Abstract
Purpose
It is plausible that statins could improve cerebral blood flow through pleiotropic mechanisms. The purpose of this investigation was to assess the contribution of statins to cerebrovascular variables in older adults with dyslipidemia and familial history of dementia. Furthermore, we explored the interaction between statin use and sex due to prevalent bias in statin trials.
Methods
Middle cerebral artery blood flow velocity (MCAv) was measured using transcranial Doppler ultrasound. Continuous supine rest recordings lasted 8 min. Participants included in analyses were statin (n = 100) or non-statin users (n = 112).
Results
MCAv and cerebrovascular conductance were significantly higher in statin users (p = 0.047; p = 0.04), and pulsatility index (PI) was significantly lower in statin users (p < 0.01). An interaction effect between statin use and sex was present for PI (p = 0.02); female statin users had significantly lower cerebrovascular resistance than the other three groups.
Conclusion
In this cross-sectional analysis, statin use was positively associated with cerebrovascular variables in older adults at risk for dementia. Female statin users had significantly higher resting MCAv and cerebrovascular conductance than female non-statin users. The greatest contribution of statin use was the association with reduced cerebrovascular resistance. Given that cerebrovascular dysregulation is one of the earliest changes in Alzheimer’s disease and related dementia pathology, targeting the cerebrovasculature with statins may be a promising prevention strategy.
Keywords: Alzheimer’s disease, Cerebral blood flow velocity, Cerebrovascular, Dementia, Statin
Introduction
As the exact causes underlying Alzheimer’s disease (AD) and related dementias (ADRD) are still unknown, it is vital to commit research efforts toward prevention strategies (Claassen 2015). ADRD is a disease of multifactorial origin, likely resulting from an interaction between genetic susceptibility and environmental risk factors (Kivipelto et al. 2002). While genetics are non-modifiable in relation to ADRD treatment, various environmental, thus modifiable, risk factors have been proposed (Kivipelto and Solomon 2006). One promising prevention strategy is to target the vascular system, as atherosclerosis due to hypercholesterolemia along with aging-related cerebrovascular alterations, constitute important independent factors for the development of ADRD (Iadecola 2010; Wiesmann et al. 2013; Kivipelto et al. 2001).
Statins are one of the most effective pharmacologic treatments for cardiovascular risk reduction (McConnachie et al. 2014; Mistry et al. 2012). As clinical use continues to surge, the effects of statins beyond just peripheral lipid reduction are being investigated. In a cross-sectional analysis from 3 different hospitals, the prevalence of AD in patients taking statins was 60% lower than in those taking other cardiovascular medications (Wolozin et al. 2000). However, 95% of patients from the 2 veterans affairs medical centers were men. Furthermore, in a case–control study, participants who were prescribed statins had a 70% lower odds for dementia (Odds Ratio = 0.29) than those who did not have hyperlipidemia or who were not on a lipid-lowering medication (Jick et al. 2000). With promising results like these, pleiotropic effects of statins are frequently being investigated (Liao 2005; Lahera et al. 2007; Wang et al. 2008; Kling et al. 2013). In animal models, experimental investigations have reported an increase in cerebral blood flow independent of lowering peripheral cholesterol with statin treatment. (Endres et al. 1998; Amin-Hanjani et al. 2001; Yamada et al. 2000). In a small pilot, randomized controlled trial of individuals with an increased familial risk for AD (n = 13), findings suggested those prescribed statins for 4 months increased regional cerebral blood flow compared to those on placebo. (Carlsson et al. 2012). Therefore, statins may be a clinically relevant treatment for the prevention of AD onset through alterations in cerebral perfusion. In contrast, a case could also be made that more needs to be known about statins and vascular health given the consistent findings that statins cause a small but statistically significant increased risk for type 2 diabetes (Preiss et al. 2011), often worsens insulin resistance (Miller and Thyfault 2020) and markers of glycemic control (Mansi et al. 2021), which are also risk factors for AD and vascular disease.
The purpose of this investigation was to assess if statin use contributed to resting cerebral blood flow velocity, cerebrovascular conductance, and cerebral pulsatility in older adults with dyslipidemia who were or were not undergoing statin therapy and a familial history of dementia or self-report cognitive decline. We hypothesized that statin use would positively contribute to resting cerebral blood flow velocity, cerebrovascular conductance, and cerebral pulsatility. Despite widespread statin use in both sexes, bias has been prevalent in statin trials (Bandyopadhyay et al. 2001); therefore, we also explored the statin use and sex interaction on resting cerebral blood flow velocity, cerebrovascular conductance, and cerebral pulsatility.
Materials and methods
This study utilized baseline data collected at the University of Kansas (KU) Medical Center, University of Kansas Alzheimer’s Disease Research Center (KU ADRC), and the University of Texas (UT) Southwestern Medical Center as part of a multi-site clinical trial (R01 AG49749).
Study population
Whereas old age is the single most important risk factor for dementia, having a first degree relative with dementia increases risk by three to fourfold (Cupples et al. 2004). Furthermore, the co-existence of vascular risk factors with a family history of dementia further increases risk (Wang et al. 2012). The individuals included in this study, therefore, represent a high prevalence and high-risk population.
