Abstract
Background
In recent years, with the development of monitoring conditions and the application of pulmonary vascular-targeted drugs, pregnancy outcomes in women with pulmonary hypertension (PH) have improved, but the maternal mortality rate is still high. The purpose of this study was to describe the maternal-foetal outcomes in pregnant women with PH.
Methods
The clinical data of 154 pregnant women with PH who were admitted to the Third Affiliated Hospital of Guangzhou Medical University from January 2011 to December 2020 were collected and descriptively analysed.
Results
Among the 154 pregnant women with PH, 6 (3.9%) had idiopathic pulmonary arterial hypertension (iPAH), 41 (26.6%) had pulmonary arterial hypertension (PAH) associated with congenital heart disease (CHD-PAH), 45 (29.2%) had PAH related to other diseases (oPAH), and 62 (40.3%) had PH related to left heart disease (LHD-PH). The systolic pulmonary artery pressure (sPAP) was 36–49 mmHg in 53.2% of the patients, 50–69 mmHg in 22.1% of the patients and ≥ 70 mmHg in 24.7% of the patients. Five (3.2%) pregnant women died within 1 week after delivery; iPAH patients had the highest mortality rate (3/6, 50%). Fifty-four patients (35.1%) were admitted to the intensive care unit (ICU), and the incidence of heart failure during pregnancy was 14.9%. A total of 70.1% of the patients underwent caesarean section; 42.9% had premature infants; 28.6% had low-birth-weight (LBW) infants; 13.0% had very-low-birth-weight (VLBW) infants; 3.2% had extremely-low-birth-weight (ELBW) infants; 61% had small for gestational age (SGA) infants; and 1.9% experienced neonatal mortality.
Conclusion
There were significant differences in the maternal-foetal outcomes in the iPAH, CHD-PAH, oPAH and LHD-PH groups. Maternal mortality was highest in the iPAH group; therefore, iPAH patients should be advised to prevent pregnancy. Standardized and multidiscipline-assisted maternal management is the key to improving maternal-foetal outcomes.
Keywords: Pregnancy, Pulmonary hypertension, Maternal-foetal outcome
Introduction
Pulmonary hypertension (PH) refers to a clinical and pathophysiological syndrome with the pulmonary vascular resistance and pulmonary arterial pressure increase due to changes in pulmonary vascular structure and function, which are caused by a variety of heterologous diseases (causes) and different pathogenic mechanisms. Patients with PH are at high risk of right heart failure and even death [1, 2]. The prevalence of PH in women is 97 cases per million [3, 4]. Although the incidence of PH in pregnancy is very low, the mortality rate of pregnant women and foetuses is extremely high. Some studies have reported that, previously, without advanced pulmonary vascular targeting drugs, the mortality rate of pregnant women with PH was as high as 30–56% [5, 6]. The preterm birth rate was 85–100%, and the foetal or neonatal loss rate was 7–13% [7, 8]. Pulmonary arterial hypertension (PAH) in pregnancy threatens the health of pregnant women and foetuses.
Women with PH have haemodynamic changes during pregnancy, such as an increased total blood volume, an increased cardiac output, a faster heart rate, reduced systemic vascular resistance, and hypercoagulable blood. These physiological changes increase the risk of refractory right heart failure, PH crisis, pulmonary embolism, and even death [9]. The European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines on the diagnosis and treatment of PH have always recommended contraception or early termination of pregnancy [3, 7]. However, during clinical work, we still face many women with PH who choose pregnancy or experience unplanned pregnancies. Especially in recent years, with the change in our country’s family planning policy and individual choices, the number of elderly pregnant women and high-risk pregnant women has increased, which brings great challenges. Thus, we have paid increasing attention to the aetiology, management, and treatment of pregnant patients with PH. Hence, by retrospectively analysing the medical records of 154 pregnant patients with PH who were admitted to the Third Affiliated Hospital of Guangzhou Medical University in the past 10 years, we aimed to explore the aetiology and pregnancy outcome in women with PH and provide clinical evidence for the management of such high-risk pregnant women.
Materials and methods
Clinical information
This study identified 154 pregnant women with PH who were admitted to the Third Affiliated Hospital of Guangzhou Medical University from January 2011 to December 2020 as the research subjects (Fig. 1). The clinical data, including cardiac function, systolic pulmonary artery pressure (sPAP), regular obstetric examinations, birth method, anaesthesia method, postpartum complications, foetal or neonatal status and other indicators, were analysed. In this study, it was inevitable that some clinical data would be incomplete; missing data were supplemented by telephone follow-up.
Fig. 1.
