Abstract
The in vitro glycolipid binding specificity of clinical strains of nontypeable Haemophilus influenzae is altered to include sulfated glycolipids following a brief heat shock. We have constructed, expressed, and purified a recombinant protein of H. influenzae Hsp70, which showed significant specific binding to sulfated galactolipids in vitro. Furthermore, indirect immunofluorescence demonstrates that Hsp70 proteins are surface exposed in H. influenzae only after heat shock and are contained in the outer membrane protein fractions.
Haemophilus influenzae is a respiratory pathogen that colonizes the upper respiratory mucosal surfaces, especially the nasopharynx (25). The organism is able to cause otitis media, sinusitis, conjunctivitis, bronchitis, pneumonia, meningitis, epiglottitis, and cellulitis (11, 34).
Previously, we have shown that after heat shock treatment, clinical strains of nontypeable H. influenzae show a long-lasting change in the binding specificity for sulfogalactoglycolipids and a markedly enhanced growth rate in vitro (8). Sulfated glycolipids are present in a variety of mammalian tissues. High levels of sulfogalactosylceramide (SGC) are found in the respiratory tract (12), gastric mucosa (28), and kidney and brain (14). Sulfogalactosylglycerolipid (SGG) has been found as the major sulfated glycolipid of the mammalian male germ cells (27) and as a minor component of the mammalian brain tissues (10). Sulfogalactolipid receptor recognition by eukaryotic cell receptors has been implicated for mycoplasma (1, 12, 15, 18, 29), for heat-stressed H. influenzae (8) and acid-stressed Helicobacter pylori (9), and for the mechanism of sperm-egg binding (19).
As we have shown previously (8), cell binding to SGC and SGG can be inhibited by anti-Hsp70 antibody, which reacts with two protein bands at 82 and 62 kDa, indicating the presence of two members of the heat shock protein family Hsp70. The functions of Hsp70 proteins as chaperones aiming for the stabilization of the cell physiology under environmental stress conditions are well characterized (2, 7); however, their role as mediators of intercellular adhesion, under both normal and stress conditions, is less well recognized. Our previous data (8) imply that cell surface Hsp70-related heat shock proteins could potentially mediate H. influenzae attachment to sulfoglycolipids following heat shock. To begin to define the role of Hsp70 in H. influenzae adhesion and sulfatide recognition, we have generated and purified a recombinant Hsp70 protein, a DnaK homolog of H. influenzae, and we show preliminary data of glycolipid binding specificity of that recombinant protein.
Cloning and expression of the H. influenzae hsp70 (dnaK) gene.
All restriction enzymes, ligases, and their buffers were purchased from Gibco BRL (Burlington, Ontario, Canada) and Bio-Rad Labs (Richmond, Calif.). A 1.9-kbp PCR DNA fragment was amplified (Taq polymerase; Pharmacia Biotech, Baie D'urfe, Quebec, Canada), utilizing oligonucleotides dnaK5′ (5′-ATGGGAAAAATTATTGGTATTGACT-3′) and dnaK3′ (5′-TTTATTATCTTTCACTTCTTCAAAT-3′). The oligonucleotide specificity was determined according to the 5′ and 3′ regions containing the open reading frame of an hsp70 (dnaK) homolog of H. influenzae that was annotated HI1237 (6) and which corresponds to an 82-kDa protein. As template, chromosomal DNA of strain H. influenzae Rd (KW-20) was used. The PCR DNA fragment was ligated into a TA cloning vector (pCR II; Invitrogen). Plasmid construction was performed in Escherichia coli strain LE392 (22). Plasmid DNA was recovered, digested with the restriction enzyme EcoRI, and ligated into the complementary restriction enzyme site of the pTrcHisA expression vector (Invitrogen). Expression of the corresponding protein was carried out with E. coli strain INFα (Invitrogen).
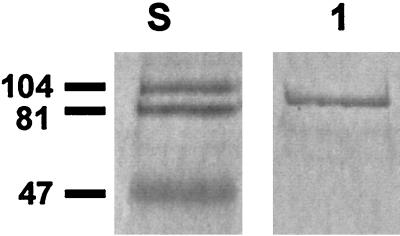
The recombinant N-terminal His6-tagged fusion protein was purified from the E. coli lysate under denaturing conditions by batch-gravity flow column purification nickel affinity chromatography (TALON metal affinity resin and TALON 2-ml disposable gravity column; Clontech, Palo Alto, Calif.), according to the user manual. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis of the purified protein showed a single protein band of 85 kDa, which corresponds to the 82-kDa protein of the two Hsp70-like proteins detected in cell extracts of nontypeable H. influenzae after heat shock as described previously (8). The His6 tag connected with the recombinant expressed DnaK accounts for an increase in apparent size of the protein (Fig. 1).