Inclusion criteria: (1) age 60–85; (2) family history of AD defined as having at least one first-degree relative (siblings or biological parents) with a clinical diagnosis of possible or probable AD with an onset age between 60 and 80 years (based on the medical records and/or the databases) or self-report of cognitive decline (Honea et al. 2012); (3) AD8 < 2 and Mini-Mental State Exam (MMSE) > 27 (based on age and education adjusted norms) to exclude dementia (Crum et al. 1993); (4) a sedentary lifestyle defined as < 90 min of moderate aerobic exercise per week over the past year; (5) hypertension defined as systolic blood pressure (sBP) ≥ 140 mmHg (James et al. 2014), subjects on anti-hypertensives were eligible; (6) dyslipidemia defined as those who have an estimate 10-year cardiovascular disease score ≥ 7.5% (calculated using pooled cohort equations) and LDL-C ≥ 95 mg/dL (Goff et al. 2013), subjects on low-intensity statin therapy were eligible.
Exclusion criteria: (1) history of stroke or other major cerebrovascular diseases by clinical diagnosis or magnetic resonance imaging/computerized tomography scans; (2) diagnosis of AD or other type of dementia and significant neurologic diseases; (3) patients with current evidence of severe depression or other DSM-V Axis I psychopathology; (4) major and unstable heart (e.g., heart attack/cardiac arrest, severe atrial fibrillation, cardiac bypass procedures and congestive heart failure), liver, renal and other medical conditions; (5) orthostatic hypotension; (6) history of significant autoimmune disorders; (7) clinical diagnosis of type 1 or 2 diabetes (Zhang et al. 2015); (8) patients with resistant hypertension (SBP ≥ 140 or diastolic blood pressure (dBP) ≥ 90 mmHg despite 3 antihypertensive drugs at maximal doses); (9) current use of warfarin or anticoagulant therapy; (10) Regularly smoking cigarettes within the past year; (11) any conditions judged by the study investigators to be medically contraindicated, risky for the participant, or likely to have poor study adherence.
Prior to study commencement, the protocol was reviewed and approved by the individual sites respective institutional review boards (IRB number STUDY00004510 at the KU Medical Center and STU052016-076 at the UT Southwestern Medical Center). All participants provided written informed consent.
Extensive details have been previously published describing screening and fasting clinical chemistry (Szabo-Reed et al. 2019).
Cerebrovascular and hemodynamic measurements
Once in the supine position, participants completed a rest period of at least 10 min in a quiet, temperature-controlled room (22–24 °C) before data collection. During the rest period, participants were instrumented for the hemodynamic and cerebrovascular measurements. Heart rate was measured via a 5-lead electrocardiogram at KU Medical Center (Cardiocard, Nasiff Associates, Central Square, NY, USA) or a 3-lead electrocardiogram at UT Southwestern Medical Center (Hewlett-Packard, Palo Alto, CA, USA). Mean arterial pressure (MAP) was measured using a brachial sphygmomanometer (Tango M2 Stress Test Monitor, Sun-Tech Medical, Inc., Morrisville, NC, USA; Finapres Medical Systems, Amsterdam, The Netherlands). End-tidal CO2 was measured via a nasal cannula attached to a gas analyzer (BCI Capnocheck 9004, Smiths Medical, Columbia, SC, USA; Carpnograd, Novamatrix, Wallingford, CT, USA). Transcranial Doppler ultrasound (TCD; RobotoC2MD System, Multigon Industries, Yonkers, NY, USA; Multi-Dop X2, Compumedics/DWL, Singen, Germany) was used to measure mean middle cerebral artery blood flow velocity (MCAv) with a 2 Hz probe placed on the right or left trans-temporal window using a signal depth between 40 and 65 mm. The TCD probe was secured with headgear to maintain the optimal position and angle.
Heart rate, end-tidal CO2, and TCD rest recordings lasted for eight minutes. At KU Medical Center, data were collected with a sampling frequency of 500 Hz (National Instruments, Austin, TX) with data acquisition and analysis completed with a custom MATLAB script (The Math Works, Inc., Version 2019a, Natick, MA, USA). At UTSW, data were collected with a sampling frequency of 1000 Hz and stored in a computer for off-line analysis using a data acquisition and analysis software (Acknowledge, BIOPAC systems, Goleta, CA, USA). Three brachial blood pressure measurements were taken before recordings started. MCAv was calculated by averaging the MCAv within each cardiac cycle. Heart rate, end-tidal CO2 and MCAv were averaged over the recorded duration for each participant. Cerebrovascular conductance index (CVCi) was calculated by dividing mean MCAv by MAP. Pulsatility index (PI) was calculated as the difference between systolic and diastolic MCAv divided by the mean MCAv ([MCAvsystolic – MCAvdiastolic]/mean MCAv) (Gosling and King 1974).
Statistical analysis
All statistical analyses were performed using R language for statistical computing (R Core Team, version 4.0.3, 2020). Continuous variables are reported as mean ± standard deviation and all categorical variables as count (%) unless otherwise stated. Differences were considered significant at p < 0.05.
