Abstract
PspC was found to bind human complement factor H (FH) by Western blot analysis of D39 (pspC+) and an isogenic mutant TRE108 (pspC). We confirmed that PspA does not bind FH, while purified PspC binds FH very strongly. The binding of FH to exponentially growing pneumococci varied among different isolates when analyzed by fluorescence activated cell sorting analysis.
Streptococcus pneumoniae causes a variety of diseases such as pneumonia, bacteremia, meningitis, upper respiratory infections, otitis media, and sinusitis in both adults and children worldwide (1, 13). The pneumococcus has acquired components such as the polysaccharide capsule and surface proteins which prevent opsonization mediated by the complement system (17). The role of PspA in pneumococcal virulence has been well established (6, 9). PspC is another choline-binding protein that is structurally similar to PspA, with shared amino acid sequences in the alpha-helix, proline-rich, and choline-binding regions (2). Immunization with PspC of strain D39 (PspC/D39) elicits antibodies that cross-react with PspA/D39 and is able to protect against pneumococcal infection.
PspC, also designated SpsA and CbpA (5, 12), interacts with the immune system in a variety of ways. PspC binds specifically to the secretory component of immunoglobulin A (4). PspC may regulate the complement system by either adhering to glycoconjugates, sialic acid, and lactotetraoses on the surface of activated human epithelial cells or binding to the C3 component of complement system (12, 14).
Activation of the alternative pathway of the complement system results in the deposition of C3b on the bacterial surface, which leads to opsonophagocytosis of the pneumococcus. Unidentified proteins on the surface of type 3 pneumococci have been reported to bind factor H (FH), a 150-kDa protein that functions in regulating the alternative pathway of complement (10). In this study, we provide evidence that PspC binds FH.
Bacterial strains, growth conditions, and cell lysates.
S. pneumoniae strains used in this study are listed in Tables 1 and 2 and have been previously described (8, 15). These strains include capsular type 2 strain D39 and three insertion duplication mutants (9) derived from D39: LM91 (PspA− PspC+ Eryr) (9), TRE108 (PspA+ PspC− Eryr), and TRE144 (PspA+ PspC+ Tetr; the insertion was downstream of pspC). Pneumococci were cultured and cell lysates were prepared as previously described (7). Escherichia coli Y1090 was grown in Luria-Bertani medium and used as a control lysate. Cell lysates were stored at −20°C until use. The amount of protein in each lysate was determined by the Bio-Rad protein assay (Bio-Rad Laboratories, Richmond, Calif.).
TABLE 1.
FACS analysis of binding of FH to capsular type 2 S. pneumoniae D39-related strains
| Strain | Relevant phenotype | Mean fluorescent intensitya |
|---|---|---|
| D39 | PspC+ PspA+ | 25.2 ± 2.4 |
| TRE108 | PspC− PspA+ | 4.2 ± 0.3 |
| TRE121 | PspC− PspA− | 5.3 ± 0.2 |
| LM91 | PspC+ PspA− | 23.7 ± 2.9 |
The Geometric mean of fluorescent intensity ± standard error of the mean from at least three experiments.
TABLE 2.
FACS analysis of binding of FH to viable encapsulated pneumococci
| Strain | Capsular type | Mean fluorescent intensitya |
|---|---|---|
| WU2 | 3 | 38.1 ± 1.1 |
| EF10197 | 3 | 17.5 ± 2.4 |
| A66 | 3 | 35.6 ± 0.3 |
| EF3296 | 4 | 40.7 ± 1.2 |
| L81905 | 4 | 217.5 ± 8.2 |
| DBL5 | 5 | 11.8 ± 2.2 |
| DBL1 | 6 | 7.8 ± 0.5 |
| DBL6A | 6A | 7.4 ± 1.1 |
| BG7401 | 6A | 7.1 ± 0.9 |
| EF6796 | 6A | 38.1 ± 4.1 |
| BG3011 | 6B | 9.8 ± 0.7 |
| BG7322 | 6B | 23.1 ± 0.3 |
| AC122 | 9V | 13.8 ± 2.3 |
| L82013 | 19A | 104.7 ± 7.0 |
Geometric mean of fluorescent intensity ± standard error of the mean from at least three experiments.