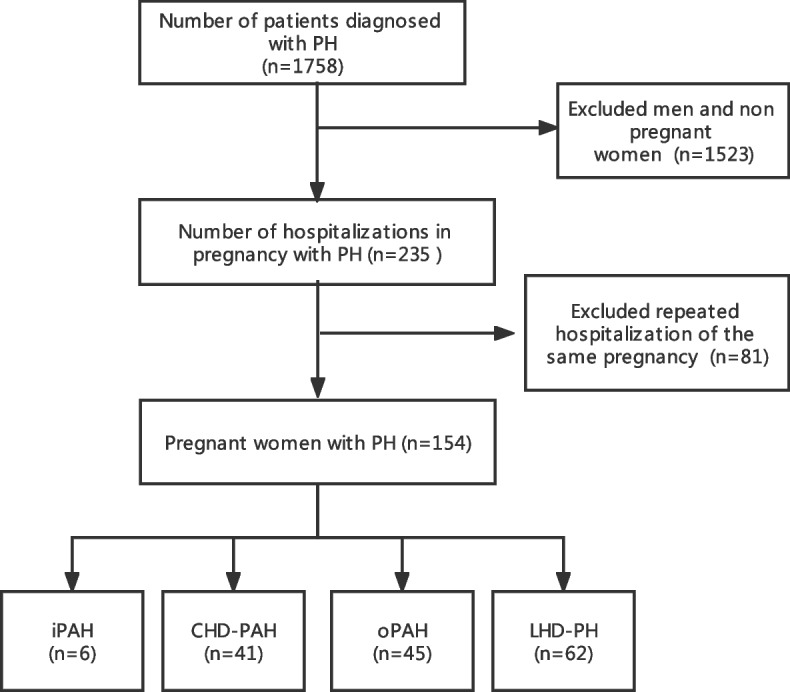
Flow chart of the study. iPAH, idiopathic pulmonary arterial hypertension; CHD-PAH, pulmonary arterial hypertension associated with congenital heart disease; oPAH, pulmonary arterial hypertension associated with other diseases; LHD-PH, pulmonary hypertension caused by left heart disease
Diagnosis, inclusion and exclusion criteria of PH
According to the diagnostic criteria of the “2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension”, the haemodynamic diagnostic criteria are that the mean pulmonary artery pressure (mPAP) measured by right heart catheterization at sea level and at rest ≥25 mmHg, pulmonary artery wedge pressure (PAWP) ≤15 mmHg and pulmonary vascular resistance (PVR) > 3 Wood units; the diagnostic criteria for idiopathic pulmonary arterial hypertension (iPAH) should not only meet the PAH criteria diagnosis but also exclude a family history of PAH and all possible secondary factors that may cause PAH [10]. Strict diagnostic criteria should be based on right heart catheterization data. However, considering that the right cardiac catheterization is an invasive examination and the particularity of pregnant women, it is impossible to routinely perform cardiac catheterization to obtain pulmonary artery pressure in pregnant women.
In obstetrics, the sPAP is often indirectly evaluated by the tricuspid regurgitation pressure difference under cardiac colour Doppler ultrasound, and an sPAP ≥36 mmHg (1 mmHg = 0.133 kPa) is used as the diagnostic standard of PH [10]. In this study, the inclusion criteria were as follows: all subjects had undergone cardiac echocardiography, which met the diagnostic criteria of echocardiography, been transferred to the ICU for hemodynamic monitoring during the perinatal period, or had a clinical diagnosis of PH that was confirmed by right cardiac catheterization in the cardiovascular department after delivery [11]. The exclusion criteria were as follows: patients with an elevated sPAP caused by outflow tract obstruction/pulmonary stenosis.
In this study, the timing of pregnancy termination and the mode of delivery of the study subjects were determined by a multidisciplinary team, which comprised experts in obstetrics, critical care medicine, anaesthesiology, and cardiovascular medicine. After discussion, they selected the timing and mode of delivery that was most conducive to the maternal and foetal outcomes based on the level of pulmonary artery pressure, cardiac function, and foetal conditions of the pregnant women.
PH subgroups
According to the recent expert consensus, PH can be divided on the basis of its aetiology into idiopathic pulmonary arterial hypertension (iPAH), congenital heart disease-related pulmonary arterial hypertension (CHD-PAH), pulmonary arterial hypertension caused by other diseases (oPAH) and PH due to left heart disease (LHD-PH) [2, 10, 12]. Conditions involving LHD-PH include left ventricular systolic or diastolic dysfunction and valve disease. The other diagnoses mentioned in the consensus document were not present in our study population. According to the severity of sPAP, patients with an sPAP of 36–49 mmHg had mild PH, those with an sPAP of 50–69 mmHg had moderate PH, and those with an sPAP ≥70 mmHg had severe PH [8, 11].
Statistical methods
All analyses were performed using Free Statistics software (version 1.4) (www.freestatistics.tk) and SPSS statistical software (version 25.0) (SPSS Inc., Chicago, IL, USA). Count data are described by frequencies and percentages, and the statistical differences among groups were compared by χ2 tests or Fisher’s exact tests. The Shapiro-Wilk (S-W) method was used to test measurement data with a normal distribution; if the data conformed to a normal distribution, which are represented by the mean ± standard deviation (SD), the mean difference among groups was tested by one-way ANOVA, and the LSD-t test was used for pairwise multiple comparisons. If the variance was not uniform, the Dunnett-t test was used. If the measurement data did not conform to a normal distribution, which are expressed by the median (interquartile interval), the mean difference among groups was tested by the rank sum (Kruskal-Wallis H) test, and the Nemenyi method was used for pairwise multiple comparisons. A P value of < 0.05 was considered statistically significant (two-sided test).