Statin vs. no statin groups were assessed using a one-way analysis of variance (ANOVA) or a Kruskal–Wallis test depending on whether the assumption of normality was violated. Normality was assessed using the Shapiro–Wilk test and Q-Q plot visualization. Group differences for categorical variables was assessed using χ2 test. The statin use by sex groups were assessed using a one-way ANOVA with Bonferroni correction for multiple comparisons.
A forward and backward stepwise model selection based on Akaike Information Criterion (AIC) was used for the linear regression to determine if statin use contributed to the cerebrovascular outcomes. Within the initial model, the independent variables included statin use, age, sex, beta-blocker use, LDL-C levels, HDL-C levels, heart rate, mean arterial pressure and end-tidal CO2. Variance inflation factor was checked for all initial models and final models. To assess statin and sex interaction effects, linear regression models without forward and backward stepwise AIC selections were completed. The independent variables that remained in the model after the AIC model selections were included in the models for the statin and sex interaction analyses.
Results
Analyses were conducted in 212 participants with 100 in the statin group and 112 in the non-statin group (Tables 1 and 2). As expected, those undergoing statin therapy had lower cholesterol values (LDL-C, HDL-C, and total cholesterol) compared to those not undergoing statin therapy. Additionally, significantly fewer participants were taking beta-blockers in the statin group compared to the non-statin group. When controlling for other potentially confounding variables (stepwise AIC selection models), statin use significantly contributed to the cerebrovascular variables (Table 3). Statin use was positively and significantly associated with MCAv (p = 0.047) and CVCi (p = 0.04), indicating that within statin users cerebral blood flow velocity and conductance tended to be higher than non-statin users. Furthermore, statin use was negatively and significantly associated with PI (p < 0.01), indicating that within the statin users cerebrovascular resistance tended to be lower than non-statin users.
Table 1.
Participant characteristics
| Statin n = 100 | No statin n = 112 | p | |
|---|---|---|---|
| Age | 69 ± 6 | 69 ± 6 | 0.68 |
| Female, n (%) | 51 (51%) | 74 (66%) | 0.03 |
| Height, cm | 170 ± 9 | 168 ± 10 | 0.13 |
| Weight, kg | 88 ± 18 | 84 ± 19 | 0.12 |
| Body mass index, kg m−2 | 30 ± 5 | 30 ± 6 | 0.32 |
| Heart rate, bpm | 58 ± 7 | 57 ± 8 | 0.27 |
| End-tidal CO2, mmHg | 35 ± 3 | 35 ± 4 | 0.93 |
| sBP, mmHg | 130 ± 15 | 132 ± 15 | 0.19 |
| dBP, mmHg | 73 ± 9 | 76 ± 9 | 0.08 |
| MAP, mmHg | 92 ± 10 | 95 ± 10 | 0.08 |
| LDL-C, mg dL−1 | 93 ± 29 | 119 ± 31 | < 0.0001 |
| HDL-C, mg dL−1 | 53 ± 16 | 62 ± 20 | < 0.001 |
| Total cholesterol, mg dL−1 | 170 ± 36 | 203 ± 37 | < 0.0001 |
| Beta-blocker, n (%) | 75 (75%) | 100 (89%) | < 0.01 |
Bold font indicates significance at p < 0.05
Continuous variables are mean ± standard deviation. Categorical variables are count (%)
dBP diastolic blood pressure, HDL-C high-density lipoprotein cholesterol, LDL-C low density lipoprotein cholesterol, MAP, mean arterial pressure, sBP systolic blood pressure
Table 2.
Cerebrovascular outcomes
| No statin n = 112 | Statin n = 100 | p | |
|---|---|---|---|
| MCAv, cm s−1 | 52.22 ± 13.63 | 52.50 ± 13.20 | 0.88 |
| CVCi, cm s−1·mmHg−1 | 0.56 ± 0.15 | 0.58 ± 0.16 | 0.42 |
| PI, a.u | 1.03 ± 0.28 | 0.97 ± 0.23 | 0.09 |
Variables are mean ± standard deviation. p values are unadjusted and a comparison between groups
CVCi cerebrovascular conductance index, MCAv middle cerebral artery blood flow velocity, PI pulsatility index
Table 3.