FIG. 1.
Western blotting (3, 13, 35) of the purified recombinant N-terminal His6-tagged fusion protein with monoclonal anti-bovine brain Hsp70 antibody (H 5147; Sigma). Lanes: S, prestained SDS-PAGE standards (sizes in kilodaltons), low range (Bio-Rad Labs); 1, purified recombinant N-terminal His6-tagged fusion protein (1 μg).
Glycolipid binding specificity.
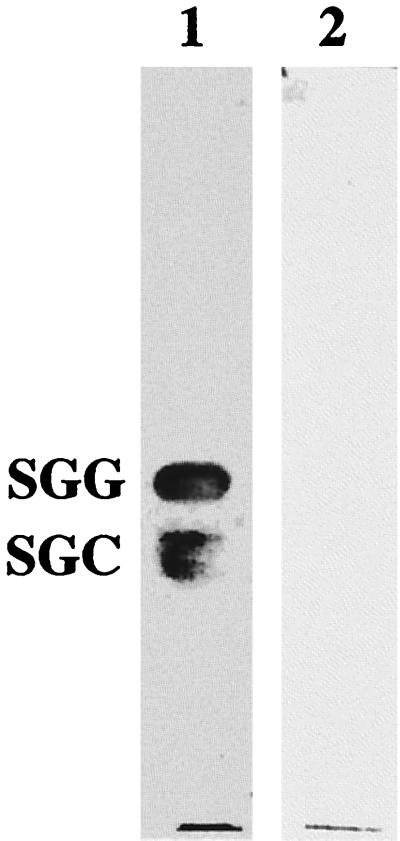
The glycolipid binding specificity of the purified Hsp70 protein is shown in Fig. 2. Phosphatidylethanolamine from soybeans and SGC from bovine brain tissue were obtained from Sigma (St. Louis, Mo.). Gangliosytetraocylceramide (Gg4), gangliotriosylceramide (Gg3), and SGG were prepared as described elsewhere (16, 17, 36). Thin-layer chromatography (TLC) overlay assays were performed as described in the legend of Fig. 2. The TLC overlay analysis demonstrated that purified recombinant Hsp70 can bind specifically to the sulfated galactolipids SGC and SGG, which are also recognized by both cell extracts and whole cells of H. influenzae (8). Neither phosphatidylethanolamine nor the neutral glycolipids Gg3 and Gg4 were bound. This result clearly shows that the 82-kDa Hsp70 (DnaK) protein of H. influenzae is one candidate responsible for the acquired ability of this organism to bind to sulfated galactolipids following heat shock. The role of a second Hsp70 protein (60 kDa) still has to be examined; however, we cannot presently exclude that this might represent a breakdown product of the DnaK gene product.
FIG. 2.
Binding specificity of the purified recombinant N-terminal His6-tagged fusion protein. Glycolipids (5 μg) were separated by TLC and incubated with purified recombinant N-terminal His6-tagged fusion proteins (5 μg ml−1), and binding was detected immunologically with polyclonal anti-GST70K antibody (Hsp70 from H. pylori) (4) (lane 1). As a negative control, a TLC overlay assay without the recombinant protein was performed, showing no signal (lane 2).
Cell surface localization of Hsp70 (DnaK).