Results
Baseline characteristics
A total of 1758 patients with PH were included in the analysis of the earlier study. After step-by-step exclusion, 154 pregnant women were ultimately identified as the study subjects. (Fig. 1).
In the iPAH group, 6 patients (3.9%) had a median sPAP value of 107.0 mmHg (Q1-Q3 = 78.8–125.5). In the CHD-PAH group, 41 patients (26.6%) had a median sPAP value of 59.0 mmHg (Q1-Q3 = 39.0–92.0). Among them, 25 patients were diagnosed with atrial septal defects, 9 were diagnosed with ventricular septal defects, 4 were diagnosed with patent ductus arteriosus, 1 was diagnosed with atrioventricular septal defects, 1 was diagnosed with congenital single ventricle and 1 was diagnosed with congenital single atrium. In the 54 patients (29.2%) in the oPAH group, the median sPAP value was 40.0 mmHg (Q1-Q3 = 36.0–49.0); 12 women were diagnosed with SLE and 3 women were diagnosed with typical or atypical antiphospholipid antibody syndrome. Among the 62 patients (40.3%) with LHD-PH, the median sPAP value was 50.0 mmHg (Q1-Q3 = 38.2–62.8). There were 38 pregnant women diagnosed with heart valve disease, 6 pregnant women diagnosed with myocardial disease, 15 pregnant women diagnosed with preeclampsia and 3 pregnant women diagnosed with chronic hypertension (Table 1).
Table 1.
Classification of pregnant women with PH
| Aetiology of PH | n | % |
|---|---|---|
| iPAH | 6 | 3.90% |
| CHD-PAH | 41 | 26.60% |
| Atrial septal defect | 25 | 16.20% |
| Ventricular septal defect | 9 | 5.80% |
| Atrioventricular septal defect | 1 | 0.60% |
| Patent ductus arteriosus | 4 | 2.60% |
| Congenital single atrium | 1 | 0.60% |
| Congenital single ventricle | 1 | 0.60% |
| oPAH | 45 | 29.20% |
| Systemic lupus erythematosus | 12 | 7.80% |
| Typical or atypical antiphospholipid antibody syndrome | 3 | 1.90% |
| Hyperthyroidism | 6 | 3.90% |
| Gestational diabetes mellitus | 10 | 6.50% |
| Unknown cause | 14 | 9.10% |
| LHD-PH | 62 | 40.30% |
| Heart valve disease | 38 | 25.30% |
| Cardiomyopathy | 6 | 3.90% |
| Preeclampsia | 15 | 9.70% |
| Chronic hypertension | 3 | 1.90% |
iPAH idiopathic pulmonary arterial hypertension; CHD-PAH pulmonary arterial hypertension associated with congenital heart disease; oPAH pulmonary arterial hypertension associated with other diseases; LHD-PH pulmonary hypertension caused by left heart disease
The ages of the subjects ranged from 21 to 46 years, and their average age was 30.9 ± 5.1 years. The gestational weeks during hospitalization ranged from 6 to 41 weeks, with a median of 35.5 weeks (Q1-Q3 = 31.0–38.0). A total of 129 women (83.8%) had regular obstetric examinations, but there were still 25 women (16.2%) who lacked regular obstetric examinations during pregnancy. There was a statistically significant difference between women who received regular obstetric examinations and those who received no obstetric examinations (P < 0.05). Fifty-four pregnant women were transferred to the intensive care unit because of critical conditions, accounting for 35.1% of the total study sample. The iPAH group had the highest intensive care unit (ICU) admission rate (83.3%), followed by the LHD-PH group (37.1%) and then the CHD-PAH group (36.6%). The oPAH group had the lowest ICU admission rate (24.4%), and the difference was statistically significant (P < 0.05). The overall hospital length of stay for the pregnant women ranged from 1 to 44 days, with a median value of 9 days (Q1-Q3 = 6.0–13.0). There were 82 patients (53.2%) in the mild PH group, 34 patients (22.1%) in the moderate PH group and 38 patients (24.7%) in the severe PH group (Table 2).
Table 2.