Regression results for cerebrovascular outcomes
| MCAv | CVCi | PI | |
|---|---|---|---|
| Statin | 3.88 [0.04–7.71] (0.047) |
0.04 [0.002–0.09] (0.04) |
− 0.09 [− 0.16 to − 0.03] (< 0.01) |
| Age | – | – | 0.02 [0.01–0.02] (< 0.0001) |
| Female | 6.83 [2.79–10.86] (< 0.01) |
0.08 [0.03–0.12] (< 0.001) |
– |
| Beta-blockers | − 4.69 [− 9.44 to 0.06] (0.05) |
− 0.05 [− 0.10 to 0.005] (0.07) |
– |
| LDL-C | 0.05 [− 0.01 to 0.11] (0.10) |
0.0005 [− 0.0001 to 0.011] (0.13) |
− 0.001 [− 0.002 to − 0.000002] (0.049) |
| HDL-C | 0.10 [− 0.0004 to 0.21] (0.05) |
0.001 [− 0.00002 to 0.002] (0.05) |
– |
| Heart rate | 0.24 [0.03–0.46] (0.03) |
0.003 [0.0004–0.01] (0.02) |
0.004 [0.0002–0.007] (0.04) |
| Mean arterial pressure | – | − 0.006 [− 0.008 to − 0.004] (< 0.001) |
– |
| End-tidal CO2 | 0.39 [− 0.05 to 0.84] (0.08) |
0.004 [− 0.008 to 0.009] (0.10) |
– |
| Intercept | 8.60 [− 12.89 to 30.09] (0.43) |
0.63 [0.32–0.94] (< 0.001) |
− 0.37 [− 0.77 to 0.02] (0.06) |
| R-squared | 0.17 | 0.29 | 0.29 |
| Adjusted R-squared | 0.14 | 0.27 | 0.28 |
| Residual Std. error | 12.34 | 0.13 | 0.21 |
| F statistic | 5.90 df = 7; 200 (< 0.001) |
10.33 df = 8; 199 (< 0.001) |
20.74 df = 4; 203 (< 0.001) |
Bold indicates significance at p < 0.05
For each variable the primary number is β-weight. A dash (−) indicates the variable dropped during the AIC model selection. 95% confidence intervals are reported in brackets. p-values are reported in parentheses
CVCi cerebrovascular conductance index, HDL-C high-density lipoprotein, LDL-C low-density lipoprotein, MCAv, middle cerebral blood flow velocity, PI pulsatility index
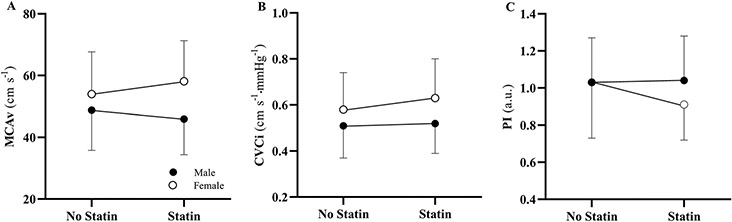
For MCAv and CVCi, the female statin group was significantly higher than the male statin group (p < 0.001; p < 0.001; Table 4). For PI, the female statin group trended towards but was not significantly lower than the male statin group (p = 0.07). Additionally, the female statin group was significantly lower than female non-statin group (p = 0.046).
Table 4.
Cerebrovascular characteristics by statin use and sex
| Males | Females | M vs. F No statin |
M vs. F statin |
Main effect | |||||
|---|---|---|---|---|---|---|---|---|---|
| No statin n = 38 | Statin n = 49 | p | No statin n = 74 | Statin n = 51 | p | p | p | p | |
| MCAv, cm s−1 | 48.78 ± 13.00 | 46.62 ± 10.53 | 0.99 | 53.99 ± 13.69 | 58.14 ± 13.11 | 0.45 | 0.25 | < 0.001 | < 0.001 |
| CVCi, cm s−1·mmHg−1 | 0.51 ± 0.14 | 0.52 ± 0.13 | 0.99 | 0.58 ± 0.16 | 0.63 ± 0.17 | 0.43 | 0.10 | < 0.001 | < 0.001 |
| PI, a.u | 1.03 ± 0.24 | 1.04 ± 0.24 | 0.99 | 1.03 ± 0.30 | 0.91 ± 0.19 | 0.047 | 0.99 | 0.07 | 0.03 |
Bold indicates significance at p < 0.05
Variables are mean ± standard deviation
CVCi cerebrovascular conductance index, F females, M males, MCAv middle cerebral artery blood flow velocity, PI pulsatility index
The interaction between statin-use and sex for MCAv and CVCi trended towards but was not significant (p = 0.08; p = 0.06; Table 5; Fig. 1). For PI, however, a significant interaction was present across sex (p = 0.02), suggesting female statin users had lower cerebrovascular resistance than men statin or non-statin users.
Table 5.