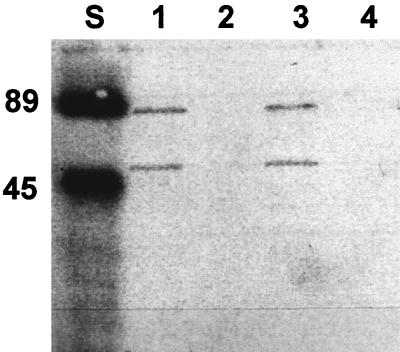
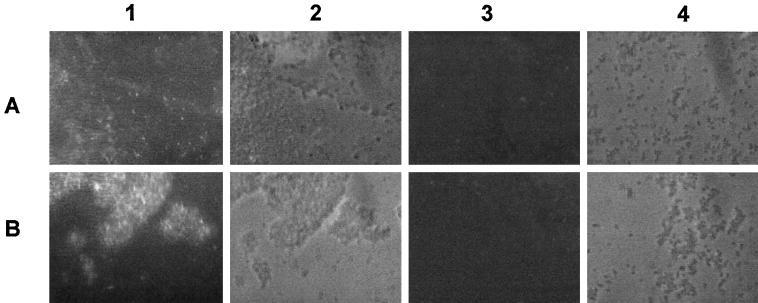
Outer membrane proteins (OMPs) were prepared as described by Carlone et al. (5) and modified by Reidl et al. (33), and OMPs were applied to H. influenzae strains Rd and nontypeable isolate 6564. OMP extracts of heat-shocked (5 min, 42°C) H. influenzae strain Rd and the clinical isolate of nontypeable 6564 were utilized in Western blot analysis with polyclonal anti-GST70K antibody. The results showed two protein bands at 60 and 82 kDa (Fig. 3), which match the previously described proteins in whole-cell extracts of nontypeable H. influenzae strains (8). No detectable Hsp70 bands appeared in OMP extracts of non-heat-shocked H. influenzae cells. To further evaluate the surface expression of H. influenzae Hsp70 proteins on whole cells, the proteins were monitored by immunofluorescence microscopy (Fig. 4). The surface expression of H. influenzae Hsp70 under post-heat shock conditions could be demonstrated by treating the bacteria with polyclonal anti-GST70K antibody (H. pylori Hsp70). A marked increase in fluorescent staining was seen following heat shock from a zero background compared to staining of non-heat-shocked organisms (Fig. 4). These studies clearly show that the Hsp70s of H. influenzae are exposed on the outer surface of the organisms after heat shock.
FIG. 3.
Hsp70 content of OMPs of two strains of H. influenzae following heat shock. Heat shock was performed as described before (8). OMPs of H. influenzae strain Rd and nontypeable isolate 6564 were separated on an SDS–12% polyacrylamide gel (13) and tested for the presence of Hsp70s by Western blotting (3, 35) with polyclonal anti-GST70K antibody (Hsp70 from H. pylori). Lanes: S, kaleidoscope prestained standard (Bio-Rad Labs); 1, strain 6564, heat shocked; 2, strain 6564, non-heat shocked; 3, strain Rd, heat shocked; 4, strain Rd, non-heat shocked. All samples were loaded at protein concentrations of about 1 μg.
FIG. 4.
Increased Hsp70 expression on the surface of H. influenzae cells following heat shock monitored by immunofluorescence. Post-heat-shocked and non-heat-shocked overnight cultures of H. influenzae were washed twice in phosphate-buffered saline (PBS) and then transferred to slides for drying. Subsequently, the bacteria were incubated for 30 min with polyclonal anti-GST70K antibody (H. pylori Hsp70), diluted 1:200 in PBS, washed several times with PBS, and incubated again for 30 min with Texas-Red-labeled goat anti-rabbit immunoglobulin G antibodies (1:150) (Dianova, Hamburg, Germany). The bacteria were washed twice with PBS and embedded with fluorescence dye (Citifluor, London, United Kingdom) for examination with a fluorescence microscope (Axioplan Zeiss). Organisms used were strain Rd (A) and nontypeable isolate 6564 (B). Lanes: 1, fluorescence, heat-shocked organisms; 2, phase contrast, heat-shocked organisms; 3, fluorescence, non-heat-shocked organisms; 4, phase contrast, non-heat-shocked organisms.
The results presented indicate that the native Hsp70 protein is contributing to the ability of H. influenzae cells to bind to sulfated galactolipids. We showed that Hsp70s are contained in the outer membrane extracts and are surface exposed on whole bacteria after heat shock induction. Since Hsp's lack a leader sequence or putative transmembrane regions, the mechanism by which they traverse the cytoplasmic membrane and remain membrane associated is unclear. Surface expression of Hsp's might result from a nonclassical type III secretory pathway in bacteria (23), autolysis and adsorption of cytoplasmic proteins on the surface (30), or coexport with other membrane proteins (31). Cell surface-exposed Hsp70s have been reported to be in a variety of prokaryotic cell (8, 9, 32) and eukaryotic cell (19, 24, 26) systems. Their function at the cell surface, however, has remained tenuous. Recent studies indicate that the Hsp70s are sulfogalactolipid-specific lectins, that sulfogalactolipid binding is conserved among Hsp70s (21), and that the binding site has been localized in the conserved, N-terminal ATPase-containing domain (20). These findings are confirmed by our study. The availability of our Hsp70 expression system will allow further investigations of the molecular basis of sulfatide binding. Since surface-displayed Hsp70s from both prokaryotic and eukaryotic organisms specifically bind sulfogalactolipids, this activity may provide a new target for intervention in bacterium-host interactions.