Baseline characteristics of the participants
| Covariates | Total (n = 154) | PH groups | P-value | |||
|---|---|---|---|---|---|---|
| iPAH (n = 6) |
CHD-PAH (n = 41) |
oPAH (n = 45) |
LHD-PH (n = 62) |
|||
| Age, mean ± SD | 30.9 ± 5.1 | 28.7 ± 4.5 | 30.6 ± 4.8 | 30.9 ± 4.6 | 31.5 ± 5.7 | 0.539 |
| Gestational week, Median (IQR) | 35.5 (31.0, 38.0) | 34.5 (30.2, 36.5) | 36.0 (30.0, 38.0) | 36.0 (29.0, 39.0) | 35.0 (32.0, 38.0) | 0.958 |
| Primipara, n (%) | 99 (64.3) | 2 (33.3) | 24 (58.5) | 28 (62.2) | 45 (72.6) | 0.175 |
| Multiparous, n (%) | 46 (29.9) | 4 (66.7) | 14 (34.1) | 13 (28.9) | 15 (24.2) | 0.173 |
| sPAP, Median (IQR) | 47.0 (37.0, 67.2) | 107.0 (78.8, 125.5) | 59.0 (39.0, 92.0) | 40.0 (36.0, 49.0) | 50.0 (38.2, 62.8) | < 0.001 |
| sPAP, n (%) | < 0.001 | |||||
| 36–49 mmHg | 82 (53.2) | 1 (16.7) | 16 (39) | 35 (77.8) | 30 (48.4) | |
| 50–69 mmHg | 34 (22.1) | 0 (0) | 7 (17.1) | 8 (17.8) | 19 (30.6) | |
| ≥ 70 mmHg | 38 (24.7) | 5 (83.3) | 18 (43.9) | 2 (4.4) | 13 (21) | |
| Place of residence, n (%) | 0.128 | |||||
| City | 79 (51.3) | 1 (16.7) | 19 (46.3) | 21 (46.7) | 38 (61.3) | |
| rural | 75 (48.7) | 5 (83.3) | 22 (53.7) | 24 (53.3) | 24 (38.7) | |
| Regular obstetric examinations, n (%) | 0.038 | |||||
| NO | 25 (16.2) | 3 (50) | 10 (24.4) | 5 (11.1) | 7 (11.3) | |
| YES | 129 (83.8) | 3 (50) | 31 (75.6) | 40 (88.9) | 55 (88.7) | |
| NYHA cardiac function classification, n (%) | < 0.001 | |||||
| I | 82 (53.2) | 2 (33.3) | 16 (39) | 36 (80) | 28 (45.2) | |
| II | 30 (19.5) | 0 (0) | 15 (36.6) | 2 (4.4) | 13 (21) | |
| III | 19 (12.3) | 1 (16.7) | 9 (22) | 0 (0) | 9 (14.5) | |
| IV | 23 (14.9) | 3 (50) | 1 (2.4) | 7 (15.6) | 12 (19.4) | |
| Length of stay (days), Median (IQR) | 9.0 (6.0, 13.0) | 6.5 (2.0, 14.0) | 8.0 (5.0, 11.0) | 8.0 (5.0, 15.0) | 10.0 (7.0, 12.8) | 0.279 |
NYHA New York Heart Association. ICU Intensive Care Unit
Pregnancy outcomes
Maternal outcomes
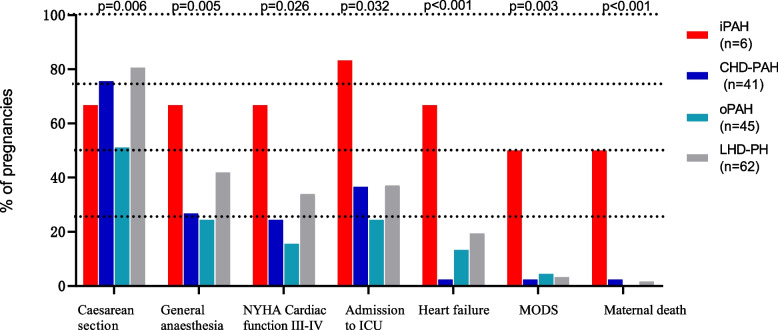
In this study, 29 (18.8%) patients delivered vaginally and 108 (70.1%) delivered by caesarean section. The proportion of caesarean sections was significantly higher than that of vaginal deliveries. Caesarean section is the main mode of delivery for pregnant women with PH. Among the patients who terminated their pregnancy by caesarean section, 52 (33.8%) were selected for general anaesthesia, and 56 (36.4%) were selected for intraspinal anaesthesia. In this study, 26 (16.9%) pregnant women had uterine asthenia after delivery, 23 (14.9%) had heart failure during the perinatal period, 8 (5.2%) had multiple organ dysfunction syndrome (MODS), and 1 (0.6%) had cardiac arrest during the perinatal period, but the rescue was timely and effective, saving her life (Table 3).
Table 3.