Regression results for statin use and sex interaction effect for cerebrovascular outcomes
| MCAv | CVCi | PI | |
|---|---|---|---|
| Statin*female | 6.32 [− 0.64 to 13.29] (0.08) |
0.07 [− 0.004 to 0.15] (0.06) |
− 0.14 [− 0.26 to − 0.02] (0.02) |
| Statin | 0.26 [− 5.26 to 5.78] (0.93) |
0.002 [− 0.06 to 0.06] (0.96) |
− 0.01 [− 0.10 to 0.09] (0.87) |
| Female | 3.50 [− 1.93 to 8.94] (0.20) |
0.04 [− 0.02 to 0.10] (0.19) |
0.07 [− 0.01 to 0.16] (0.10) |
| Age | – | – | 0.02 [0.01–0.02] (< 0.001) |
| Beta-blockers | − 4.81 [− 9.53 to − 0.09] (0.046) |
− 0.05 [− 0.10 to 0.003] (0.06) |
– |
| LDL-C | 0.05 [− 0.01 to 0.19] (0.09) |
0.001 [− 0.0001 to 0.001] (0.12) |
− 0.001 [− 0.002 to 0.00002] (0.05) |
| HDL-C | 0.11 [0.01–0.22] (0.04) |
0.001 [0.00007–0.002] (0.04) |
– |
| Heart Rate | 0.23 [0.02–0.45] (0.03) |
0.003 [0.0002–0.005] (0.03) |
0.004 [0.0004–0.008] (0.03) |
| Mean Arterial Pressure | – | − 0.01 [− 0.008 to − 0.004] (< 0.001) |
|
| End-tidal CO2 | 0.41 [− 0.03 to 0.85] (0.07) |
0.004 [− 0.0006 to 0.009] (0.09) |
– |
| Constant | 10.18 [− 11.26 to 31.63] (0.35) |
0.67 [0.36–0.98] (< 0.001) |
− 0.45 [− 0.85 to − 0.05] (0.03) |
| R-squared | 0.18 | 0.31 | 0.31 |
| Adjusted R-squared | 0.15 | 0.27 | 0.29 |
| Residual Std. error | 12.28 | 0.13 | 0.21 |
| F statistic | 5.62 df = 8; 199 (< 0.001) |
9.69 df = 9; 198 (< 0.001) |
15.02 df = 6; 201 (< 0.001) |
Bold indicates significance at p < 0.05
For each variable the primary number is β-weight. A dash (−) indicates the variable dropped during the AIC model selection. 95% confidence intervals are reported in brackets. p values are reported in parentheses
CVCi cerebrovascular conductance index, HDL-C high-density lipoprotein, LDL-C low-density lipoprotein, MCAv middle cerebral blood flow velocity, PI pulsatility index
Fig. 1.
Interaction plots for statin use by sex for each cerebrovascular outcome. A MCAv, middle cerebral artery blood flow velocity. B CVCi, cerebrovascular conductance index. C PI, pulsatility index
Discussion
Our primary findings are that statin use was a significant contributing factor for resting cerebrovascular variables in older adults with dyslipidemia and a familial history of dementia. Statin users tended to have higher cerebral blood flow velocity and cerebrovascular conductance as well as lower cerebrovascular resistance than non-statin users. Furthermore, there was a statin and sex interaction effect, such that female statin users had lower cerebrovascular resistance than female non-statin users as well as male statin and non-statin users.
In relation to statin effects on cerebral blood flow, many of the investigations have been completed using magnetic resonance imaging. In a pilot clinical trial of asymptomatic middle-aged adults at risk for AD (n = 16), participants were randomized to four months of atorvastatin or placebo (Carlsson et al. 2012). At baseline and month 4, regional cerebral blood flow was measured using arterial spin-labeling magnetic resonance imaging. They reported greater increases in regional cerebral blood flow in bilateral hippocampi, fusiform gyrus, putamen, and insular cortices compared to placebo. Other studies have demonstrated relative hypoperfusion in the posterior cingulate, and the parietal and medial temporal lobes in adults with mild cognitive impairment (Johnson et al. 2005), cognitively healthy apolipoprotein E4 allele carriers (Scarmeas et al. 2003), and non-demented persons with memory complaints who later develop AD (El Fakhri et al. 2003). These previous studies provide support for our findings of increased cerebral blood flow velocity and cerebral conductance within the middle cerebral artery within statin users.
Sex differences in vascular risk factors associated with ADRD have not been studied systematically, with the majority of studies adjusting for sex; preventing the assessment of sex related effects on outcomes (Ferretti et al. 2018). Differences in aging related brain structure (Ruigrok et al. 2014) and function (Laws et al. 2016) between sexes are emerging, as are sex effects on the manifestation and progression of neurological conditions (Cordonnier et al. 2017). Previous research indicates that sex can modulate not only the prevalence of risk factors for AD but also the susceptibility to AD conferred by a given risk factor. For example, the first manifestation of cardiovascular disease differs between sexes, which could differentially influence the risk of AD (Ruitenberg et al. 2005). Given the evidence that vascular factors contribute to AD risk and our evidence suggesting statin contribution significantly supports cerebrovascular health in females, sex differences in prevention and treatment strategies for ADRD deserve further examination.
The brain is one of the most cholesterol enriched organs in the body and contains about 25% of total body cholesterol. As cholesterol is essential for cell membranes and plays a crucial role in the development and maintenance of neuronal plasticity and function, cholesterol homeostasis within the brain is strictly regulated (Pfrieger 2003). The exchange rate between peripheral and brain cholesterol is predominantly, but not fully, independent of blood cholesterol levels (Dietschy and Turley 2004). When blood cholesterol levels are not regulated and hypercholesterolemia occurs, individuals increase their risk for the development of ADRD related pathologies. This conclusion has been supported by previous investigations. Arvanitakis et al. (2016) reported that large and small cerebral vessel pathology (i.e., atherosclerosis) were associated with lower cognitive performance and increased risk for AD. Beach et al. (2007) demonstrated that individuals with AD exhibit substantial atherosclerosis in the arteries of the circle of Willis, with lesions being more severe and more stenotic than in age-matched controls. Importantly, these finding have been validated within a large cohort, reporting that common pathophysiological mechanisms promote both vascular pathology and amyloid plaque as well as tau tangle pathology (Yarchoan et al. 2012).