Acknowledgments
We thank D. Marmelak for technical assistance. Polyclonal anti-GST70K antibody (Hsp70 from H. pylori) and clinical strains of H. influenzae were kindly provided by the Department Pediatrics Laboratory Medicine, The Hospital for Sick Children, Toronto, Ontario, Canada.
This work was funded in part by BMBF grant 01KI8906 and MRC grant no. MT 14367 to C.A.L.
REFERENCES
- 1.Boulanger J, Faulds D, Eddy E M, Lingwood C A. Members of the 70 kDa heat shock protein family specifically recognize sulfoglycolipids: role in gamete recognition and mycoplasma-related infertility. J Cell Physiol. 1995;165:7–17. doi: 10.1002/jcp.1041650103. [DOI] [PubMed] [Google Scholar]
- 2.Bukau B, Horwich A L. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 3.Burnette W N. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 4.Busse J, Hartmann E, Lingwood C A. Receptor affinity purification of a lipid-binding adhesin from Haemophilus influenzae. J Infect Dis. 1997;175:77–83. doi: 10.1093/infdis/175.1.77. [DOI] [PubMed] [Google Scholar]
- 5.Carlone A C Y, Myrtle L T, Rumschlag H S, Sottner F O. Rapid microprocedure for isolating detergent-insoluble outer membrane proteins from Haemophilus species. J Clin Microbiol. 1986;24:330–332. doi: 10.1128/jcm.24.3.330-332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Frichman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 7.Hartl F U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann E, Lingwood C. Brief heat shock treatment induces a long-lasting alteration in the glycolipid receptor binding specificity and growth rate of Haemophilus influenzae. Infect Immun. 1997;65:1729–1733. doi: 10.1128/iai.65.5.1729-1733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huesca M, Borgia S, Hoffman P, Lingwood C A. Acidic pH changes receptor binding specificity of Helicobacter pylori: a binary adhesion model in which surface heat shock (stress) proteins mediate sulfatide recognition in gastric colonization. Infect Immun. 1996;64:2643–2648. doi: 10.1128/iai.64.7.2643-2648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishizuka I, Inomata M. Sulfated glycoglycerolipids in rat brain: decrease and disappearance after developmental age. J Neurochem. 1979;33:387–388. doi: 10.1111/j.1471-4159.1979.tb11749.x. [DOI] [PubMed] [Google Scholar]
- 11.Korones D N, Marshall G S, Shapiro E D. Outcome of children with occult bacteremia caused by Haemophilus influenzae type b. Pediatr Infect Dis J. 1992;11:516–520. doi: 10.1097/00006454-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Krivan H C, Olson L D, Barile M F, Ginsburg V, Roberts D D. Adhesion of Mycoplasma pneumoniae to sulfated glycolipids and inhibition by dextran sulfate. J Biol Chem. 1989;264:9283–9288. [PubMed] [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Lingwood C, Schachter H. Localization of sulfatoxygalactosylacylalkylglycerol at the surface of rat testicular germinal cells by immunocytochemical techniques: pH dependence of a nonimmunological reaction between immunoglobulin and germinal cells. J Cell Biol. 1981;89:621–630. doi: 10.1083/jcb.89.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lingwood C, Schramayr S, Quinn P. Male germ cell specific sulfogalactoglycerolipid is recognized and degraded by mycoplasmas associated with male infertility. J Cell Physiol. 1990;142:170–176. doi: 10.1002/jcp.1041420121. [DOI] [PubMed] [Google Scholar]
- 16.Lingwood C A, Murray R K, Schachter H. The preparation of rabbit antiserum specific for mammalian testicular sulfogalactoglycerolipid. J Immunol. 1980;124:769–774. [PubMed] [Google Scholar]
- 17.Lingwood C A, Nutikka A. A novel chemical procedure for the selective removal of nonreducing terminal N-acetyl hexosamine residues from glycolipids. Anal Biochem. 1994;217:119–123. doi: 10.1006/abio.1994.1091. [DOI] [PubMed] [Google Scholar]
- 18.Lingwood C A, Quinn P A, Wilansky S, Nutikka A, Ruhnke H L, Miller R B. Common sulfoglycolipid receptor for mycoplasmas involved in animal and human infertility. Biol Reprod. 1990;43:694–697. doi: 10.1095/biolreprod43.4.694. [DOI] [PubMed] [Google Scholar]