Maternal and foetal outcomes
| Covariates | PH groups | P-value | ||||
|---|---|---|---|---|---|---|
| Total (n = 154) | iPAH (n = 6) |
CHD-PAH (n = 41) |
oPAH (n = 45) |
LHD-PH (n = 62) |
||
| Maternal outcomes | ||||||
| Mode of delivery, n (%) | 0.006 | |||||
| Caesarean section | 108 (70.1) | 4 (66.7) | 31 (75.6) | 23 (51.1) | 50 (80.6) | |
| Vaginal delivery | 29 (18.8) | 2 (33.3) | 3 (7.3) | 16 (35.6) | 8 (12.9) | |
| Way of anaesthesia, n (%) | 0.005 | |||||
| General anaesthesia | 52 (33.8) | 4 (66.7) | 11 (26.8) | 11 (24.4) | 26 (41.9) | |
| Spinal anaesthesia | 56 (36.4) | 0 (0) | 20 (48.8) | 12 (26.7) | 24 (38.7) | |
| Complications | ||||||
| NYHA cardiac function III-IV | 42 (27.3) | 4 (66.7) | 10 (24.4) | 7 (15.6) | 21 (33.9) | 0.026 |
| Admission to ICU, n (%) | 54 (35.1) | 5 (83.3) | 15 (36.6) | 11 (24.4) | 23 (37.1) | 0.032 |
| Postpartum haemorrhage, n (%) | 16 (10.4) | 1 (16.7) | 3 (7.3) | 8 (17.8) | 4 (6.5) | 0.197 |
| Uterine fatigue, n (%) | 26 (16.9) | 1 (16.7) | 5 (12.2) | 10 (22.2) | 10 (16.1) | 0.642 |
| Heart failure, n (%) | 23 (14.9) | 4 (66.7) | 1 (2.4) | 6 (13.3) | 12 (19.4) | < 0.001 |
| MODS, n (%) | 8 (5.2) | 3 (50) | 1 (2.4) | 2 (4.4) | 2 (3.2) | 0.003 |
| Cardiac arrest, n (%) | 1 (0.6) | 0 (0) | 0 (0) | 1 (2.2) | 0 (0) | 0.607 |
| Maternal death, n (%) | 5 (3.2) | 3 (50) | 1 (2.4) | 0 (0) | 1 (1.6) | < 0.001 |
| Foetal outcome | ||||||
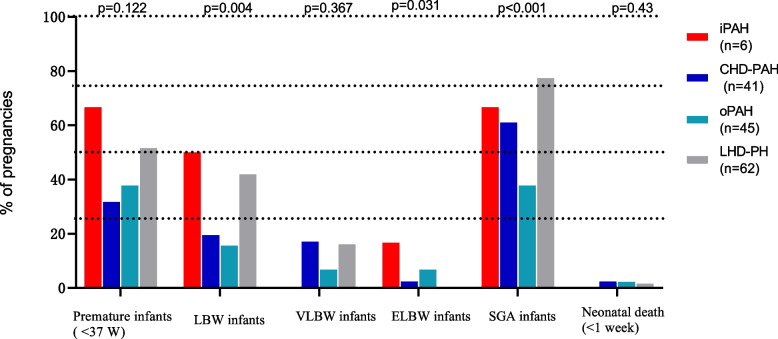
| Premature infants (< 37 weeks), n (%) | 66 (42.9) | 4 (66.7) | 13 (31.7) | 17 (37.8) | 32 (51.6) | 0.122 |
| (34 ~ 37 weeks), n (%) | 30 (19.5) | 2 (33.3) | 4 (9.8) | 7 (15.6) | 17 (27.4) | 0.088 |
| (< 34 weeks), n (%) | 36 (23.4) | 2 (33.3) | 9 (22) | 10 (22.2) | 15 (24.2) | 0.897 |
| Birth weight of the newborn, mean ± SD | 2334.9 ± 814.6 | 2124.0 ± 837.4 | 2341.3 ± 799.3 | 2522.6 ± 974.0 | 2243.8 ± 718.8 | 0.374 |
| LBW, n (%) | 44 (28.6) | 3 (50) | 8 (19.5) | 7 (15.6) | 26 (41.9) | 0.004 |
| VLBW, n (%) | 20 (13.0) | 0 (0) | 7 (17.1) | 3 (6.7) | 10 (16.1) | 0.367 |
| ELBW, n (%) | 5 (3.2) | 1 (16.7) | 1 (2.4) | 3 (6.7) | 0 (0) | 0.031 |
| SGA, n (%) | 94 (61.0) | 4 (66.7) | 25 (61) | 17 (37.8) | 48 (77.4) | < 0.001 |
| Therapeutic abortion | 15 (9.7) | 0 (0) | 7 (17.1) | 5 (11.1) | 3 (4.8) | 0.204 |
| Intrauterine death, n (%) | 7 (4.5) | 0 (0) | 1 (2.4) | 5 (11.1) | 1 (1.6) | 0.132 |
| Missed abortion, n (%) | 1 (0.6) | 0 (0) | 0 (0) | 1 (2.2) | 0 (0) | 0.589 |
| Neonatal death (< 1 week), n (%) | 3 (1.9) | 0 (0) | 1 (2.4) | 1 (2.2) | 1 (1.6) | 0.43 |
LBW low birth weight (> 1500 g, < 2500 g); VLBW very low birth weight (1000–1500 g); ELBW extremely low birth weight (< 1000 g); SGA small for gestational age; MODS multiple organ dysfunction syndrome
In this study, 149 patients (96.8%) survived, and 5 patients (3.2%) died. There were no deaths during pregnancy. All 5 women died in the postpartum period. Maternal details are shown in Table 4. None of the 5 women who died had PH diagnosed before pregnancy; all of them were diagnosed with PH during pregnancy and hospitalization. They did not have regular obstetric examinations before admission and did not pay enough attention to their own health management during pregnancy. The New York Heart Association (NYHA) cardiac function classification of the pregnant women who died was IV at admission. The maternal mortality rate was the highest in the iPAH group (3/6, 50%), followed by the CHD-PAH group (1/41, 2.4%) and the LHD-PH group (1/62, 1.6%). Among the six pregnant women in the iPAH group, only 3 improved because they were treated with targeted drugs (Treprostinil) in the perioperative period, which helped dilate pulmonary blood vessels and reduce the cardiac load and pulmonary arterial pressure. However, the other 3 pregnant women in the iPAH group (patients 1, 2, and 4) who died were all in critical condition and transferred from other hospitals. They did not undergo regular prenatal examinations during pregnancy. Due to postpartum haemorrhage complicated with the induction of a PH crisis, even though the patients were under positive rescue, their disease still could not be reversed, and they died. Another case of LHD-PH occurred in a pregnant woman who presented heart valve disease complicated by thrombosis, cerebrovascular embolism, sepsis, etc., and then multiple organ dysfunction. Ultimately, she died of respiratory and circulatory failure on the 6th day after a miscarriage operation; another pregnant woman with CHD-PAH who died (patient 5) suffered from massive haemorrhage and respiratory and circulatory failure after vaginal delivery in a primary hospital. She was in critical condition when transferred from another hospital and died before targeted drug therapy was performed (Table 4).
