain barrier which allows the detection of brain tissue inflammation [16]. Furthermore, studies have shown that rCBV with the use of ferumoxytol is a good indicator of tumor vascularity that could allow improved distinction between tumor progression and treatment-related inflammation, pseudoprogression [17,18]. Specifically, one study demonstrated that the use of ferumoxytol and gadolinium contrast mismatch ratios on T1WI may improve diagnostic capability of distinguishing pseudoprogression from disease recurrence in IDH-1 wild-type GBM. Patients with the development of pseudoprogression showed reduced mismatch ratio compared with disease recurrence [19]. As such, ferumoxytol has the potential to be used as a biomarker to distinguish tumor activity from treatment related inflammation, which may have important clinical implications.
In summary, we report a case with confounded interpretation of post-treatment follow-up MRI in patient with GBM due to inadvertent administration of IV ferumoxytol. Herein, we propose a new guideline for pre-MRI screening to include querying the patient regarding recent ferumoxytol treatment. Furthermore, radiologists and physicians caring patients with ferumoxytol medication must be aware of its anticipated effects to the contrast enhanced MRI and DSC-PWI.
Conclusion
Ferumoxytol injection prior to contrast enhanced MRI studies can obscure enhancement and interfere with diagnostic interpretation. Hence, screening for patients’ previous ferumoxytol injection is recommended prior to MRI evaluations.
Footnotes
Author contributions
D Park and J Kim drafted and edited the manuscript. M Lobbous, L Nabors and J Markert contributed to the interpretation of data and editing of the manuscript. All authors have approved the final manuscript.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
This is a case report with no experimental intervention performed as part of the study. Our retrospective review of de-identified MRI data and patient's medical record without elements of protected health information was carried out in accordance with the Declaration of Helsinki. The University of Alabama at Birmingham does not require IRB approval for this study.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Davis ME. Glioblastoma: overview of disease and treatment. Clin. J. Oncol. Nurs. 20(Suppl. 5), S2–S8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke JL, Chang S. Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr. Neurol. Neurosci. Rep. 9(3), 241–246 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Hygino Da Cruz LC Jr, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. Am. J. Neuroradiol. 32(11), 1978–1985 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reviews pseudoprogression and pseudoresponse.
- 4.Cha J, Kim ST, Kim HJ et al. Differentiation of tumor progression from pseudoprogression in patients with posttreatment glioblastoma using multiparametric histogram analysis. Am. J. Neuroradiol. 35(7), 1309–1317 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Discusses the usefulness of multiparametric histogram analysis of post-treatment glioblastoma in predicting true tumor progression.
- 5.Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ. Multiparametric MRI as a potential surrogate endpoint for decision-making in early treatment response following concurrent chemoradiotherapy in patients with newly diagnosed glioblastoma: a systematic review and meta-analysis. Eur. Radiol. 28(6), 2628–2638 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Seeger A, Braun C, Skardelly M et al. Comparison of three different MR perfusion techniques and MR spectroscopy for multiparametric assessment in distinguishing recurrent high-grade gliomas from stable disease. Acad. Radiol. 20(12), 1557–1565 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Thakkar JP, Dolecek TA, Horbinski C et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomarkers Prev. 23(10), 1985–1996 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanderi T, Gupta V. Glioblastoma multiforme. In: StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC, FL, USA: (2021). [PubMed] [Google Scholar]
- 9.Strauss SB, Meng A, Ebani EJ, Chiang GC. Imaging glioblastoma posttreatment: progression, pseudoprogression, pseudoresponse, radiation necrosis. Neuroimaging Clin. N. Am. 31(1), 103–120 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Hu LS, Baxter LC, Smith KA et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. Am. J. Neuroradiol. 30(3), 552–558 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu M, Cohen MH, Rieves D, Pazdur R. FDA report: ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am. J. Hematol. 85(5), 315–319 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Vasanawala SS, Nguyen KL, Hope MD et al. Safety and technique of ferumoxytol administration for MRI. Magn. Reson. Med. 75(5), 2107–2111 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reports administration methods of ferumoxytol and the risks of use in MRI.
- 13.Bashir MR, Bhatti L, Marin D, Nelson RC. Emerging applications for ferumoxytol as a contrast agent in MRI. J. Magn. Reson. Imaging 41(4), 884–898 (2015). [DOI] [PubMed] [Google Scholar]; •• Summarizes the use of ferumoxytol in clinical imaging and its limitations.
- 14.Toth GB, Varallyay CG, Horvath A et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int. 92(1), 47–66 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the properties of ferumoxytol and its use in MRI.
- 15.AMAG, Pharmaceuticals, Inc. Feraheme Safety. https://www.feraheme.com/safety-side-effects
- 16.Mcconnell HLBS, Schwartz DLBA, Richardson BEPD, Woltjer RLMDPD, Muldoon LLPD, Neuwelt EAMD. Ferumoxytol nanoparticle uptake in brain during acute neuroinflammation is cell-specific. Nanomedicine 12(6), 1535–1542 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Identifies that ferumoxytol MRI may be used to assess the inflammatory lesions of brain.
- 17.Dósa E, Guillaume DJ, Haluska M et al. Magnetic resonance imaging of intracranial tumors: intra-patient comparison of gadoteridol and ferumoxytol. Neuro. Oncol. 13(2), 251–260 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Discusses ferumoxytol use in further assessment of brain tumor activity in imaging.
- 18.Gahramanov S, Raslan AM, Muldoon LL et al. Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int. J. Radiat. Oncol. Biol. Phys. 79(2), 514–523 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reports the benefits of ferumoxytol use in dynamic susceptibility contrast MRI.
- 19.Barajas R, Hamilton B, Schwartz D et al. Combined iron oxide nanoparticle ferumoxytol and gadolinium contrast enhanced MRI define glioblastoma pseudoprogression. Neuro. Oncol. 21(4), 517–526 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Suggests the use of mismatch ratio for differentiating pseudoprogression from disease recurrence.