The pleiotropic effect of statins on cerebral blood flow are not well understood but may be attributed to several processes resulting from the inhibition of 3-hydroxy-3-methyl-glutarylo-coenzyme A (HMG-CoA) reductase. Statins main effect is the inhibition of cholesterol and isoprenoid synthesis, which may result in lower circulating endothelial nitric oxide synthase, an enzyme responsible for vascular endothelial function, and thus improved pathophysiologic response (Liao and Laufs 2005). In addition to this main effect, statins may also induce antioxidant effects via the decreased production of NADPH oxidase and resulting reactive oxidant species in circulation (Endres et al. 1998). Inflammatory markers such as C-reactive protein have also been shown to be reduced by statins, leading to the hypothesis that statins have anti-inflammatory properties (Li et al. 2010). By impairing production of β-amyloid proteins and apolipoprotein E and exerting anti-inflammatory effects, statins are proposed to decrease the amount of tau fibrillization in the pathophysiologic process of Alzheimer’s disease. Studies are underway to clarify the impact of statins on these ADRD-related processes and whether modifying these potential mechanisms of disease leads to long-term improved cognition and reduced risk for AD. In contrast, statins have been implicated in worsening glycemic control which itself is a risk factor for vascular complications and AD. However, this study excluded those with type 2 diabetes or elevated glycemia. Thus, a possible negative effect of statins on vascular effects through glycemia was likely avoided in this study. A future study examining the impact of statins on cerebrovascular blood flow control in those with pre-diabetes or type 2 diabetes is warranted.
There are several limitations to the investigation that should be considered. First, using transcranial Doppler ultrasound to quantify cerebral blood flow requires the assumption that vessel length, vessel radius and blood viscosity remain constant for velocity to be a surrogate for cerebral blood flow (Serrador et al. 2000). Evidence suggests changes in MAP and end-tidal CO2 are associated with changes in the diameter of the MCA (Verbree et al. 2017). However, transcranial Doppler ultrasound measurements for the study were collected in a resting state and MCA diameter most likely was not affected. Additionally, only unilateral MCAv was assessed, therefore, results may not reflect the trajectory of age-related change in posterior cerebral circulation and/or global cerebral circulation. Secondly, the statin and beta-blocker medication doses as well as duration of medication use were not recorded. It is unknown, therefore, within our results if medication doses play a role in the cerebrovascular outcomes, especially within the statin and sex interaction assessment. Third, intracranial atherosclerosis was not analyzed, thus, we could not account for varying degrees of stenosis in the common and internal carotid arteries. Lastly, this investigation was a cross-sectional analysis and the effects of statin use on cerebrovascular outcomes cannot be directly surmised, however, we will explore this further once the clinical trial is complete (NCT02913664). Moreover, we cannot directly tell if the statin drug itself or the statin induced change in circulating cholesterol is the primary driver of the differences observed between groups. However, the significant effects from the current study along with the previous investigations discussed, further investigation into the longitudinal effects with a focus on statin therapy dose and duration, and the impact of sex on cerebrovascular function should be completed.
Conclusions
In this cross-sectional analysis, statin use positively contributed to cerebrovascular outcomes in older adults at risk for dementia. Specifically, females showed the greatest contribution to cerebrovascular outcomes from statin use. Given that cerebrovascular dysregulation is one of the earliest changes in ADRD pathology, (Iadecola 2004) targeting cerebral perfusion with statins may be a promising prevention strategy to delay or prevent ADRD. Intervention studies are currently underway that may highlight the effects of, as well as mechanisms behind, statin use on cerebrovascular function in individuals at a high-risk for ADRD.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work has been supported under the Clinical and Translational Science Award (CTSA) grant from the National Center for Advancing Translational Sciences (NCATS) awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (# TL1TR002368) and National Institute of Health (NIH) grants R01AG049749, R01AR071263, and P30AG072973. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.
Abbreviations
- AIC
Akaike information criterion
- ANOVA
Analysis of variance
- AD
Alzheimer’s disease
- AD8
Eight-item informant interview to differentiate aging and dementia
- ADRD
Alzheimer’s disease and related dementias
- CVCi
Cerebrovascular conductance index
- dBP
Diastolic blood pressure
- DSM-V
Diagnostic and statistical manual of mental disorders fifth edition
- HDL-C
High-density lipoprotein
- LDL- C
Low-density lipoprotein
- MAP
Mean arterial pressure
- MCAv
Middle cerebral artery blood flow velocity
- MMSE
Mini-mental state exam
- PI
Pulsatility index
- sBP
Systolic blood pressure
- TCD
Transcranial Doppler ultrasound
Footnotes
Conflict of interest The authors have no competing interests to declare that are relevant to the content of this article.
Ethics approval The protocol was reviewed and approved by the individual site’s respective institutional review boards (IRB number STUDY00004510 at the KU Medical Center and STU052016-076 at the UT Southwestern Medical Center).
Consent to participate All participants provided written informed consent before study commencement.
Data availability
Data related to this manuscript will be available upon reasonable request to the corresponding author.