Table 4.
Clinical characteristics of the 5 pregnant women who died
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Age (years) | 35 | 32 | 32 | 30 | 25 |
| Gestational weeks | 39+ 1 | 34 | 16 | 29 | 30 |
| Causes of disease | iPAH | iPAH | LHD-PH | iPAH | CHD-PAH |
| PH diagnosed during pregnancy | NO | NO | NO | NO | NO |
| sPAP (mmHg) | 75 | 152 | 43 | 90 | 148 |
| Parity | G2P2 | G1P1 | G1P0 | G3P2 | G1P1 |
| Regular prenatal examinations | NO | NO | NO | NO | NO |
| NYHA cardiac function classification | IV | IV | IV | IV | IV |
| Complication | Postpartum haemorrhage | Postpartum haemorrhage | Respiratory and circulatory failure | Postpartum haemorrhage | Respiratory and circulatory failure |
| Foetal outcome | Live birth | Live birth | Iatrogenic abortion | Live birth | Foetal death |
| Time of maternal death | 2 days postpartum | 7 days postpartum | 6 days postpartum | 7 days postpartum | 2 days postpartum |
Maternal outcomes were compared in the iPAH, CHD-PAH, oPAH, and LHD-PH groups (Table 3 and Fig. 2). There were significant differences in the delivery mode and anaesthesia mode among the four groups (p < 0.05). NYHA cardiac function classifications of III-IV, admission to the ICU, heart failure, multiple organ dysfunction syndrome (MODS) and maternal death all showed significant differences (P < 0.05). However, there was no significant difference in postpartum haemorrhage (P > 0.05).
Fig. 2.
Maternal outcomes in iPAH, CHD-PAH, OPAH, and LHD-PH groups. MODS: multiple organ dysfunction syndrome. NYHA: New York Heart Association. ICU: Intensive Care Unit
Foetal outcomes
Among the 154 foetuses of the pregnant patients with PH, 66 (42.9%) were preterm infants (< 37 weeks), including 30 (19.5%) preterm infants born at 34–37-weeks and 36 (23.4%) preterm infants born at < 34 weeks, and the foetal birth weight was 2334.9 ± 814.6 g. There were 44 infants (28.6%) with low birth weight (LBW) (> 1500 g, < 2500 g), 20 infants (13%) with very low birth weight (VLBW) (1000 g–1500 g), 5 infants (3.2%) with extremely low birth weight (ELBW) (< 1000 g), and 94 infants (61.0%) who were small for gestational age (SGA). Three (1.9%) neonates died within 1 week after birth; their mothers were diagnosed with congenital heart disease, rheumatic heart disease and systemic lupus erythematosus. There were 15 cases of therapeutic abortion, accounting for 9.7% of the foetuses. There were 7 cases (4.5%) of intrauterine death, and the average gestational age was (27.7 ± 4.8) weeks; there was 1 case (0.65%) of missed abortion, and the gestational age was 14 weeks (Table 3). Foetal outcomes were compared in the iPAH, CHD-PAH, oPAH, and LHD-PH groups. As shown in Table 3 and Fig. 3, LBW, ELBW and SGA were significantly different (P < 0.05) among the groups. However, there were no significant differences in premature infants (< 37 weeks), therapeutic abortion, intrauterine death, and neonatal death (< 1 week) among the four groups (P > 0.05).
Fig. 3.
Foetal outcomes in the iPAH, CHD-PAH, OPAH, and LHD-PH groups. LBW: low birth weight (> 1500 g, < 2500 g); VLBW: very low birth weight (1000–1500 g); ELBW: extremely low birth weight (< 1000 g); SGA: small for gestational age; MODS: multiple organ dysfunction syndrome
Discussion
This was a single-centre retrospective study in southern China, analysing 154 pregnant women regarding maternal, foetal or neonatal complications of PH. Previous studies reported that the median length of survival of PH patients was only 2.8 years [13]. With the application of targeted drug therapy in recent years, the prognosis of PAH patients has been significantly improved. However, some studies have shown that the mortality rate of patients with PH is still as high as 13–18% in the first year [14]. In 2016, Karen Sliwa reported that the mortality rate of pregnant women with pulmonary hypertension within 1 week after delivery was 3.3% [8]. In our study, the 1-week postpartum mortality rate among pregnant women with PH was 3.2%. However, our study was based on data from a single centre and has limitations.