FH, monoclonal antibodies, and purified proteins.
FH was purified from human serum to greater than 95% purity as determined by Coomassie blue staining (11) and biotinylated according to protocol for the EZ-Link sulfo-NHS-LC-biotinylation kit (Pierce, Rockford, Ill.). The only exception was that the solution was dialyzed overnight at 4°C with three changes of phosphate-buffered saline (PBS; pH 7.2). The concentration of FH was 1.0 mg/ml, and biotinylation of FH was confirmed by an enzyme-linked immunosorbent assay.
PspA-specific monoclonal antibodies Xi126 and XiR278 (3), purified PspA (6), and purified PspC and sera from rabbits immunized with PspC have been described elsewhere (2).
Western blot analysis.
Western blot analyses were conducted as previously described (7). In all cases, equivalent protein concentrations were loaded for each lysate. In some cases, the membranes were incubated with biotinylated FH (1/20,000). Bound antibodies and FH were detected by incubation with strepavidin-conjugated horseradish peroxidase and visualized using a chemiluminescent substrate (Pierce).
Fluorescence-activated cell sorting (FACS) analysis.
Various capsular serotypes of exponentially growing pneumoccoci (3 × 108 CFU/ml) in 100 μl of 1% bovine serum albumin–PBS were incubated with 100 μl of FH (1/30) for 1 h at 37°C. The bacteria were washed three times with PBS. The pellet was suspended with fluorescein isothiocyanate-strepavidin in PBS (Southern Biotechnology Associates, Birmingham, Ala.) and incubated for 30 min on ice. After three washes with PBS, pneumococci were suspended in 2 ml of PBS. Fluorescence was analyzed by a FACScan cytometer (Beckton Dickinson), and the results were expressed as the means of two or more runs with each sample.
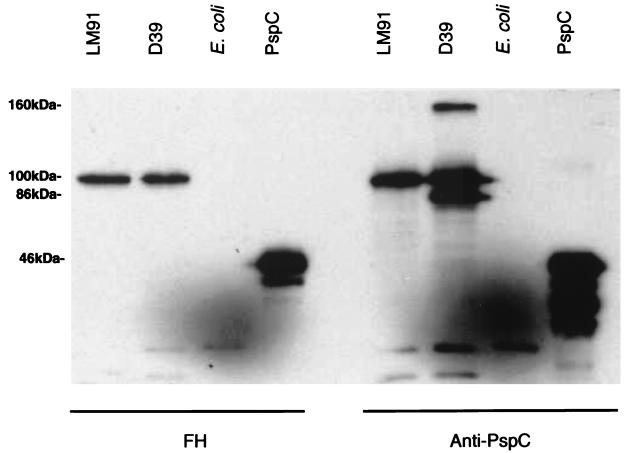
A pneumococcal protein that binds FH was identified by incubating Western blots of capsular type 2 strain D39 with biotinylated FH and anti-PspC antiserum. Figure 1A was developed with an anti-PspC serum that is known to cross-react with both PspC and PspA (2). In the lane with D39, there are three prominent bands of 160, 100, and 86 kDa. The 160- and 86-kDa bands correspond to known positions of PspA/D39 polymeric and monomeric forms (16). The 100-kDa band corresponds to PspC (2). In Fig. 1B, a duplicate blot reacted with FH, it was observed that PspC but not PspA bound FH. The identity of this band as PspC is confirmed by the binding of FH to a recombinant (amino acid numbers 1 to 445) purified fragment of PspC.
FIG. 1.
Western blot analysis of biotinylated FH binding to PspC. Cell lysates of the indicated strain or purified PspC were transferred to nitrocellulose and reacted with FH (A) or anti-PspC antiserum (B).
The studies are extended in Fig. 2, where it was observed that the 100-kDa band detected by FH in strain D39 was not seen in isogenic strain TRE108 (PspC−). Additional experiments with purified PspA confirmed that PspA did not bind FH (data not shown).