- 19.Mamelak D, Lingwood C. Expression and sulfogalactolipid binding specificity of the recombinant testis-specific cognate heat shock protein 70. Glycoconj J. 1997;14:715–722. doi: 10.1023/a:1018569417218. [DOI] [PubMed] [Google Scholar]
- 20.Mamelak D, Lingwood C A. The ATPase domain of Hsp70 possesses a unique binding specificity for 3′ sulfated galactolipids. J Biol Chem. 2001;276:449–456. doi: 10.1074/jbc.M006732200. [DOI] [PubMed] [Google Scholar]
- 21.Mamelak, D., M. Mylvaganam, H. Whetstone, E. Hartmann, W. Lennarz, P. B. Wyrick, J. Raulston, H. Han, P. Hoffmann, and C. A. Lingwood. Hsp70s contain a specific sulfoglycolipid binding site. Differential aglycone influence on sulfogalactosyl ceramide binding by recombinant prokaryotic and eukaryotic hsp70 family members. Biochemistry, in press. [DOI] [PubMed]
- 22.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 23.Michiels T, Wattiau P, Brasseur R, Ruysschaert J M, Cornelis G. Secretion of Yop proteins by yersiniae. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller D, Brough S, al-Harbi O. Characterization and cellular distribution of human spermatozoal heat shock proteins. Hum Reprod. 1992;7:637–645. doi: 10.1093/oxfordjournals.humrep.a137711. [DOI] [PubMed] [Google Scholar]
- 25.Moxon R E. Haemophilus influenzae. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, NY: Churchill Livingstone; 1995. pp. 2039–2045. [Google Scholar]
- 26.Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R. Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J Immunol. 1997;158:4341–4350. [PubMed] [Google Scholar]
- 27.Murray R K, Narasimhan R, Levine N, Pinteric L, Shirley M, Lingwood C A, Schachter H. Galactoglycerolipids of mammalian testis, spermatozoa and nervous tissue. In: Sweely E C, editor. Cell surface glycolipids. Washington, D.C.: American Chemical Society; 1980. pp. 105–125. [Google Scholar]
- 28.Natomi H, Saitoh T, Sugano K, Iwamori M, Fukayama M, Nagai Y. Systematic analysis of glycosphingolipids in the human gastrointestinal tract: enrichment of sulfatides with hydroxylated longer-chain fatty acids in the gastric and duodenal mucosa. Lipids. 1993;28:737–742. doi: 10.1007/BF02535996. [DOI] [PubMed] [Google Scholar]
- 29.Olson L D, Gilbert A A. Characteristics of Mycoplasma hominis adhesion. J Bacteriol. 1993;175:3224–3227. doi: 10.1128/jb.175.10.3224-3227.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phadnis S H, Parlow M H, Levy M, Ilver D, Caulkins C M, Connors J B, Dunn B E. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect Immun. 1996;64:905–912. doi: 10.1128/iai.64.3.905-912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raab L S, Polakoski K L, Hancock L W, Hamilton D W. Characterization of the heat shock protein P70 in rat spermatogenic cells. Mol Reprod Dev. 1995;40:186–195. doi: 10.1002/mrd.1080400207. [DOI] [PubMed] [Google Scholar]
- 32.Raulston J E, Davis C H, Paul T R, Wayrick P B. Heat shock protein 70 kDa and the chlamydial envelope: an entry level position? In: Stephens R S, Byrne G I, Christiansen M D, Clarke I N, Graystone J T, editors. Chlamydial infections. San Francisco, Calif: University of California—Berkeley Press; 1998. pp. 83–86. [Google Scholar]
- 33.Reidl J, Schloer S, Kraiss A, Schmidt-Brauns J, Kemmer G, Soleva E. NADP and NAD utilization in Haemophilus influenzae. Mol Microbiol. 2000;35:1573–1581. doi: 10.1046/j.1365-2958.2000.01829.x. [DOI] [PubMed] [Google Scholar]
- 34.Smith A L. Haemophilus influenzae. In: Schaechter G M M, Schlesinger D, editors. Mechanism of microbial disease. 2nd ed. Baltimore, Md: Williams & Wilkins; 1989. pp. 242–244. [Google Scholar]
- 35.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uemura K, Yuzawa M, Taketomi T. Preparation and properties of antisera to glycolipid of guinea pig erythrocyte membrane. J Biochem (Tokyo) 1978;83:1199–1201. doi: 10.1093/oxfordjournals.jbchem.a132011. [DOI] [PubMed] [Google Scholar]