The mechanisms of PH caused by different aetiologies are different, and the treatment and prognosis are also different. Left heart diseases are acquired heart diseases; congenital left heart diseases and acquired heart diseases are the most common causes of PH. Hypoxia in patients with CHD-PAH is a chronic process that is well tolerated by the body, and most of these individuals can adapt to the haemodynamic changes after pregnancy. Left heart diseases are acquired heart diseases; the physiological blood volume increases during pregnancy and is more likely to induce heart failure in patients with LHD-PH, but with timely control of blood volume, most patients can avoid sudden death [12, 15]. Moreover, patients with pre-existing CHD and heart valve disease before pregnancy should focus on health management and regular prenatal examinations during pregnancy. It is easy to achieve early detection and early diagnosis of pregnancy with PH. With early intervention, the prognosis is mostly good. The patients in the oPAH group were generally mildly ill, and the sPAP was usually mild to moderate. For example, the prognosis of patients with systemic lupus erythematosus-related PH is usually better after glucocorticoid pulse therapy and immunosuppressive therapy [16]. In this study, the iPAH group had the worst outcomes, with a mortality rate of 50%. IPAH is caused by multiple factors leading to vasoconstriction, vascular reconstruction and the formation of in situ thrombosis, resulting in haemodynamic and pathophysiological changes, poor cardiac function, and an extremely poor prognosis [17].
Heart failure is a serious complication in pregnant women with PH. Haemodynamic changes in pregnant women are an important reason for the increased risk during pregnancy in this population. First, the blood pressure decreases in early pregnancy but increases in late pregnancy, and this fluctuation of blood pressure increases the risk of adverse pregnancy outcomes; second, with the increase in gestational age, the total blood volume and cardiac output of pregnant women will also gradually increase [13]. The median gestational age of the 5 patients who died was 30 weeks, and the average time of death was 4.8 days after delivery. However, during the 32–34 weeks of pregnancy, the period of delivery and the postpartum period is the period with the most obvious changes in maternal blood volume and haemodynamics, and it is also the period when pregnant women with PH are at high risk of heart failure, pulmonary hypertensive crisis or sudden death [18]. Therefore, for patients with cardiac function classifications of III-IV, our multidisciplinary team suggests that that the pregnancy be terminated at an early stage. Caesarean section should be selected to terminate pregnancy in the middle and late trimesters. Caesarean section can end delivery in a short time, avoid hemodynamic changes caused by prolonged uterine contractions, and reduce the increase in oxygen consumption caused by fatigue and pain.
During normal pregnancy in healthy women, although the blood circulation volume increases, the mean pulmonary arterial pressure (mPAP) does not change significantly, which may be due to pulmonary vasodilation to reduce vascular resistance and thereby adapt to this physiological change [19]. However, in pregnant women with PH, the pulmonary vasodilation capacity decreases, while the pulmonary vascular resistance increases, the pulmonary artery pressure increases, and the blood flow perfusion to the lung decreases, the effect on placental blood flow is unclear [8]. Compared with those of healthy women, foetuses of mothers with CHD are at greater risk of preterm birth and LBW [20]. Foetal and neonatal complications are increased in pregnant women with PH, and the rate of foetal death and neonatal mortality are approximately 10% [8]. Our study showed that the higher the pulmonary arterial pressure was, the higher the rate of LBW infants, and the incidence of LBW infants was higher in the iPAH and LHD-PH groups than in the other two groups. The long-term effects of maternal chronic hypoxia on foetal outcomes are unclear, but the literature reports that LBW infants are at increased risk for future complications such as heart disease, diabetes, and hypertension [21].
Limitations
This study was a single-centre retrospective investigation conducted in China, with a limited sample of the study populations, and there may be bias in the population representation. Second, since our study population consisted of pregnant women cardiac colour Doppler ultrasonography, but not heart catheterization, was used as a diagnostic tool. This is a common limitation of similar studies.
Conclusion
There were significant differences in maternal and foetal outcomes in the iPAH, CHD-PAH, oPAH and LHD-PH groups. Maternal mortality was highest in the iPAH group. Therefore, iPAH patients should avoid pregnancy; for high-risk pregnant women who insist on continuing pregnancy, we recommend they see a doctor as soon as possible and standardize treatment. At the same time, we suggest that comprehensive hospitals should establish multidisciplinary assistance teams, including obstetrics, cardiovascular medicine, critical medicine, anaesthesiology and neonatology specialists. Clinicians must balance the pros and cons of maternal and foetal outcomes and choose the most appropriate treatment plan to improve maternal and foetal pregnancy outcomes.
Acknowledgements
Not applicable.
Authors’ contributions
CTL and YWH were responsible for analysing all the data and writing the manuscript. GYL, LCW, and DJC participated in the design of the study and the analysis of the patients. YMG conceived of the whole study and carried out the study design and correction of the manuscript. CTL and YWH contributed equally to this work. All authors read and approved the final manuscript.
Funding
The publication cost of this article is sponsored by the Natural Science Foundation of Guangdong Province (2017A030313857). This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The raw data supporting this study can be requested from the corresponding author.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of The Third Affiliated Hospital of Guangzhou Medical University. Written informed consent was obtained from patients or their representatives for participation in this study. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chengtian Lv and Yuwen Huang contributed equally to this work.