References
- Amin-Hanjani S, Stagliano NE, Yamada M, Huang PL, Liao JK, Moskowitz MA (2001) Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke 32(4):980–986. 10.1161/01.str.32.4.980 [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA (2016) Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol 15(9):934–943. 10.1016/s1474-4422(16)30029-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S, Bayer AJ, O’Mahony MS (2001) Age and gender bias in statin trials. QJM 94(3):127–132. 10.1093/qjmed/94.3.127 [DOI] [PubMed] [Google Scholar]
- Beach TG, Wilson JR, Sue LI, Newell A, Poston M, Cisneros R, Pandya Y, Esh C, Connor DJ, Sabbagh M, Walker DG, Roher AE (2007) Circle of Willis atherosclerosis: association with Alzheimer’s disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol 113(1):13–21. 10.1007/s00401-006-0136-y [DOI] [PubMed] [Google Scholar]
- Carlsson CM, Xu G, Wen Z, Barnet JH, Blazel HM, Chappell RJ, Stein JH, Asthana S, Sager MA, Alsop DC, Rowley HA, Fain SB, Johnson SC (2012) Effects of atorvastatin on cerebral blood flow in middle-aged adults at risk for Alzheimer’s disease: a pilot study. Curr Alzheimer Res 9(8):990–997. 10.2174/156720512803251075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen JA (2015) New cardiovascular targets to prevent late onset Alzheimer disease. Eur J Pharmacol 763(Pt A):131–134. 10.1016/j.ejphar.2015.05.022 [DOI] [PubMed] [Google Scholar]
- Cordonnier C, Sprigg N, Sandset EC, Pavlovic A, Sunnerhagen KS, Caso V, Christensen H (2017) Stroke in women - from evidence to inequalities. Nat Rev Neurol 13(9):521–532. 10.1038/nrneurol.2017.95 [DOI] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF (1993) Population-based norms for the mini-mental state examination by age and educational level. JAMA 269(18):2386–2391 [PubMed] [Google Scholar]
- Cupples LA, Farrer LA, Sadovnick AD, Relkin N, Whitehouse P, Green RC (2004) Estimating risk curves for first-degree relatives of patients with Alzheimer’s disease: the REVEAL study. Genet Med 6(4):192–196. 10.1097/01.gim.0000132679.92238.58 [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD (2004) Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res 45(8):1375–1397. 10.1194/jlr.R400004-JLR200 [DOI] [PubMed] [Google Scholar]
- El Fakhri G, Kijewski MF, Johnson KA, Syrkin G, Killiany RJ, Becker JA, Zimmerman RE, Albert MS (2003) MRI-guided SPECT perfusion measures and volumetric MRI in prodromal Alzheimer disease. Arch Neurol 60(8):1066–1072. 10.1001/archneur.60.8.1066 [DOI] [PubMed] [Google Scholar]
- Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK (1998) Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 95(15):8880–8885. 10.1073/pnas.95.15.8880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher Dimech A, Santuccione Chadha A, Baracchi F, Girouard H, Misoch S, Giacobini E, Depypere H, Hampel H (2018) Sex differences in Alzheimer disease - the gateway to precision medicine. Nat Rev Neurol 14(8):457–469. 10.1038/s41582-018-0032-9 [DOI] [PubMed] [Google Scholar]
- Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson J, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW (2013) 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 10.1161/01.cir.0000437741.48606.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling RG, King DH (1974) Arterial assessment by Doppler-shift ultrasound. Proc R Soc Med 67(6 Pt 1):447–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Vidoni ED, Swerdlow RH, Burns JM (2012) Maternal family history is associated with Alzheimer’s disease biomarkers. J Alzheimers Dis 31(3):659–668. 10.3233/jad-2012-120676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C (2004) Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5(5):347–360. 10.1038/nrn1387 [DOI] [PubMed] [Google Scholar]
- Iadecola C (2010) The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 120(3):287–296. 10.1007/s00401-010-0718-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E (2014) 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee (JNC 8). JAMA 311(5):507–520. 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA (2000) Statins and the risk of dementia. Lancet 356(9242):1627–1631. 10.1016/s0140-6736(00)03155-x [DOI] [PubMed] [Google Scholar]
- Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N (2005) Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology 234(3):851–859. 10.1148/radiol.2343040197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Solomon A (2006) Cholesterol as a risk factor for Alzheimer’s disease - epidemiological evidence. Acta Neurol Scand Suppl 185:50–57. 10.1111/j.1600-0404.2006.00685.x [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A (2001) Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ 322(7300):1447–1451. 10.1136/bmj.322.7300.1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Iivonen S, Mannermaa A, Tuomilehto J, Nissinen A, Soininen H (2002) Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med 137(3):149–155. 10.7326/0003-4819-137-3-200208060-00006 [DOI] [PubMed] [Google Scholar]
- Kling MA, Trojanowski JQ, Wolk DA, Lee VM, Arnold SE (2013) Vascular disease and dementias: paradigm shifts to drive research in new directions. Alzheimers Dement 9(1):76–92. 10.1016/j.jalz.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahera V, Goicoechea M, de Vinuesa SG, Miana M, de las Heras N, Cachofeiro V, Luño J (2007) Endothelial dysfunction, oxidative stress and inflammation in atherosclerosis: beneficial effects of statins. Curr Med Chem 14(2):243–248. 10.2174/092986707779313381 [DOI] [PubMed] [Google Scholar]