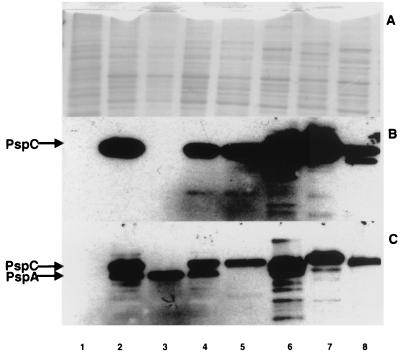
FIG. 2.
Binding of FH to PspC from different pneumococcal isolates. Cell lysates were loaded at equivalent protein concentrations onto three gels and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. (A) Blot stained with Coomassie blue; (B) Western blot reacted with biotinylated FH; (C) Western blot reacted with anti-PspC antibody. Lane 1, E. coli Y1090; lanes 2 to 8, S. pneumoniae strains D39, TRE108, TRE144, LM91, L82013, L81905, and DBL6A, respectively. TRE108 is an isogenic mutant of D39 that lacks PspC. TRE144 has a insertion downstream of the pspC gene in D39. LM91 is an isogenic mutant of D39 that lacks PspA.
The reactivity of biotinylated FH with S. pneumoniae was also tested on cell lysates prepared from different capsular serotypes by Western blot analysis (Fig. 2). All strains tested bound FH. The size of bands varied among various strains.
The binding of FH by exponentially growing pneumococci was determined by flow cytometry. Table 1 shows the results from assays using isogenic strains derived from S. pneumoniae D39. The only variable among the strains is the expression of PspC, PspA, or both surface proteins. The data indicate that in the absence of PspC, only background levels of fluorescence were observed. This was confirmed by fluorescence microscopy (data not shown).
The effect of capsular polysaccharide on the binding of FH was examined by flow cytometry using pneumococci of different capsular types. Table 2 shows that 11 of 14 different strains bound FH significantly better than did the PspC mutant TRE108 (Table 1). There did not appear to be any correlation between the capsular types examined and binding of FH. Additionally, while most pneumococcal isolates bound FH, the isolates varied in their ability to do so. Enzyme-linked immunosorbent assay inhibition studies demonstrated that lysates of several pneumococcal strains could block the binding of FH to heat-killed D39 (data not shown).
Our data demonstrate that the clade B PspC from strain D39 binds human FH. Flow cytometry indicates that clade A PspC also binds FH because the mean fluorescent intensity of two strains, EF6796 and BG7322, is greater than background levels. EF6796 and BG7322 contain clade A pspC genes which encode a larger PspC molecule that has homology to PspA within the alpha helix (2). PspA/D39, which contains structural domains similar to PspC/D39 and reacts with anti-PspC antibody (2), does not react with FH. Therefore, it is unlikely that binding of FH by PspC results from structural similarities or conformation but is a specific result of the primary sequence of PspC. PspC is a regulated protein, and it is preferentially expressed on transparent as opposed to opaque colonies of S. pneumoniae (12). Furthermore, the transcript of pspC is also regulated during the growth phase. The variation in the mean fluorescent intensities of pneumococcal strains may reflect differences in the amount of regulated PspC.
Studies have shown that most type 3 strains, including WU2, do not contain a gene that encodes PspC homologous to PspC/D39 (2, 15). However, we observed significant binding of FH to WU2 and all type 3 pneumococcal isolates tested, suggesting that other molecules bind FH in these strains. Variants of PspC that differ in the anchor and proline-rich region have been described (F. Iannelli, M. R. Spinosa, M. R. Oggioni, and G. Pozzi, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. B-42, 2000) and may account for the binding seen in the type 3 strains. Identification of pneumococcal proteins that bind FH should provide insight into the mechanisms by which pneumococci resist complement activation and phagocytosis.
Acknowledgments
This study was supported by NIH grants AI43653, DK35081, and AI21548.
We are grateful to Edwin Swiatlo and David Briles for suggestions, discussion, and critical reading of the manuscript. We thank Nan Harvey for assistance with the FACS analysis.