References
- 1.Galiè N, McLaughlin VV, Rubin LJ, Simonneau G. An overview of the 6th world symposium on pulmonary hypertension. Eur Respir J. 2019;53(1):1802148. doi: 10.1183/13993003.02148-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galiè N, Humbert M, Vachiery J, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2015;46(4):903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk NA, Beghetti M, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 4.Meng M, Landau R, Viktorsdottir O, Banayan J, Grant T, Bateman B, Smiley R, Reitman E. Pulmonary hypertension in pregnancy: a report of 49 cases at four tertiary north. Obstet Gynecol. 2017;129(3):511–520. doi: 10.1097/AOG.0000000000001896. [DOI] [PubMed] [Google Scholar]
- 5.Gei A, Montufar-Rueda C. Pulmonary hypertension and pregnancy: an overview. Clin Obstet Gynecol. 2014;57(4):806–826. doi: 10.1097/GRF.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 6.Pitkin RM, Perloff JK, Koos BJ, Beall MH. Pregnancy and congenital heart disease. Ann Intern Med. 1990;112(6):445–454. doi: 10.7326/0003-4819-76-3-112-6-445. [DOI] [PubMed] [Google Scholar]
- 7.Regitz-Zagrosek V, Blomstrom LC, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, et al. ESC guidelines on the management of cardiovascular diseases during pregnancy: the task force on the Management of Cardiovascular Diseases during pregnancy of the European Society of Cardiology (ESC) Eur Heart J. 2011;32(24):3147–3197. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 8.Sliwa K, van Hagen IM, Budts W, Swan L, Sinagra G, Caruana M, Blanco MV, Wagenaar LJ, Johnson MR, Webb G, et al. Pulmonary hypertension and pregnancy outcomes: data from the registry of pregnancy and cardiac disease (ROPAC) of the European Society of Cardiology. Eur J Heart Fail. 2016;18(9):1119–1128. doi: 10.1002/ejhf.594. [DOI] [PubMed] [Google Scholar]
- 9.Martin SR, Edwards A. Pulmonary hypertension and pregnancy. Obstet Gynecol. 2019;134(5):974–987. doi: 10.1097/AOG.0000000000003549. [DOI] [PubMed] [Google Scholar]
- 10.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk NA, Beghetti M, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 11.Yang M, Wang J, Zhang X, Zhuang Q, Wang R, Shen J, Lin J. Incidence and long-term outcomes of pregnant women complicated with pulmonary arterial hypertension during different pregnancies: a prospective cohort study from China. Int J Cardiol. 2021;326:178–183. doi: 10.1016/j.ijcard.2020.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez SM, Krishna KR, Landzberg M, Machado RF, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q, Peng P, Liu X, Liu J, Gao J, Chen W. Evaluation of maternal and fetal outcomes in pregnancy complicated with pulmonary arterial hypertension. Ann Palliat Med. 2021;10(2):1404–1410. doi: 10.21037/apm-20-551. [DOI] [PubMed] [Google Scholar]
- 14.Hemnes AR, Kiely DG, Cockrill BA, Safdar Z, Wilson VJ, Al HM, Preston IR, MacLean MR, Lahm T. Statement on pregnancy in pulmonary hypertension from the pulmonary vascular research institute. Pulm Circ. 2015;5(3):435–465. doi: 10.1086/682230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liew N, Rashid Z, Tulloh R. Strategies for the management of pulmonary arterial hypertension in patients with congenital heart disease. J Congenit Cardiol. 2020;4(S1):1–8.
- 16.Al-Riyami N, Salman B, Al-Rashdi A, Al-Dughaishi T, Al-Haddabi R, Hassan B. Pregnancy outcomes in systemic lupus erythematosus women: a single tertiary Centre experience. Sultan Qaboos Univ Med J. 2021;21(2):e244–e252. doi: 10.18295/squmj.2021.21.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rashidi F, Sate H. Pregnancy outcome in a pregnant patient with idiopathic pulmonary arterial hypertension: a case report and review of the literature. J Med Case Rep. 2018;12(1):31. doi: 10.1186/s13256-017-1547-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koutrolou-Sotiropoulou P, Parikh PB, Miller C, Lima FV, Butler J, Stergiopoulos K. Impact of heart disease on maternal and fetal outcomes in pregnant women. Am J Cardiol. 2015;116(3):474–480. doi: 10.1016/j.amjcard.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 19.Martin SR, Edwards A. Pulmonary hypertension and pregnancy. Obstet Gynecol. 2019;134(5):974–987. doi: 10.1097/AOG.0000000000003549. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Li Y, Zhang J, Zhao W, Bao Z, Ma X, Zhao Y, Zhao C, Liu K, Ye Q, et al. Pregnancy complications and outcomes among women with congenital heart disease in Beijing China. Front Cardiovasc Med. 2021;8:765004. doi: 10.3389/fcvm.2021.765004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker DJP, Gelow J, Thornburg K, Osmond C, Kajantie E, Eriksson JG. The early origins of chronic heart failure: impaired placental growth and initiation of insulin resistance in childhood. Eur J Heart Fail. 2010;12(8):819–825. doi: 10.1093/eurjhf/hfq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting this study can be requested from the corresponding author.