- Laws KR, Irvine K, Gale TM (2016) Sex differences in cognitive impairment in Alzheimer’s disease. World J Psychiatry 6(1):54–65. 10.5498/wjp.v6.i1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li JJ, He JG, Nan JL, Guo YL, Xiong CM (2010) Atorvastatin decreases C-reactive protein-induced inflammatory response in pulmonary artery smooth muscle cells by inhibiting nuclear factor-kappaB pathway. Cardiovasc Ther 28(1):8–14. 10.1111/j.1755-5922.2009.00103.x [DOI] [PubMed] [Google Scholar]
- Liao JK (2005) Clinical implications for statin pleiotropy. Curr Opin Lipidol 16(6):624–629. 10.1097/01.mol.0000191913.16321.60 [DOI] [PubMed] [Google Scholar]
- Liao JK, Laufs U (2005) Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 45:89–118. 10.1146/annurev.pharmtox.45.120403.095748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansi IA, Chansard M, Lingvay I, Zhang S, Halm EA, Alvarez CA (2021) Association of statin therapy initiation with diabetes progression: a retrospective matched-cohort study. JAMA Intern Med 181(12):1562–1574. 10.1001/jamainternmed.2021.5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnachie A, Walker A, Robertson M, Marchbank L, Peacock J, Packard CJ, Cobbe SM, Ford I (2014) Long-term impact on healthcare resource utilization of statin treatment, and its cost effectiveness in the primary prevention of cardiovascular disease: a record linkage study. Eur Heart J 35(5):290–298. 10.1093/eurheartj/eht232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Thyfault JP (2020) Exercise-pharmacology interactions: metformin, statins, and healthspan. Physiology (Bethesda) 35(5):338–347. 10.1152/physiol.00013.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry H, Morris S, Dyer M, Kotseva K, Wood D, Buxton M (2012) Cost-effectiveness of a European preventive cardiology programme in primary care: a Markov modelling approach. BMJ Open. 10.1136/bmjopen-2012-001029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrieger FW (2003) Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci 60(6):1158–1171. 10.1007/s00018-003-3018-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK (2011) Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 305(24):2556–2564. 10.1001/jama.2011.860 [DOI] [PubMed] [Google Scholar]
- Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J (2014) A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev 39(100):34–50. 10.1016/j.neubiorev.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM (2005) Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann Neurol 57(6):789–794. 10.1002/ana.20493 [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Habeck CG, Stern Y, Anderson KE (2003) APOE geno-type and cerebral blood flow in healthy young individuals. JAMA 290(12):1581–1582. 10.1001/jama.290.12.1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL (2000) MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31(7):1672–1678. 10.1161/01.str.31.7.1672 [DOI] [PubMed] [Google Scholar]
- Szabo-Reed AN, Vidoni E, Binder EF, Burns J, Cullum CM, Gahan WP, Gupta A, Hynan LS, Kerwin DR, Rossetti H, Stowe AM, Vongpatanasin W, Zhu DC, Zhang R, Keller JN (2019) Rationale and methods for a multicenter clinical trial assessing exercise and intensive vascular risk reduction in preventing dementia (rrAD Study). Contemp Clin Trials 79:44–54. 10.1016/j.cct.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbree J, Bronzwaer A, van Buchem MA, Daemen M, van Lieshout JJ, van Osch M (2017) Middle cerebral artery diameter changes during rhythmic handgrip exercise in humans. J Cereb Blood Flow Metab 37(8):2921–2927. 10.1177/0271678x16679419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Liu PY, Liao JK (2008) Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med 14(1):37–44. 10.1016/j.molmed.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Roe CM, Snyder AZ, Brier MR, Thomas JB, Xiong C, Benzinger TL, Morris JC, Ances BM (2012) Alzheimer disease family history impacts resting state functional connectivity. Ann Neurol 72(4):571–577. 10.1002/ana.23643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann M, Kiliaan AJ, Claassen JA (2013) Vascular aspects of cognitive impairment and dementia. J Cereb Blood Flow Metab 33(11):1696–1706. 10.1038/jcbfm.2013.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G (2000) Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol 57(10):1439–1443. 10.1001/archneur.57.10.1439 [DOI] [PubMed] [Google Scholar]
- Yamada M, Huang Z, Dalkara T, Endres M, Laufs U, Waeber C, Huang PL, Liao JK, Moskowitz MA (2000) Endothelial nitric oxide synthase-dependent cerebral blood flow augmentation by L-arginine after chronic statin treatment. J Cereb Blood Flow Metab 20(4):709–717. 10.1097/00004647-200004000-00008 [DOI] [PubMed] [Google Scholar]
- Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, Van Deerlin V, Lee VM, Trojanowski JQ, Arnold SE (2012) Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain 135(Pt 12):3749–3756. 10.1093/brain/aws271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Vongpatanasin W, Levine BD (2015) Faster brain shrinkage in the ACCORD MIND study: an unexpected result? JAMA Intern Med 175(1):144. 10.1001/jamainternmed.2014.6971 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data related to this manuscript will be available upon reasonable request to the corresponding author.