REFERENCES
- 1.Briles D E, Tart R C, Swiatlo E, Dillard J P, Smith P, Benton K A, Ralph B A, Brooks-Walter A, Crain M J, Hollingshead S K, McDaniel L S. Pneumococcal diversity: considerations for new vaccine strategies with an emphasis on pneumococcal surface protein A (PspA) Clin Microbiol Rev. 1998;11:645–657. doi: 10.1128/cmr.11.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks-Walter A, Briles D E, Hollingshead S K. The pspC gene of Streptococcus pneunmoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect Immun. 1999;67:6533–6542. doi: 10.1128/iai.67.12.6533-6542.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crain M J, Waltman W D, II, Turner J S, Yother J, Talkington D E, McDaniel L M, Gray B M, Briles D E. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990;58:3293–3299. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammerschmidt S, Talay S R, Brandtzaeg P, Chhatwal G S. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol Microbiol. 1997;25:1113–1124. doi: 10.1046/j.1365-2958.1997.5391899.x. [DOI] [PubMed] [Google Scholar]
- 5.Hammerschmidt S, Tillig M P, Wolff S, Vaerman J, Chhatwal G S. Species-specific binding of human secretory component to SpsA protein of Streptococcus pneumoniae via a hexapeptide motif. Mol Microbiol. 2000;36:726–736. doi: 10.1046/j.1365-2958.2000.01897.x. [DOI] [PubMed] [Google Scholar]
- 6.McDaniel L S, McDaniel D O, Hollingshead S K, Briles D E. Comparison of the PspA sequence from Streptococcus pneumoniae EF5668 to the previously identified PspA sequence from strain Rx1 and ability of PspA from EF5668 to elicit protection against pneumococci of different capsular types. Infect Immun. 1998;66:4748–4754. doi: 10.1128/iai.66.10.4748-4754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDaniel L S, Sheffield J S, Delucchi P, Briles D E. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular type. Infect Immun. 1991;59:222–228. doi: 10.1128/iai.59.1.222-228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDaniel L S, Sheffield J S, Swiatlo E, Yother J, Crain M J, Briles D E. Molecular localization of variable and conserved regions of pspA, and identification of additional pspA homologus sequences in Streptococcus pneumoniae. Microb Pathog. 1992;13:261–269. doi: 10.1016/0882-4010(92)90036-n. [DOI] [PubMed] [Google Scholar]
- 9.McDaniel L S, Yother J, Vijayakumar M, McGarry L, Guild W R, Briles D E. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA) J Exp Med. 1987;165:381–394. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neeleman C, Geelen S P M, Aerts P C, Daha M R, Mollnes T E, Roord J J, Posthuma G, van Dijk H, Fleer A. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect Immun. 1999;67:4517–4524. doi: 10.1128/iai.67.9.4517-4524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pangburn M K, Schreiber R D, Muller-Eberhard H J. Human Ceb inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta-1H for cleavage of C3b and C4b in solution. J Exp Med. 1977;146:257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenow C, Ryan P, Weiser J N, Johnson S, Fontan P, Ortqvist A, Masure R. Contribution of novel choline-binding proteins to adherence, colonization, and immunigenicity of Streptococcus pneumoniae. Mol Microbiol. 1997;25:819–829. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 13.Siber G R. Pneumococcal disease: prospects for a new generation of vaccines. Science. 1994;265:1385–1387. doi: 10.1126/science.8073278. [DOI] [PubMed] [Google Scholar]
- 14.Smith B L, Hostetter M K. C3 as substrate for adhesion of Streptococcus pneumoniae. J Infect Dis. 2000;182:497–508. doi: 10.1086/315722. [DOI] [PubMed] [Google Scholar]
- 15.Swiatlo E, Brooks-Walter A, Briles D E, McDaniel L S. Oligonucleotides identify conserved and variable regions of pspA and pspA-like sequences of Streptococcus pneumoniae. Gene. 1997;188:279–294. doi: 10.1016/s0378-1119(96)00823-2. [DOI] [PubMed] [Google Scholar]
- 16.Talkington D F, Voellinger D C, McDaniel L S, Briles D E. Analysis of pneumococcal PspA microheterogeneity in SDS polyacrylamide gels and the association of PspA with the cell membrane. Microb Pathog. 1992;13:343–355. doi: 10.1016/0882-4010(92)90078-3. [DOI] [PubMed] [Google Scholar]
- 17.Tu A T, Fulgham R L, McCrory M A, Briles D E, Szalai A J. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect Immun. 1999;67:4720–4724. doi: 10.1128/iai.67.9.4720-4724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]